Introduction
Small cell lung cancer (SCLC) is the most aggressive
type of primary lung cancer characterized by neuroendocrine
features, rapid tumor growth, early metastatic dissemination and a
poor prognosis (1). In general,
SCLC is highly sensitive and responsive to initial chemotherapy;
however, most patients eventually relapse and finally succumb to
the disease. While SCLC has been treated as a single disease
historically, recent studies have suggested that SCLCs can be
classified based on the expression of lineage factors, such as
achaete-scute homolog 1 (ASCL1), neurogenic differentiation factor
1 (NEUROD1), POU domain class 2 transcription factor 3 (POU2F3) and
transcriptional coactivator YAP1 (YAP1); SCLCs defined by the high
expression of either one of the first two factors are categorized
as neuroendocrine-high, while the latter two factors categorize the
disease as neuroendocrine-low (2-7).
However, the associations between these subtypes and clinical
characteristics, including tailored treatment options, have not
been well studied.
The present study reports a case of
neuroendocrine-low SCLC, which was resistant to standard
chemotherapy for SCLC, but responded to a regimen for non-SCLC
(NSCLC). Pathological assessment of the autopsy specimens after the
death of the patient revealed a dynamic transition of
differentiation status in a neuroendocrine cancer.
Case report
A 60-year-old man with a 20-pack-year history of
smoking visited Keiyu Hospital (Yokohama, Japan) due to chest pain
and dysphagia in December 2019. The patient had been treated for
hypertension and hyperuricemia at a previous hospital. Computed
tomography revealed a 71-mm mass in the lower lobe of the left
