Introduction
Over 90% of testicular tumors are derived from the
germ cells, which represent the main cell population of the testis.
Testicular germ cell tumors (TGCTs) tend to occur most frequently
in the age range of 15-35 years. The most commonly encountered type
of histological pattern of the disease is the non-seminomatous TGCT
(NS-TGCT) type, which peaks at 25 years (1). Among the risk factors associated with
developing testicular cancer are conditions such as Klinefelter
syndrome, cryptorchidism, a family history of testicular tumors,
previous history of a testicular tumor, sperm abnormalities and
infertility (1-3).
In TGCTs, there are at least two possible histological types.
Often, the combinations include seminoma and embryonal carcinoma,
teratoma and yolk sac tumor. The prognosis becomes even poorer when
aggressive histotypes are present (1). Spontaneous tumor regression has been
reported in various neoplasias (2,4). One
such case of regression is the so-called ‘burned-out’ testicular
tumor. This is a TGCT that has regressed without intervention, and
usually presents as metastasis to other sites (manifesting as
secondary symptoms), such as the retroperitoneal and mediastinal
regions (5). This occurrence of
metastasis can present as raised tumor markers and as a dubious
ultrasound image for the testis. The most likely condition to burn
out is seminoma with the next most likely to burn out being
embryonal carcinoma; however, it has previously been reported this
process is unlikely to occur in the case of teratoma (6). Nevertheless, such an occurrence of a
burned-out testicular teratoma that metastasized to the
retroperitoneal and mediastinal areas without any palpable lesions
on testicular examination, nor without ultrasound evidence of
tumor, is documented in the present case report.
Case report
A 29-year-old male was admitted to the Internal
Medicine Unit of another hospital due to symptoms of incomplete
ileus (diffuse abdominal discomfort, nausea and abdominal
distension), associated with fever and loss of appetite; the
patient had no relevant past medical history, without common risk
factors for testicular cancer (such as the presence of tumor in the
contralateral testis, history of cryptorchidism or undescended
