Introduction
Epithelial ovarian carcinoma generally has poor
prognosis; an estimated 313,959 new cases and 207,252 deaths were
registered worldwide in 2020(1).
Among epithelial ovarian carcinomas, ovarian clear cell carcinoma
(OCCC) demonstrates chemoresistance (2,3) and
is more frequent in Japan than in other countries (4). Thus, a novel therapeutic approach for
OCCC is required, particularly in Japan.
Although there are several reports on the influence
of systematic lymphadenectomy on the prognosis of stage I OCCC, the
data are controversial (5,6). A previous study by our group reported
that removing more lymph nodes in patients with stage I OCCC
increased progression-free survival (PFS), suggesting that the
number of removed lymph nodes is an independent predictor of
progression for stage I OCCC (7).
In that study, the population was confined to patients with stage I
OCCC. To the best of our knowledge, there has been no study
examining whether the number of removed lymph nodes is an
independent predictor of progression for stage II or higher
OCCC.
The present article provided a single-institutional
retrospective study to investigate whether specific conditions,
particularly the number of removed lymph nodes, are independent
predictors of progression in patients with stage II or higher OCCC
with systematic lymphadenectomy. Furthermore, the significance of
the influence of the number of removed lymph nodes was evaluated
according to the International Federation of Gynecology and
Obstetrics (FIGO) stage of OCCC.
Materials and methods
Subjects
This study was approved by the ethics committee of
Jichi Medical University Hospital (Tochigi, Japan; no. Rindai
15-121). The subjects were patients with stage I to IV OCCC who
underwent total hysterectomy, bilateral salpingo-oophorectomy,
omentectomy and systematic retroperitoneal lymphadenectomy between
January 1993 and December 2015 at Jichi Medical University Hospital
(Tochigi, Japan). Patients who had >20 lymph nodes removed were
included. Exclusion criteria were active double cancer and/or
neo-adjuvant chemotherapy. Their medical records were
retrospectively reviewed. Those patients with stage I up to
December 2013 were the same as those in the previous study by our
group (7).
Clinical data
Clinicopathological variables, including age,
Eastern Cooperative Oncology Group performance status (ECOG PS),
complications, tumor diameter, endometriosis, FIGO stage, number of
removed lymph nodes, operator, residual tumor, lymph node
metastasis, adjuvant chemotherapy, progression, PFS and overall
survival (OS) were obtained from the medical records of the
subjects. The recurrence site was distinguished between regional
node recurrence and others. Cases of simultaneous regional node
recurrence and others were assigned to the regional node recurrence
group. PFS was defined as the time elapsed between the date of
operation and the date of progression or the date of the last
follow-up. Progression was defined by the RECIST 1.1 criteria
(8). OS was defined as the time
elapsed between the date of operation and the date of death or last
follow-up. According to internal clinical practice guidelines, the
patients were followed up for >10 years and follow-up was
performed every 1-2 months in the first year, every 2-3 months in
the second year, every 3-6 months in the third year, every 4-6
months in the fourth year, every 6 months in the fifth year and
yearly thereafter.
Statistical analyses
The best cut-off value of the number of removed
lymph nodes for predicting progression was calculated using the
receiver operating characteristic curve. The median values were
adopted as the cut-off values of age and tumor diameter. The values
of age, tumor diameter and number of removed lymph nodes were
expressed as median (total range). PFS was estimated using the
Kaplan-Meier method. The significance of the difference in survival
distribution between subgroups was evaluated using the log-rank
test. Variables with P<0.05 in the log-rank test were
subsequently entered into the multivariate analysis. Multivariate
analysis was performed using Cox's proportional hazards model.
P<0.05 was considered to indicate statistical significance. EZR
software version 1.54 (Saitama Medical Center, Jichi Medical
University) (9) was used for
statistical analyses.
Results
Patients' characteristics
The patient characteristics are presented in
Table I. A total of 113 patients
were enrolled. The median age was 54 (range, 25-72) years. As for
the ECOG PS, 105 patients (93%) had an ECOG PS of 0 or 1 and the
remaining 8 (7%) had an ECOG PS of 2 or 3. The number of patients
with FIGO stage I, II, III and IV was 77 (68%), 13 (12%), 17 (15%)
and 6 (5%), respectively. The main complications were hypertension,
hyperlipidemia, diabetes mellites and thrombosis. The others
included one case each of a condition such as asthma or
hyperthyroidism. The median tumor diameter was 13 (range, 4-28) cm.