lung, enlarged left hilar and mediastinal lymph nodes, and left
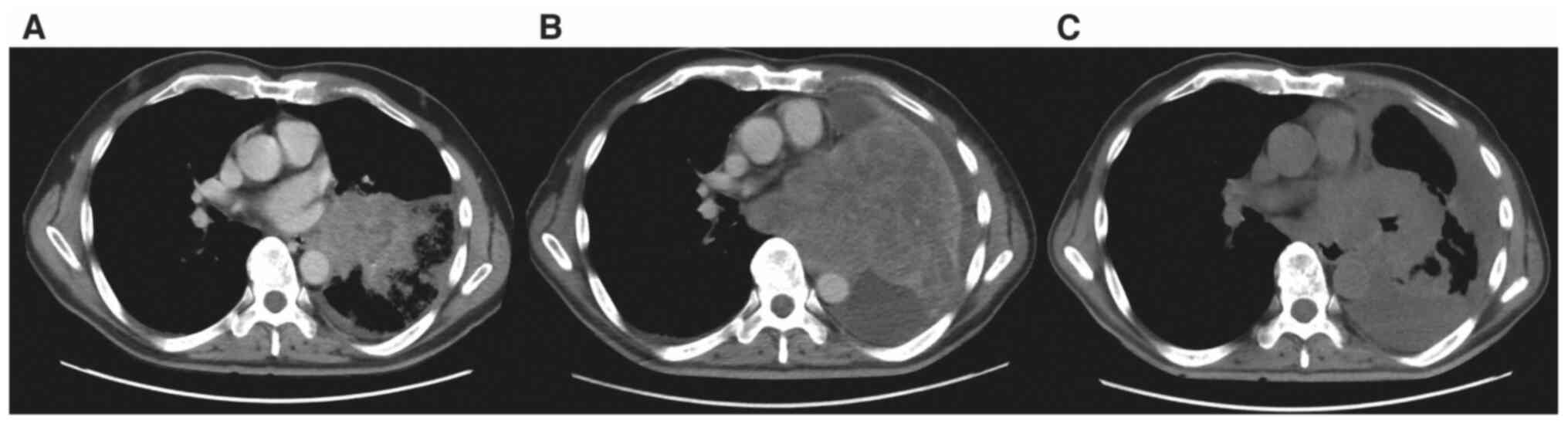
pleural effusion (Fig. 1A). A
transbronchial biopsy was performed and the pathological findings
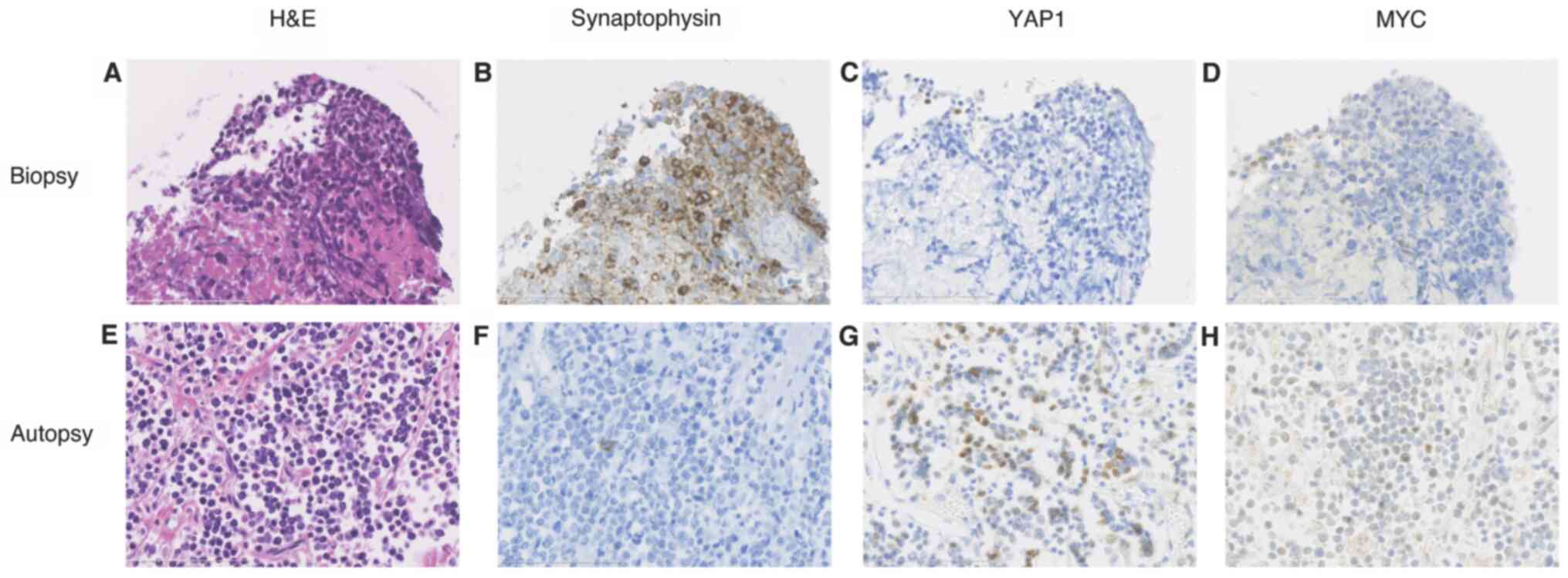
are shown in Fig. 2. The tumor was
considered to be small cell carcinoma as the tumor presented with
cells that were small and round in shape, with scant cytoplasm and
finely granular nuclear chromatin (Fig. 2A). Immunostaining was positive for
synaptophysin (Fig. 2B), but
negative for chromogranin, CD56 and thyroid transcription factor 1.
No brain metastasis was found by head magnetic resonance imaging
and thus, the patient was diagnosed with primary stage IVA SCLC
(cT4N2M1a), according to the eighth edition of the TNM
Classification for Lung Cancer (8). The biopsy specimens were negative for
ASCL1, NEUROD1, POU2F3 and YAP1. The presence of Myc proto-oncogene
protein (MYC)-expressing tumor cells was almost negative in most
areas in the biopsy specimens (Fig.
2D). Immunostaining method details are provide in Table SI.
First-line chemotherapy with carboplatin [area under
the blood concentration time curve (AUC) 5 on day 1] plus etoposide
(100 mg/m2 on days 1-3) was started. However, computed
tomography showed tumor progression in the primary lesion, and the
hilar and mediastinal lymph nodes. Although, atezolizumab (1,200 mg
on day 1) was administered in addition to carboplatin (AUC 5 on day
1) plus etoposide (100 mg/m2 on days 1-3) in the second
cycle, the tumor did not respond to the treatment, growing further
to 121 mm, and solid food intake was disturbed due to the
esophageal invasion (Fig. 1B).