testis, hypotrophic testis and Klinefelter syndrome). Furthermore,
no family history of testicular cancer was reported among first
degree relatives. A Computed Tomography (CT) scan revealed the
presence of lymphadenopathies affecting the neck, mediastinum and
retroperitoneum. In particular, the lymphadenopathies of the
retroperitoneum and of the left external iliac site were
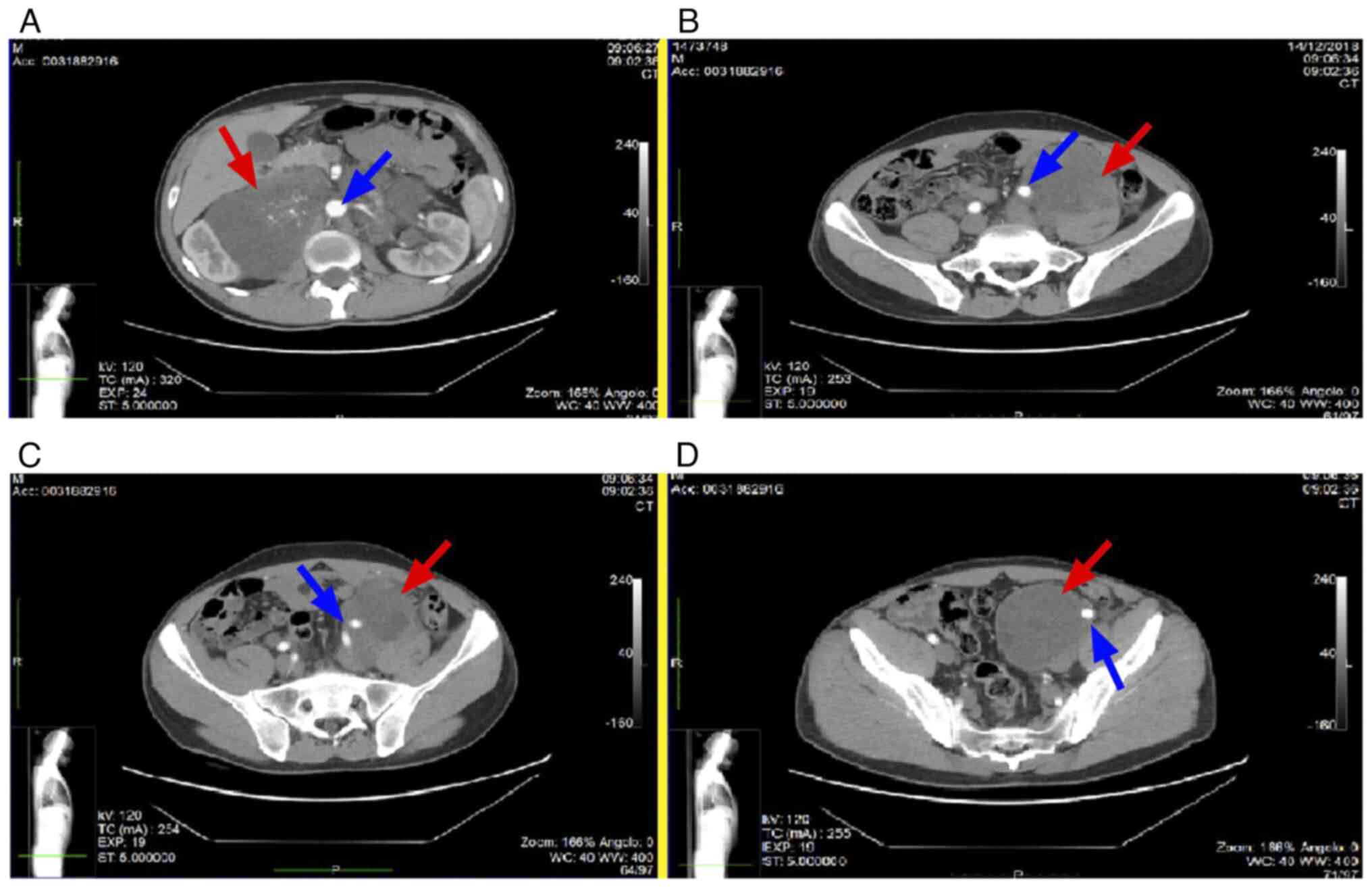
voluminous, forming packages up to 18 cm in diameter, with
infiltration of the large retroperitoneal vessels (Fig. 1A), the left common iliac vessel
(Fig. 1B) and the left external
iliac vessel (Fig. 1C and D).
The levels of baseline plasmatic specific biomarkers
were as follows: Lactate dehydrogenase (LDH), 3006 U/l (normal
values: 122-222 U/l); α-fetoprotein (α-FP), 8160 ng/ml (normal
values <10 ng/ml); and β-human chorionic gonadotropin (β-HCG),
47382 mIU/ml (normal values: 0-5 mIU/ml). Physical examination
revealed a palpable abdominal mass, although both testicles
appeared normal. Testicular ultrasound revealed no focal
alterations, although the right testis was atrophic, with a
rarefied eco-structure. An excisional biopsy of the left
sternocleidomastoid lymph nodes revealed a mixed non-seminomatous
germ cell neoplasm, with prevalent aspects of mature teratoma while
the minority component was represented by embryonic carcinoma
(CD30+). The patient started a 3-cycle bleomycin, etoposide and
cisplatin (PEB) chemotherapy. At the end of the course, the
plasmatic biomarker levels were as follows: β-HCG, 11,9 mIU/ml;
α-FP, 162 ng/ml; and LDH, 144 U/l.
A post-chemo CT scan disclosed a negligible decrease
in the lymph node mass of the mediastinum, confirming the presence
of voluminous pathological lymph nodes in the retroperitoneum (with
bilateral hydronephrosis). A fourth cycle of PEB chemotherapy was
scheduled but, despite a further decrease in the levels of the
tumor markers (β-HCG, 9,5 mIU/ml and α-FP, 4,6 ng/ml), the lymph
node masses did not decrease in size. Following an endoscopic
positioning of a double J stent bilaterally, an open
retroperitoneal lymph node dissection (RPLND) was performed,
including nodes of the left renal hilum and intercaval-aortic,
para-caval, para-aortic and left iliac and obturator nodes.