The median number of removed lymph nodes was 55 (range, 21-135).
Endometriosis was diagnosed in 59 patients (52%). Postoperative
adjuvant chemotherapy was performed in 92 patients (81%).
Progression was noted in 38 patients (34%). The calculated cut-off
values for the number of removed lymph nodes for predicting
progression for stage I and stage II or higher were 33 and 49,
respectively.
 | Table IPatients' characteristics. |
Table I
Patients' characteristics.
| Characteristic | Value |
|---|
| Age, years | 54 (25-72) |
| ECOG PS | |
|
0,1 | 105(93) |
|
2,3 | 8(7) |
| Stage | |
|
I | 77(68) |
|
II | 13(12) |
|
III | 17(15) |
|
IV | 6(5) |
| Complications | |
|
HT | 8(7) |
|
HL | 7(6) |
|
DM | 3(3) |
|
DVT | 3(3) |
|
PE | 2(2) |
|
Others | 32(28) |
| Tumor diameter,
cm | 13 (4-28) |
| Number of removed
lymph nodes | 55 (21-135) |
| Association with
endometriosis | |
|
Yes | 59(52) |
|
No | 54(48) |
| Adjuvant
chemotherapy | |
|
Yes | 92(81) |
|
No | 21(19) |
| Progression | |
|
Yes | 38(34) |
|
No | 75(66) |
PFS and OS analyses
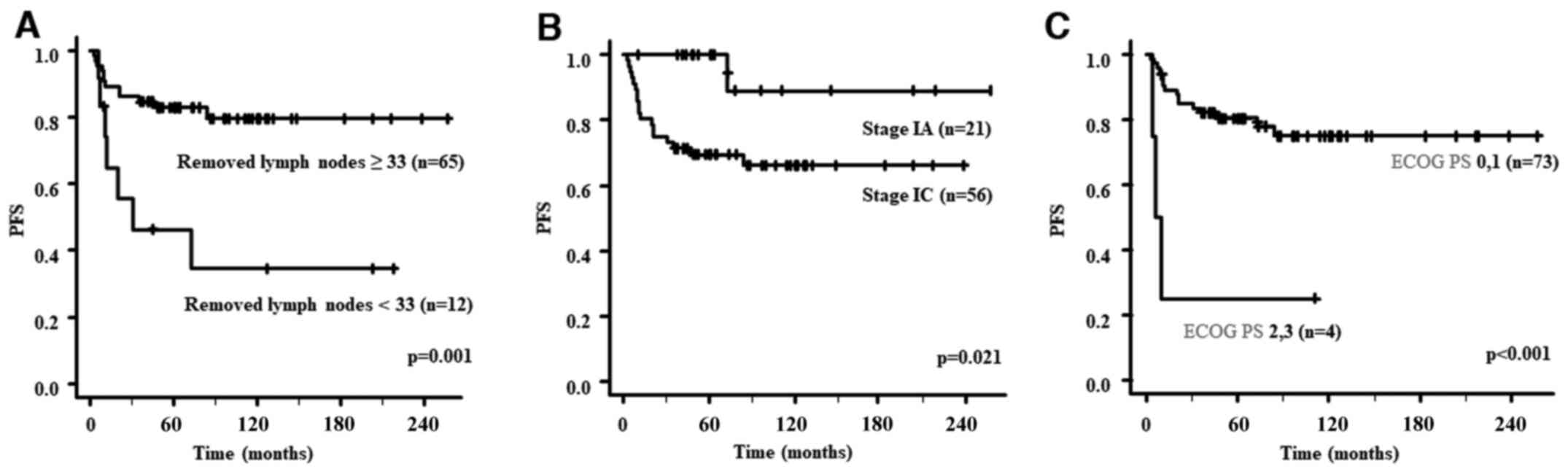
PFS analyses for patients with stage I are presented
in Table II. The PFS of the group
with ≥33 removed lymph nodes was significantly better than that of
the group with <33 removed lymph nodes (P=0.001). Similarly, the
PFS of patients with stage IA was significantly better than that of
patients with stage IC (P=0.021) and that of the group with ECOG PS
0 or 1 was significantly better than that of the group with ECOG PS
2 or 3 (P<0.001). The PFS curves according to the number of
removed lymph nodes, stage and ECOG PS are presented in Fig. 1. OS analyses for patients with
stage I are presented in Table
II. Only ECOG PS 0 or 1 was a good prognostic factor for OS
(P=0.005).