Since the immunostaining patterns in the biopsy
specimens were atypical for SCLC, with only one positive
neuroendocrine marker, and the pathology represented only a small
part of the tumor due to the biopsy size, the patient was treated
with the NSCLC regimen of carboplatin (AUC 4.5 on day 1),
nanoparticle albumin-bound (nab) paclitaxel (100 mg/m2
on days 1, 8 and 15) and pembrolizumab (200 mg on day 1),
considering the possibility that a NSCLC component was present.
After one cycle, the symptom of dysphagia improved, and computed
tomography showed that the primary tumor was decreased in size to
84 mm (Fig. 1C). A second cycle of
chemotherapy (same dose as first cycle) was administered. At 11
days after the start of the second cycle, dyspnea developed,
leading to a diagnosis of bacterial pneumonia. Despite antibiotic
treatment, the patient succumbed 5 months after the initial
diagnosis.
A pathological autopsy was performed. The 95-mm
primary lesion was found to have direct involvement with the
pericardium, left atrium and esophagus. The tumor had metastasized
to the hilar and mediastinal lymph nodes, but no hematogenous
metastasis was found. The tumor contained small round cells and was
morphologically consistent with an SCLC (Fig. 2E). Immunostaining of the autopsy
specimens showed consistently negative results for chromogranin A,
CD56 and TTF-1, and almost all cells were negative for
synaptophysin (Fig. 2F). The
autopsy specimens were negative for ASCL1, NEUROD1 and POU2F3,
identical to the biopsy results. A negative result had been found
for YAP1 in the biopsy (Fig. 2C);
however, positive focal staining was detected in the autopsy
specimens (Fig. 2G). The
proportion of MYC-expressing tumor cells increased in the autopsy
samples compared with that in the biopsy specimens (Fig. 2D and H). This alteration in the expression of
neuroendocrine markers may reflect a dynamic transition to
neuroendocrine-low SCLC.
Discussion
In the present case of standard therapy-resistant
SCLC with a temporal response to a nab-paclitaxel-containing
regimen, pathological assessment of autopsy specimens enabled the
capture of the transition of its neuroendocrine fate. To the best
of our knowledge, evidence for neuroendocrine differentiation
shifting during SCLC evolution have been lacking in the clinical
setting.
Recent studies have suggested that SCLC is composed
of at least four molecular subtypes based on the expression of the
transcription factors ASCL1, NEUROD1, POU2F3 and YAP1 (2,6), and
that more than four-fifths of SCLC tumors belong to the
neuroendocrine-high SCLC-ASCL1 or SCLC-NEUROD1 subtypes (6,9,10).
The present case was originally negative for ASCL1 and NEUROD1, as
well as TTF-1 and two out of three neuroendocrine markers in the
biopsy specimens, suggesting a neuroendocrine-low status. The
biopsy specimens were also negative for POU2F3 and YAP1; therefore,
this case could not be categorized into one of those SCLC
subtypes.
Notably in the present case, the autopsy specimens
showed loss of synaptophysin expression and an increase in the
number of MYC-positive tumor cells that were focally positive for
YAP1, different from the results found in the biopsy specimens. In
this regard, using genetically engineered mouse models, a recent
study demonstrated that the SCLC subtypes are not independent but
rather different stages of the dynamic evolution of SCLC (11). Expression of the classic oncogene
MYC is hypothesized to be involved in this dynamic, with MYC
promoting lineage shifts from SCLC-ASCL1 to SCLC-NEUROD1 and from
SCLC-NEUROD1 to SCLC-YAP1 (11,12).
We hypothesize that the present case may be in the dynamic
transition stage of its neuroendocrine fate, possibly under the
influence of the anticancer drug treatments. It has been reported
that the expression of MYC is elevated in tumor cells surviving
cisplatin chemotherapy (13). The
neuroendocrine differentiation state may have shifted due to the
upregulation of MYC expression after platinum chemotherapy in the
present case. Future investigations are needed to further
illustrate the cell lineage plasticity of human SCLCs.