Histological evaluation revealed a disorder of normal architecture
of nodes structure due the presence of widespread mature teratoma
(immunohistochemical examination, OCT3/4-, CD30-).
A post-operative CT scan was repeated 2 months
later, which revealed no abdominal residual masses. At 3 months
after surgery, the ureteral stents were removed. Subsequently, the
patient was admitted to the Thoracic Surgery Unit for an open
removal of thymus, subcarinal lymph node extending up to the arc of
the azygos, left latero-cervical lymph node and neoformation of the
anterior mediastinum. The thymic parenchyma resulted in partial
adipose involution, but the other three samples were affected by
germinal neoplasia, consisting of both cystic and solid areas, with
elements of derivation of the three embryonic sheets. Respiratory,
gastro-enteric and Mullerian epithelium were identified. There were
no structures referable to the lymph node. At 6 months'
post-operation, a control CT scan revealed a hypodense formation in
the anterior para-aortic site, strictly adjacent to the aortic
arch. Furthermore, the retroperitoneum was affected by tumor
recurrence. In particular, the following tumors were documented: in
the lumbar aortic left region, long and close to the common iliac
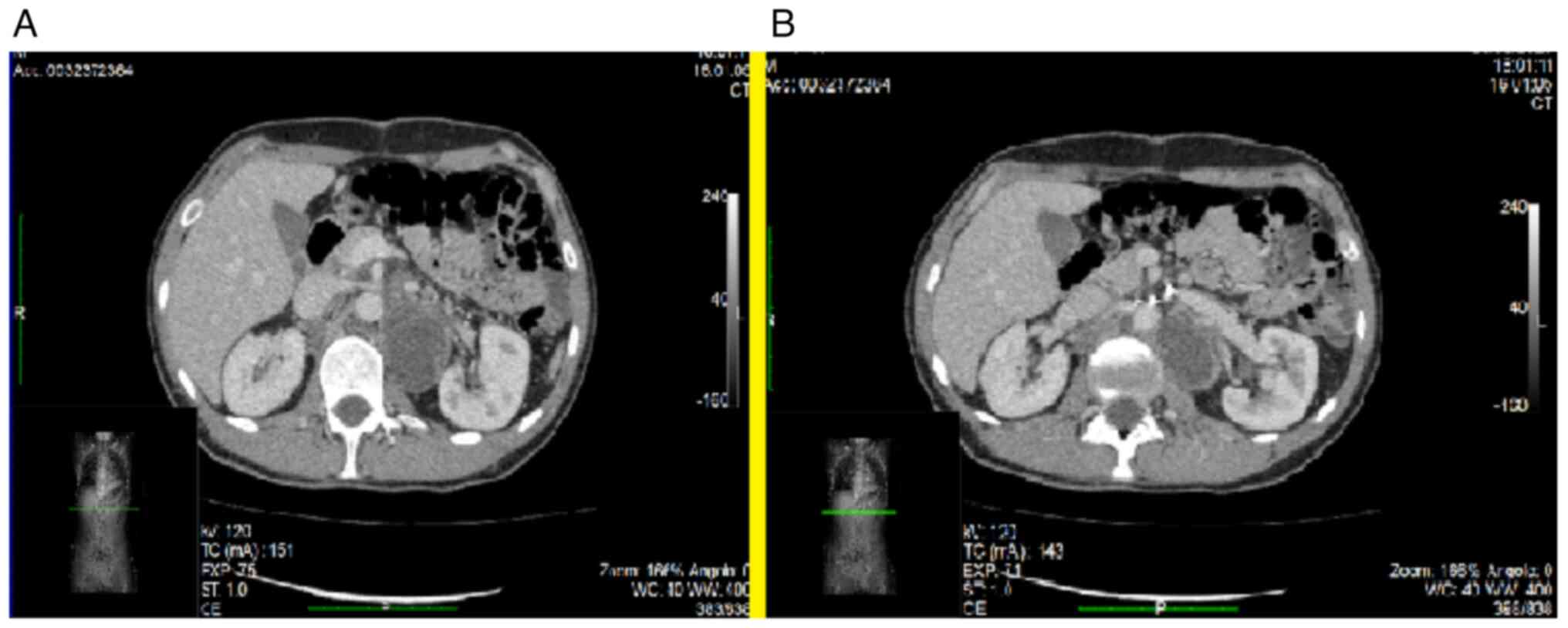
and external left vascular axis (Figs.