 | Table IILog-rank test in the survival analysis
for stage I patients based on the Kaplan-Meier method. |
Table II
Log-rank test in the survival analysis
for stage I patients based on the Kaplan-Meier method.
| Variable | Number of
patients | 5-year PFS (%) | P-value | 5-year OS (%) | P-value |
|---|
| Age, years | | | 0.223 | | 0.891 |
|
<54 | 40 | 70 | | 82 | |
|
≥54 | 37 | 86 | | 88 | |
| ECOG PS | | | <0.001 | | 0.005 |
|
0,1 | 73 | 80 | | 87 | |
|
2,3 | 4 | 25 | | 33 | |
| Complication | | | 0.855 | | 0.782 |
|
Yes | 40 | 77 | | 86 | |
|
No | 37 | 78 | | 83 | |
| Tumor diameter,
cm | | | 0.657 | | 0.710 |
|
<13 | 33 | 79 | | 81 | |
|
≥13 | 44 | 77 | | 88 | |
| Association with
endometriosis | | | 0.306 | | 0.226 |
|
Yes | 47 | 72 | | 80 | |
|
No | 30 | 87 | | 93 | |
| Stage | | | 0.021 | | 0.079 |
|
IA | 21 | 100 | | 100 | |
|
IC | 56 | 69 | | 79 | |
| Number of removed
lymph nodes | | | 0.001 | | 0.148 |
|
≥33 | 65 | 83 | | 87 | |
|
<33 | 12 | 46 | | 70 | |
| Operator | | | 0.220 | | 0.202 |
|
Gynecological
oncologist | 60 | 75 | | 82 | |
|
Other | 17 | 88 | | 94 | |
| Adjuvant
chemotherapy | | | 0.669 | | 0.647 |
|
Yes | 58 | 76 | | 86 | |
|
No | 19 | 83 | | 83 | |
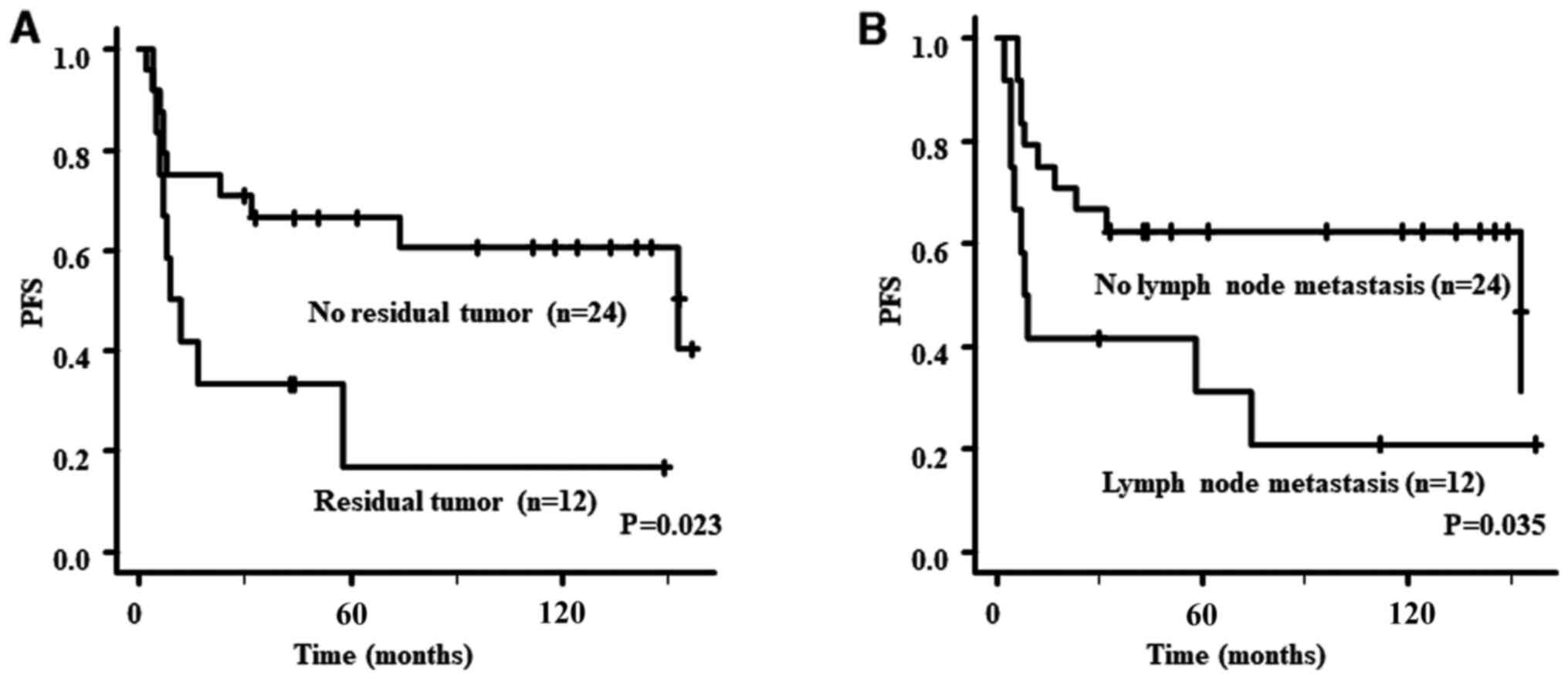
PFS analyses for patients with stage II or higher
are presented in Table III. The
PFS of patients with no residual tumor or no lymph node metastasis
was significantly better than that of those with residual tumor
(P=0.023) or lymph node metastasis (P=0.035). However, there were
no significant differences in PFS based on the number of removed
lymph nodes. PFS curves according to residual tumor and lymph node
metastasis are provided in Fig. 2.