Associations between the SCLC subtypes and clinical
characteristics have been reported. NEUROD1 expression was found to
be higher in extensive SCLC than in limited SCLC (14); SCLC-YAP1 was found to be associated
with a shorter patient survival time and increased chemotherapy
resistance (3). It is possible
that the undetermined neuroendocrine-low status of the SCLC in the
present study was related to its resistance to the standard
platinum-based chemotherapy. However, it responded to the
nab-paclitaxel-containing regimen. There have been several reports
of paclitaxel or nab-paclitaxel showing antitumor activity against
SCLC (15,16). In the present case, nab-paclitaxel
may have contributed to the therapeutic effect, as it is unlikely
that continuous use of carboplatin or the switch from atezolizumab
to pembrolizumab resulted in the marked antitumor effect. However,
nab-paclitaxel shows modest antitumor activity only in a small
proportion of relapsed SCLC (17).
Further investigations are needed to elucidate the background of
SCLC tumors that are sensitive to nab-paclitaxel.
In summary, the present case was an atypical SCLC
that responded only to the nab-paclitaxel-containing regimen.
Immunostaining suggested its original neuroendocrine-low status and
a dynamic shift of its neuroendocrine fate during the disease
trajectory. Establishment of precision medicine, considering the
heterogeneity and plasticity of SCLC differentiation states, is
warranted.
Supplementary Material
Immunohistochemical antibodies and
protocols.a
Acknowledgements
The authors would like to thank Ms. Ayushi Patel
(New York University School of Medicine, New York, USA) for helpful
discussions and English language editing.
Funding
This study is supported in part by the Grants-in-Aid for
Scientific Research from the Japan Society for the Promotion of
Science (grant no. 20K17192).
Availability of data and materials
The data that support the findings in this case are
available from the corresponding author upon reasonable
request.
Authors' contributions
FI, TSa, NK, KY, RW, MH and TSh made the diagnosis
and treated the patient. TSa designed the study. FI, TSa, KE, HN,
YI, KT, HD collected and analyzed the data. FI and TSa wrote the
manuscript. All authors contributed to the interpretation of the
data and reviewed the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent to publish the case
information and related images was obtained from the wife of the
deceased patient.
Competing interests
Dr Takashi Sato reports personal fees from Chugai
Pharmaceutical (Tokyo, Japan) and Bristol Myers Squibb (Tokyo,
Japan), outside of the submitted work. Dr Hironori Ninomiya reports
personal fees from Merck Sharp and Dohme (Tokyo, Japan) outside the
submitted work. Dr Kentaro Tanaka reports grants and personal fees
from Chugai Pharmaceutical, and personal fees from Taiho
Pharmaceutical (Tokyo, Japan), outside the submitted work. Dr
Tetsuya Shiomi reports personal fees from Boehringer Ingelheim
(Tokyo, Japan), Bristol Myers Squibb, Chugai Pharmaceutical, Pfizer
(Tokyo, Japan) and Taiho Pharmaceutical, outside the submitted
work. The remaining authors declare no conflicts of interest.
References
|
1
|
Jackman DM and Johnson BE: Small-cell lung
cancer. Lancet. 366:1385–1396. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rudin CM, Poirier JT, Byers LA, Dive C,
Dowlati A, George J, Heymach JV, Johnson JE, Lehman JM, MacPherson
D, et al: Molecular subtypes of small cell lung cancer: A synthesis
of human and mouse model data. Nat Rev Cancer. 19:289–297.
2019.PubMed/NCBI View Article : Google Scholar : Erratum in: Nat Rev
Cancer 19: 415, 2019.