2A and B and 3B); and in the retrocaval region that
compressed and displaced the venous vascular lumen anteriorly
(Fig. 3A).
No evolutionary densitometric changes affecting the
parenchymatous organs of the abdomen were identified. Neither were
any suspicious focal lesions evidently due to secondary reactions
of the skeleton included in the volume under examination. Given the
low efficacy of pre-intervention chemotherapy in reducing the size
of the tumor masses, it was decided against treating the patient
with further chemotherapy cycles. Instead, at 12 months after the
first operation, a second RPLND was performed, during which the
left retro-ilar lymph nodes, para-caval lymph nodes, common right
iliac lymph nodes and common and external left iliac lymph nodes
were removed. Upon anatomopathological examination, the samples
were found to be formed from fibro-adipose tissue in which no
defined lymph node structures were recognized, and which also
formed the site of massive secondary localization from germinal
neoplasia of the teratoma type (with areas of necrosis and
calcification), showing (in immunohistochemical examination)
negativity for OCT3/4 and CD30. Six months have passed since the
last surgery and, at CT follow-up checks, no neoplastic recurrences
have been identified in the abdomen. At present, the patient is
preparing for a second chest surgery.
Discussion
GCTs represent about 1-1,5% of all human cancers,
being the most frequent malignancy in males between the ages of 15
and 40(7). The incidence of
testicular cancer is 3-6 new cases per year per 100,000 males in
Western countries, with an increase in the incidence observed in
the last 30 years (8). About 95%
are primary testicular neoplasms, while a primitive extragonadal
site is less common. Although not common, EGCTs are, nevertheless,
well-known neoplasms. Between 3-5% of all GCTs are primary EGCTs.
Of these tumors, in excess of 60% of them are seminomas originating
in the anterior mediastinum and retroperitoneum. Instead, in total,
~60% of all cases of testicular mixed GCTs present with an advanced
disease stage (stage II or III). Metastases most frequently occur
at an early stage in the retroperitoneal lymph nodes. These can
then develop to associate with mediastinal and supraclavicular
lymph nodes (1,9). Occasionally, TGCTs can recede without
intervention after their metastatic extension to the lymph nodes.
The term ‘burned-out’ is often used to describe this unusual
regression, and the burn out may be either partial or total
(10). Previously, numerous of
these cases were categorized as primary extragonadal GCTs (EGCTs),
although in a number of cases spontaneous regression of the TGCT
was recorded. An addition to the 2016 WHO classification is
expanded discussion of ‘burnt-out’ germ cell tumour (11). Findings in the testis of such
patients typically include a scar, reduced spermatogenesis and
microlithiasis. However, findings that have been proposed as
specific for germ cell tumour regression rather than non-neoplastic
scarring are limited to: i) Germ Cell Neoplasia in Situ
(GCNIS) in the adjacent parenchyma; and ii) coarse, large
intratubular calcifications. However, these coarse calcifications
must be distinguished from microlithiasis (small, rounded
calcifications), which may be found in the adjacent parenchyma of
germ cell tumour patients but are not specific for the presence of
tumour. Overall, pathologists must be aware in the setting of
scarring that, even if careful search does not reveal these highly
specific lesions (GCNIS or coarse calcifications), the possibility
of germ cell tumour regression remains a consideration for any
testicular ‘scar’ and this must be communicated to clinical
colleagues to ensure appropriate follow-up. As also highlighted in
their review by Astigueta et al (12), there are not many publications on
the regression of testicular GCTs and this entity has only recently
been recognised in the last edition of the WHO's book on Tumours of
the Urinary System and Male Genital Organs (2016), in the chapter
on testicular tumours and paratesticular tissues (11). It is considered that the first to
describe this phenomenon was Prym in 1927(13); he, during an autopsy, recorded
testicular scarring in a patient who had an extragonadal tumor.