OS analyses for patients with stage II or higher are presented in
Table II. There was no
significant prognostic factor for OS.
 | Table IIILog-rank test in the survival
analysis for patients with stage II or higher based on the
Kaplan-Meier method. |
Table III
Log-rank test in the survival
analysis for patients with stage II or higher based on the
Kaplan-Meier method.
| Variable | Number of
patients | 5-year PFS (%) | P-value | 5-year OS (%) | P-value |
|---|
| Age, years | | | 0.641 | | 0.745 |
|
<54 | 16 | 50 | | 59 | |
|
≥54 | 20 | 50 | | 70 | |
| ECOG PS | | | 0.586 | | 0.455 |
|
0,1 | 32 | 50 | | 64 | |
|
2,3 | 4 | 75 | | 75 | |
| Complication | | | 0.116 | | 0.537 |
|
Yes | 15 | 40 | | 66 | |
|
No | 21 | 59 | | 66 | |
| Tumor diameter,
cm | | | 0.834 | | 0.594 |
|
<13 | 20 | 50 | | 65 | |
|
≥13 | 16 | 56 | | 64 | |
| Association with
endometriosis | | | 0.282 | | 0.225 |
|
Yes | 12 | 58 | | 75 | |
|
No | 24 | 47 | | 60 | |
| Stage | | | 0.082 | | 0.233 |
|
II | 13 | 69 | | 77 | |
|
III, IV | 23 | 40 | | 59 | |
| Number of removed
lymph nodes | | | 0.468 | | 0.090 |
|
≥49 | 21 | 56 | | 76 | |
|
<49 | 15 | 47 | | 50 | |
| Lymph node
metastasis | | | 0.035 | | 0.083 |
|
Yes | 12 | 31 | | 50 | |
|
No | 24 | 63 | | 74 | |
| Operator | | | 0.111 | | 0.417 |
|
Gynecological
oncologist | 29 | 53 | | 68 | |
|
Other | 7 | 43 | | 57 | |
| Residual tumor | | | 0.023 | | 0.100 |
|
Yes | 12 | 17 | | 44 | |
|
No | 24 | 66 | | 75 | |
Multivariate analyses for predictors
of progression
Multivariate analyses were performed to identify
independent predictors of progression. For patients with stage I,
the number of removed lymph nodes, stage and ECOG PS were compared.