|
|
3
|
McColl K, Wildey G, Sakre N, Lipka MB,
Behtaj M, Kresak A, Chen Y, Yang M, Velcheti V, Fu P, et al:
Reciprocal expression of INSM1 and YAP1 defines subgroups in small
cell lung cancer. Oncotarget. 8:73745–73756. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Borromeo MD, Savage TK, Kollipara RK, He
M, Augustyn A, Osborne JK, Girard L, Minna JD, Gazdar AF, Cobb MH,
et al: ASCL1 and NEUROD1 reveal heterogeneity in pulmonary
neuroendocrine tumors and regulate distinct genetic programs. Cell
Rep. 16:1259–1272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang YH, Klingbeil O, He XY, Wu XS, Arun
G, Lu B, Somerville TDD, Milazzo JP, Wilkinson JE, Demerdash OE, et
al: POU2F3 is a master regulator of a tuft cell-like variant of
small cell lung cancer. Genes Dev. 32:915–928. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Poirier JT, George J, Owonikoko TK, Berns
A, Brambilla E, Byers LA, Carbone D, Chen HJ, Christensen CL, Dive
C, et al: New approaches to SCLC therapy: From the Laboratory to
the Clinic. J Thorac Oncol. 15:520–540. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Horie M, Saito A, Ohshima M, Suzuki HI and
Nagase T: YAP and TAZ modulate cell phenotype in a subset of small
cell lung cancer. Cancer Sci. 107:1755–1766. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: International Association for the
Study of Lung Cancer Staging and Prognostic Factors Committee,
Advisory Boards, and Participating Institutions: The IASLC lung
cancer staging project: Proposals for revision of the TNM stage
groupings in the forthcoming (eighth) edition of the TNM
classification for lung cancer. J Thorac Oncol. 11:39–51.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pearsall SM, Humphrey S, Revill M, Morgan
D, Frese KK, Galvin M, Kerr A, Carter M, Priest L, Blackhall F, et
al: The rare YAP1 subtype of SCLC revisited in a biobank of 39
circulating tumor cell patient derived explant models: A brief
report. J Thorac Oncol. 15:1836–1843. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Baine MK, Hsieh MS, Lai WV, Egger JV,
Jungbluth AA, Daneshbod Y, Beras A, Spencer R, Lopardo J, Bodd F,
et al: SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A
comprehensive immunohistochemical and histopathologic
characterization. J Thorac Oncol. 15:1823–1835. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ireland AS, Micinski AM, Kastner DW, Guo
B, Wait SJ, Spainhower KB, Conley CC, Chen OS, Guthrie MR, Soltero
D, et al: MYC drives temporal evolution of small cell lung cancer
subtypes by reprogramming neuroendocrine Fate. Cancer Cell.
38:60–78.e12. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Patel AS, Yoo S, Kong R, Sato T, Sinha A,
Karam S, Bao L, Fridrikh M, Emoto K, Nudelman G, et al:
Prototypical oncogene family Myc defines unappreciated distinct
lineage states of small cell lung cancer. Sci Adv.
7(eabc2578)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Walker TL, White JD, Esdale WJ, Burton MA
and DeCruz EE: Tumour cells surviving in vivo cisplatin
chemotherapy display elevated c-myc expression. Br J Cancer.
73:610–614. 1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ikematsu Y, Tanaka K, Toyokawa G, Ijichi
K, Ando N, Yoneshima Y, Iwama E, Inoue H, Tagawa T, Nakanishi Y, et
al: NEUROD1 is highly expressed in extensive-disease small cell
lung cancer and promotes tumor cell migration. Lung Cancer.
146:97–104. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sugiyama K, Kogure Y, Torii A, Shiraishi
K, Yamada A, Ishida A, Shigematsu F, Nozawa K, Niwa H, Oka S, et
al: Solvent-based paclitaxel or nab-paclitaxel for heavily treated
relapsed/refractory small cell lung cancer: Retrospective
single-institution observational study. Medicine (Baltimore).
98(e14758)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Naito Y, Tamiya A, Tamiya M, Kimura Y,
Hamaguchi M, Saijo N, Kanazu M, Tokura S, Shiroyama T, Morisita N,
et al: Efficacy of nanoparticle albumin-bound paclitaxel regimens
for relapsed small cell lung cancer: A retrospective analysis.
Medicine (Baltimore). 96(e7884)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gelsomino F, Tiseo M, Barbieri F, Riccardi
F, Cavanna L, Frassoldati A, Delmonte A, Longo L, Dazzi C, Cinieri
S, et al: Phase 2 study of NAB-paclitaxel in SensiTivE and
refractory relapsed small cell lung cancer (SCLC) (NABSTER TRIAL).
Br J Cancer. 123:26–32. 2020.PubMed/NCBI View Article : Google Scholar : Erratum in: Br J
Cancer 125: 306, 2021.
|