Azzopardi et al (14) have
documented the presence of cell-poor, collagen-rich fibrous scars
in some otherwise substantially normal testes of patients with
metastatic NS-TGCTs. The authors uncovered hematoxyphilic deposits
in seminiferous tubuli, which they identified as hematoxyphilic
bodies. Comiter et al (15)
recorded widespread degeneration of the testis with hematoxyphilic
and psammoma forms in a number of cases, which they concluded as
being representative of echogenic foci. In all instances, a fibrous
scar was found in the testis, a condition which brings to an end
the spontaneous reversal of a primary GCT after metastasis has
diffused to different locations. A plausible explanation is the
occurrence of an immune response, with an ischemia resulting from
the high metabolic rate of the neoplasm outgrowing its blood
supply. Burned-out tumors can be difficult to diagnose, as
secondary tumors are often erroneously diagnosed as primary tumors.
Discriminatory diagnosis of burned-out TGCT leads to a
consideration of EGCTs as far as the diagnosis is concerned,
although their occurrence is rare (between 5-10% of all TGCTs); the
most common location of these is in the retroperitoneal region.
However, if scarring in the testis is observed, the possibility of
the reversal of the GCT must also be considered. The main
difficulty in reaching a correct diagnosis is that of deciding
whether a neoplasm under investigation is either primary or
secondary in origin. In these situations, performing a testicular
ultrasound is obligatory. The same prognosis applies to metastatic
disease, which occurs secondarily to burned-out lesion (as the
primary testicular malignancy); accordingly, it is essential that
it is accurately diagnosed. A ‘burned-out’ TGCT is an exceptional
finding, and it is important to recognize that many burned-out TGCT
cases lack molecular sequencing. This fact can hinder the
understanding of this phenomenon; accordingly, further research is
required. It is well established that all germinal tumors have the
potential for regression; however, there is disagreement in the
literature regarding the frequency of the histological subtypes
that provide evidence of this phenomenon. Previously,
choriocarcinoma [a non-seminomatous neoplasm, included in the group
of germline tumors derived from GCNIS according to WHO 2016
classification (11)] was
considered to be the most likely to recede but, more recently,
seminoma has been identified as the most common histology, apart
from the spermatocytic type, which is now categorized as a separate
entity [in the group of germline cancers not derived from GCNIS
according to WHO 2016 classification (11)]. The teratomas [which are
non-seminomatous neoplasms, included in the group of germline
tumors derived from GCNIS according to WHO 2016 classification
(11)] remain as the histological
group with the lowest likelihood of regression (16-20).
Previous studies, as claimed also by Mosillo et al (21) in their case report, have shown that
lymph-node diffuse metastases in testicular mature teratoma tend to
be caused by aggressive germ cell components that have undergone
early regression. That is what we consider best explains what
occurred in the present case, although this is purely conjectural
as it cannot be manifestly supported by histological evaluation of
orchiectomy. However, the result of the left sternocleidomastoid
lymph node excisional biopsy (i.e., mixed, non-seminomatous germ
cell neoplasm with prevalent aspects of mature teratoma) is
supportive of the present hypothesis. Furthermore, the scrotal
ultrasound detection of an atrophic and rarefied right testicle was
an important finding. In fact, two works entitled ‘Shrinking
Seminoma’ and ‘Shrinking Seminoma-Fact or Fiction?’
(22,23), published in 1990 and 2000
respectively, described a reduction in volume in a testicle with
seminoma, where the underlying mechanism was essentially
ischemia-necrosis secondary to intermittent testicular torsion.
However, other potential causes that need to be considered include
chronic inflammation and hormonal disorder. Depending on which
stage the condition has reached when it is diagnosed, a testicle
shrunken in size (with or without a residual tumor) may be found.
For this reason, when retroperitoneal, mediastinal or other types
of mass that also present as testicular shrinking is found in a
patient, a GCT should be suspected (22,23).