The results are presented in Table
IV. A number of removed lymph nodes of ≥33, stage IA and ECOG
PS 0 or 1 were independent predictors of improved PFS (P=0.004,
0.026 and <0.001, respectively).
 | Table IVMultivariate analysis for predictors
of progression for stage I. |
Table IV
Multivariate analysis for predictors
of progression for stage I.
| Variable | Number of
patients | Hazard ratio (95%
CI) | P-value |
|---|
| ECOG PS | | | <0.001 |
|
0,1 | 73 | 1 | |
|
2,3 | 4 | 14.3
(3.27-62.4) | |
| Stage | | | 0.026 |
|
IA | 21 | 1 | |
|
IC | 56 | 10.4
(1.32-82.6) | |
| Number of removed
lymph nodes | | | 0.004 |
|
≥33 | 65 | 1 | |
|
<33 | 12 | 4.06
(1.55-10.6) | |
For patients with stage II or higher, residual tumor
and lymph node metastasis were assessed as predictors of PFS. The
results are presented in Table V.
No residual tumor was the only independent predictor of improved
PFS (P=0.048).
 | Table VMultivariate analysis for predictors
of progression for stage II or higher. |
Table V
Multivariate analysis for predictors
of progression for stage II or higher.
| Variable | Number of
patients | Hazard ratio (95%
CI) | P-value |
|---|
| Lymph node
metastasis | | | 0.070 |
|
No | 24 | 1 | |
|
Yes | 12 | 2.32
(0.93-5.78) | |
| Residual tumor | | | 0.048 |
|
No | 24 | 1 | |
|
Yes | 12 | 2.62
(1.01-6.80) | |
Recurrence sites
Regarding stage I, the site of recurrence was
consistent with the previous study by our group (7), as an additional 9 cases did not recur
and there was no significant difference (data not shown). According
to stage II-IV, the recurrence site was clearly identified in 18
patients. Of 12 patients with ≥49 removed lymph nodes, 3 (25%) had
regional node recurrence. Recurrence sites in the other 9 patients
(75%) were peritoneal dissemination, pleural dissemination, lung,
liver, thymus and inguinal node. On the other hand, of the 6
patients with <49 removed lymph nodes, 2 (33%) had regional node
recurrence. Recurrence sites of the other 4 patients (67%) were
peritoneal dissemination, the diaphragm, liver and spleen. There
was no significant difference in the incidence of regional node
recurrence between the group with ≥49 removed lymph nodes and that
with <49.
Discussion
In the present study, it was examined whether the
number of removed lymph nodes is an independent predictor of
progression in patients with stage II or higher OCCC with
systematic lymphadenectomy and the significance of the number of
removed lymph nodes according to the FIGO stage In OCCC was
investigated. The results revealed that, in stage II or higher
OCCC, although residual tumor is an independent predictor of
progression, the number of removed lymph nodes is not. This is
contradictory to the results on stage I OCCC, as previously
reported by our group (7): The
number of removed lymph nodes was an independent predictor of
progression, which was reconfirmed by the present study including
added data of stage I OCCC.
The first novel finding of the present study was
that the number of removed lymph nodes is not a predictor of
progression for stage II or higher OCCC. There have been numerous
reports on the therapeutic significance of systematic
lymphadenectomy for ovarian cancer. A recent randomized trial (LION
trial) demonstrated that systematic lymphadenectomy in patients
with advanced ovarian cancer who underwent intraabdominal
macroscopical complete resection and had normal lymph nodes is not
associated with a longer OS or PFS than with no lymphadenectomy
(10). However, the majority of
patients in the LION trial had serous carcinoma and patients with
OCCC comprised only 2.2% (10). As
OCCC is chemoresistant, unlike serous carcinoma and endometrioid
carcinoma, it must be considered separately from other histological
types. There are several reports on the therapeutic significance of
systematic lymphadenectomy for OCCC. Systematic lymphadenectomy was
reported to both improve (11,12)
and not improve the prognosis (6,13).
Therefore, its therapeutic significance for OCCC is
controversial.
Focusing on the number of removed lymph nodes, to
the best of our knowledge, there are only two reports in which the
prognosis was investigated based on the number of removed lymph
nodes for OCCC (5,7), one of which is the previous study by
our group. Regarding the number of removed lymph nodes, >20 was
selected to avoid including resection of only swollen lymph nodes.