Where echogenicity arises in a focal area of ultrasonographic
images of burned-out tumors, this is likely to be due to calcium
deposits and fibrosis. However, when punctuate echogenic foci are
observed, but without any evidence of any hypoechoic mass lesions,
a burned-out testicular tumor should be suspected (24). Similarly, in their review, Kreydin
et al (25) claim that, on
occasion, the initial presentation of testicular cancer may be a
retroperitoneal mass, with testicular imaging not revealing an
evident lesion. In these cases, according to them, a testicular
malignancy must be considered (25). Mola Arizo et al (26) contend that the presence of
retroperitoneal tumors with ultrasonographical abnormalities
observed upon testicular examination should be considered as
metastases of a burned-out testicular cancer, and that surgical
evaluation should be conducted in an individual setting (27). Gomez Parada et al (27) state that impalpable primary
testicular tumor should be suspected in patients who present with
an EGCT and normal testes on physical examination. As also argued
by Kontos et al (28), who
described the regression of primary testicular seminoma after the
development of metastasis in the retroperitoneum, in patients with
a retroperitoneal mass, diagnosis of metastatic progression of a
germ cell neoplasia should be considered. A burned-out testicular
tumor shows a distinctive constellation of findings that usually
permits its recognition (such as a reduced volume testis and
punctuate echogenic foci on ultrasound examination). This guidance
draws attention to understanding the importance of the tumor
markers and ultrasound in making the diagnosis. Furthermore, in
conducting our own literature search, it was observed that, in all
cases of testicular tumor burn-out syndrome which were treated with
orchiectomy, tumor cells were not detected in the histological
sample of the tissue. For this reason, given the result of the
biopsy of the sternocleidomastoid lymph node and the relevance of
the ultrasound findings, as reported in the literature, our
consideration is that the diagnostic elements in the present case
were sufficient to support a diagnosis of burned-out testicular
teratoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RB made substantial contributions to conception and
design of the present study, and was the major contributor in
writing the manuscript. RS, GN and PV were involved in revising the
manuscript critically for important intellectual content and made
important contributions to interpretation of data. MA, UDM, OI, GM
and AL made substantial contributions to acquisition of data and
data analysis. All the authors read and approved the final
manuscript and agreed to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and resolved.
RS and RB confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bosl GJ and Motzer NJ: Testicular
germ-cell cancer. N Engl J Med. 337:242–253. 1997.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cole WH and Everson TC: Spontaneous
regression of cancer preliminary report. Ann Surg. 144:366–383.
1956.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Looijenga LH and Oosterhuis JW:
Pathogenesis of testicular germ cell tumors. Rev Reprod. 4:90–100.
1999.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Salman T: Spontaneous tumor regression. J
Oncol Sci. 2:1–4. 2016.
|
|
5
|
Johnson K and Brunet B: Brain metastases
as presenting feature in ‘burned out’ testicular germ cell tumor.
Cureus. 8(e551)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fabre E, Jira H, Izard V, Ferlicot S,
Hammoudi Y, Theodore C, Di Palma M, Benoit G and Droupy S:
‘Burned-out’ primary testicular cancer. BJU Int. 94:74–78.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cancer incidence in five continents, vol
VIII. IARC Sci Publ. 155:1–781. 2002.PubMed/NCBI
|
|
8
|
Huyghe E, Matsuda T and Thonneau P:
Increasing incidence of testicular cancer worldwide: A review. J
Urol. 170:5–11. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Surveillance, Epidemiology and End Result
Program (SEER): Cancer Stat Facts: Testicular Cancer. National
Cancer Institute, Bethesda, MD, 2015. https://seer.cancer.gov/statfacts/html/testis.html.
|
|
10
|
Calvo OA, Rodríguez Alonso A, Pérez García
D, Domínguez Freire F, Alonso Rodrigo A, Rodríguez Iglesias B,
Benavente Delgado J, Barros Rodríguez JM, Fiaño Valverde C and
Nogueira March JL: Extragonadal germ cell tumor with ‘burned-out’
phenomenon in the testis. Actas Urol Esp. 23:880–884.
1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ulbright T, Amin M, Balzer B, et al:
Tumors of the testis and paratesticular tissue. In: WHO
Classification of Tumors of the Urinary System and Male Genital
Organs. Moch H, Humphrey PA, Ulbright TM and Reuter VE (eds). 4th
edition. Lyon: IARC Press, pp185-258, 2016.
|
|
12
|
Astigueta JC, Abad-Licham MA, Agreda FM,
Leiva BA and De la Cruz JL: Spontaneous testicular tumor
regression: Case report and historical review.
Ecancermedicalscience. 12(888)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Prym P: Spontanheilung eines bosartigen,
wahrscheinlich chorionephiteliomatosen Gewachses im Hoden. Virchows
Arch Pathol Anat. 265:239–258. 1927.
|
|
14
|
Azzopardi JG, Mostofi FK and Theiss EA:
Lesions of testes observed in certain patients with widespread
choriocarcinoma and related tumors. The significance and genesis of
hematoxylin-staining bodies in the human testis. Am J Pathol.
38:207–225. 1961.PubMed/NCBI
|
|
15
|
Comiter CV, Renshaw AA, Benson CB and
Loughlin KR: Burned-out primary testicular cancer: Sonographic and
pathological characteristics. J Urol. 156:85–88. 1996.PubMed/NCBI
|
|
16
|
Balzer BL and Ulbright TM: Spontaneous
regression of testicular germ cell tumors: An analysis of 42 cases.
Am J SurgPathol. 30:858–865. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Extramiana J, De La Rosa F, Madero S,
Tagarro D, Díaz González R, Martínez M, Gandía V, Leiva O and
Borobia V: ‘Burned-out’ testicular tumor. Actas Urol Esp.
10:289–294. 1986.PubMed/NCBI(In Spanish).
|
|
18
|
Angulo JC, González J, Rodríguez N,
Hernández E, Núñez C, Rodríguez-Barbero JM, Santana A and López JI:
Clinicopathological study of regressed testicular tumors (apparent
extragonadal germ cell neoplasms). J Urol. 182:2303–2310.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
López JI and Angulo JC: Burned-out tumor
of the testis presenting as Retroperitoneal choriocarcinoma. Int
Urol Nephrol. 26:549–553. 1994.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tejido Sánchez A, Villacampa Aubá F,
Martín Muñoz MP, Rosino Sánchez A, Cruceyra Betriu G, Martínez
Silva V and Leiva Galvis O: Burned-out testicular tumor. Arch Esp
Urol. 53:447–452. 2000.PubMed/NCBI(In Spanish).
|
|
21
|
Mosillo C, Scagnoli S, Pomati G,
Caponnetto S, Mancini ML, Bezzi M, Cortesi E and Gelibter A:
Burned-Out testicular cancer: Really a different history. Case Rep
Oncol. 10:846–850. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Simpson AH, Calvert DG and Codling BW: The
shrinking semonima. J R Soc Med. 83(187)1990.PubMed/NCBI
|
|
23
|
Naseem S, Azzopardi A, Shrotri N and Mufti
GR: The shrinking seminoma-fact or fiction? Urol Int. 65:208–210.
2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yucel M, Kabay S, Saracoglu U, Yalcinkaya
S, Hatipoglu NK and Aras E: Burned-out testis tumor that
metastasized to retroperitoneal lymph nodes: A case report. J Med
Case Rep. 3(7266)2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kreydin EI, Barrisford GW, Feldman AS and
Preston MA: Testicular cancer: What the radiologist needs to know.
AJR Am J Roentgenol. 200:1215–1225. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mola Arizo MJ, Gonzalvo Perez V,
Torregrosa Maicas MD, Navarro Anton JA, Gomez-Ferrer Lozano A,
Estany Perez A and Polo Peris AC: Burn out bilateral testicular
tumor. Actas Urol Esp. 29:318–321. 2005.PubMed/NCBI(In Spanish).
|
|
27
|
Gomez Parada J and Puyol Pallas M: Occult
primary testicular tumor or burnt testicular tumor. Apropos of a
case. Arch Esp Urol. 49:635–638. 1996.PubMed/NCBI(In Spanish).
|
|
28
|
Kontos S, Doumanis G, Karagianni M,
Politis V, Simaioforidis V, Kachrilas S and Koritsiadis S:
Burned-out testicular tumor with retroperitoneal lymph node
metastasis: A case report. J Med Case Rep. 3(8705)2009.PubMed/NCBI View Article : Google Scholar
|