Similarly, a recent study defined systematic lymphadenectomy as at
least 20 removed lymph nodes (14). The present study added 9 patients
with stage I OCCC. Patients were enrolled during the 2 years after
the previous study by our group (7), providing a 13% increase in the
patient number, and all patients with stage I OCCC who met the
criteria were reanalyzed. This analysis reconfirmed the previous
results that the number of removed lymph nodes is an independent
predictor of progression for stage I OCCC. The two previous reports
described stage I OCCC (5,7). Therefore, the present study was the
first to examine the impact of the number of resected lymph nodes
on stage II or higher OCCC. According to the present data and
clinical experience at our clinic, in contrast to stage I, tumors
are likely to spread throughout the body in stage II or higher,
particularly in stages III and IV. Even if there are no macroscopic
tumors after surgery, microscopic tumors may remain. As OCCC is
chemoresistant, OCCC patients with microscopic tumors may have a
high risk of recurrence. Even if more lymph nodes are removed, it
may not be possible to prevent recurrence in patients with stage II
or higher OCCC with microscopic tumors.
The second finding was that residual tumor is an
independent predictor of progression for stage II or higher OCCC.
This is consistent with the previous report demonstrating that only
cytoreductive surgery resulting in no residual tumor is able to
improve the prognosis of advanced OCCC (15). Patients with no residual tumor had
significantly better PFS than those with a tumor <1 cm (P=0.04)
or those with a tumor >1 cm (P<0.01) (15). This is reasonable considering that
OCCC is chemoresistant.
Since clinical stage I ovarian CCC had about
4.5-7.5% lymph node metastasis (5,6,16),
the clinical stage I ovarian CCC cases may have included
pathological stage III cases at about 4.5-7.5%. Therefore, removing
more lymph nodes leads to accurate staging and an improved
prognosis for stage I OCCC. On the other hand, in stage II or
higher OCCC, complete surgery without macroscopic residual tumor
may be important regardless of the number of removed lymph nodes
and lymph node metastasis. Although speculative, the gynecologic
surgeon may only remove the swollen lymph nodes instead of
systematic lymphadenectomy in stage II or higher OCCC.
A limitation of present study was that the study
population was small and that this may have been a source of low
significance of the results. In future studies, larger cohorts from
multiple centers should be investigated. In particular, as the
number of patients was small, it was difficult to perform subgroup
analyses. Initially, it was intended to analyze stage II OCCC in
the same manner as stage I OCCC, as clinical stage II OCCC may
include pathological stage III cases. If more lymph nodes are
removed in patients with clinical stage II OCCC, a certain
proportion of them may have been diagnosed with pathological stage
III. Therefore, the number of removed lymph nodes may also be an
independent predictor of progression for stage II OCCC. However, as
there were only 13 cases of stage II OCCC, it was not possible to
analyze stage II. It was also considered that the number of
patients was insufficient for OS analysis. Since all patients of
the present study were subjected to systematic lymphadenectomy, it
was not possible to make a comparison between the group with
systematic lymphadenectomy and that with non-systematic
lymphadenectomy. Therefore, how systematic lymphadenectomy affects
survival in OCCC compared with non-systematic lymphadenectomy will
be the focus of future studies.
In conclusion, the present study revealed that the
number of removed lymph nodes is not a predictor of progression for
stage II or higher OCCC, differing from stage I OCCC. Residual
tumor was an independent predictor of progression for stage II or
higher OCCC. Although further studies with a large number of
patients are required to confirm the results, the present study
provides useful information on how to perform retroperitoneal
lymphadenectomy according to the FIGO stage for OCCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author upon reasonable
request.
Authors' contributions
KT, SM, HF and YT designed the present study,
critically revised the manuscript and analyzed data. ST, AT, YT,
TY, TK and YS performed the data collection. KT wrote the
manuscript. KT and YT checked and confirmed the authenticity of the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Jichi Medical University Hospital (Tochigi, Japan; no. Rindai
15-121). Patient consent was not required, as this study was a
retrospective observational study, but the patients or their
families were given the opportunity to opt out.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Takei Y, Suzuki M, Ohwada M, Saga Y, Kohno
T, Machida S and Sato I: A feasibility study of paclitaxel and
carboplatin therapy in Japanese patients with epithelial ovarian
cancer. Oncol Rep. 10:951–955. 2003.PubMed/NCBI
|
|
3
|
Sugiyama T, Kamura T, Kigawa J, Terakawa
N, Kikuchi Y, Kita T, Suzuki M, Sato I and Taguchi K: Clinical
characteristics of clear cell carcinoma of the ovary: A distinct
histologic type with poor prognosis and resistance to
platinum-based chemotherapy. Cancer. 88:2584–2589. 2000.PubMed/NCBI
|
|
4
|
Machida H, Matsuo K, Yamagami W, Ebina Y,
Kobayashi Y, Tabata T, Kanauchi M, Nagase S, Enomoto T and Mikami
M: Trends and characteristics of epithelial ovarian cancer in Japan
between 2002 and 2015: A JSGO-JSOG joint study. Gynecol Oncol.
153:589–596. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mahdi H, Moslemi-Kebria M, Levinson KL,
Gojayev A, Lockhart D, Ali-Fehmi R and Munkarah AR: Prevalence and
prognostic impact of lymphadenectomy and lymph node metastasis in
clinically early-stage ovarian clear cell carcinoma. Int J Gynecol
Cancer. 23:1226–1230. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takano M, Sugiyama T, Yaegashi N, Suzuki
M, Tsuda H, Sagae S, Udagawa Y, Kuzuya K, Kigawa J, Takeuchi S, et
al: The impact of complete surgical staging upon survival in
early-stage ovarian clear cell carcinoma: A multi-institutional
retrospective study. Int J Gynecol Cancer. 19:1353–1357.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Takei Y, Takahashi S, Machida S, Taneichi
A, Yoshiba T, Takahashi Y, Yoshida C, Saga Y, Matsubara S and
Fujiwara H: Impact of the number of removed lymph nodes on
recurrence-free survival in stage I ovarian clear cell carcinoma.
Int J Clin Oncol. 23:930–935. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargen D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kanda Y: Investigation of the freely
available easy-to-use software 'EZR' for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Harter P, Sehouli J, Lorusso D, Reuss A,
Vergote I, Marth C, Kim JW, Raspagliesi F, Lampe B, Aletti G, et
al: A randomized trial of lymphadenectomy in patients with advanced
ovarian neoplasms. N Engl J Med. 380:822–832. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yamazaki H, Todo Y, Shimada C, Takeshita
S, Minobe S, Okamoto K, Yamashiro K and Kato H: Therapeutic
significance of full lymphadenectomy in early-stage ovarian clear
cell carcinoma. J Gynecol Oncol. 29(e19)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Magazzino F, Katsaros D, Ottaiano A,
Gadducci A, Pisano C, Sorio R, Rabaiotti E, Scambia G, Cormio G,
Scarampi L, et al: Surgical and medical treatment of clear cell
ovarian cancer: Results from the multicenter Italian Trials in
Ovarian Cancer (MITO) 9 retrospective study. Int J Gynecol Cancer.
21:1063–1070. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kajiyama H, Suzuki S, Yoshikawa N,
Tamauchi S, Shibata K and Kikkawa F: The impact of systematic
retroperitoneal lymphadenectomy on long-term oncologic outcome of
women with advanced ovarian clear-cell carcinoma. J Gynecol Oncol.
31(e47)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nasioudis D, Latif NA, Haggerty AF,
Giuntoli Ii RL, Kim SH and Ko EM: Outcomes of comprehensive
lymphadenectomy for patients with advanced stage ovarian carcinoma
and rare histologic sub-types. Int J Gynecol Cancer. 31:1132–1136.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Takano M, Kikuchi Y, Yaegashi N, Kuzuya K,
Ueki M, Tsuda H, Suzuki M, Kigawa J, Takeuchi S, Tsuda H, et al:
Clear cell carcinoma of the ovary: A retrospective multicentre
experience of 254 patients with complete surgical staging. Br J
Cancer. 94:1369–1374. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mueller JJ, Holzapfel M, Han CH, Santos K,
Gunderson C, Moore K, Erickson B, Leath CA III, Diaz E, Walsh C, et
al: Staging lymphadenectomy in patients with clear cell carcinoma
of the ovary. Int J Gynecol Cancer. 26:120–124. 2016.PubMed/NCBI View Article : Google Scholar
|