Introduction
The introduction of molecular-targeted agents
notably improves the prognosis of patients with metastatic renal
cell carcinoma (mRCC) (1).
Furthermore, immune checkpoint inhibitors (ICIs), such as
programmed cell death protein-1, programmed death-ligand 1 and
cytotoxic T-lymphocyte antigen 4 antibodies, were demonstrated to
be effective against mRCC through a unique mechanism of action of
restoring T cell-mediated immune responses and have become novel
treatment options for mRCC (2).
Amongst these, nivolumab initially resulted in significant
improvements in OS compared with everolimus in previously treated
patients with mRCC (3), which led
to the approval of ICI-based combination therapies for
treatment-naïve patients with mRCC, including nivolumab plus
ipilimumab, avelumab plus axitinib and pembrolizumab plus axitinib
(4-6).
To date, well-designed models, such as the Memorial
Sloan Kettering Cancer Center (MSKCC) and the International
Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk
classification systems, have been widely accepted as
prognostication tools for both previously treated and
treatment-naïve patients with mRCC (7-10).
However, the MSKCC and IMDC systems were developed based on
patients who received cytokine and molecular-targeted therapies,
respectively (7-10);
therefore, it remains unclear whether these two conventional
prognostication models can be applied to patients with mRCC treated
using ICIs. In addition, several parameters differing from those
adopted in the MSKCC and IMDC models were identified as useful
prognostic factors for patients with mRCC receiving ICIs (11-16).
For example, Suzuki et al (11) reported that a high C-reactive
protein level and neutrophil-to-lymphocyte ratio (NLR) were
significantly associated with a poor overall survival (OS) in
patients with mRCC treated using nivolumab.
As such, a multicenter retrospective study was used
to identify reliable predictors of OS in previously treated
patients with mRCC who received nivolumab.
Patients and methods
Patients
Between October 2016 and November 2019, 114 patients
with mRCC received nivolumab after treatment using
molecular-targeted agents at one of the following four institutions
belonging to the Tokai Urologic Oncology Research Seminar:
Hamamatsu University School of Medicine (Hamatsu, Japan), Gifu
University Graduate School of Medicine (Gifu, Japan), Fujita Health
University School of Medicine (Toyoake, Japan) and Nagoya City
University Graduate School of Medical Science (Nagoya, Japan).
After excluding 37 patients who were diagnosed with non-clear cell
mRCC and/or received nivolumab as later than a fourth-line therapy,
the present study included 77 patients with clear cell mRCC who
received 1 or 2 molecular-targeted agents, followed by the
introduction of nivolumab as second- or third-line therapy at
Hamamatsu University School of Medicine (n=23), Fujita Health
University School of Medicine (n=23), Nagoya City University
Graduate School of Medical (n=17) and Gifu University Graduate
School of Medicine (n=14).
All procedures performed in the present study were
done in accordance with the ethical standards of all the
institutional and/or national research committees (approval no.
19-101), and the guidelines described in the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards (17). The need to
obtain informed consent for the publication of any associated data
and accompanying images from all patients included in this study
was waived due to its retrospective design after approval by the
ethics committees of all four institutions.
Treatment
In the recruited patients, prior to the introduction
of nivolumab, all patients were treated using either 1 or 2
molecular-targeted agents approved in Japan, and as a rule, each
agent was administered under a standard dosing schedule. After the
failure of molecular-targeted agents, nivolumab (3 mg/kg or a flat
dose of 240 mg) was generally administered intravenously every 2
weeks until the patients exhibited unacceptable toxicity, the
disease progressed or the patient declined. It was possible to
alter the dosage or postpone nivolumab treatment considering the
degree of treatment-associated adverse events. Depending on the
general condition and preference of each patient, a
molecular-targeted agent was further introduced after the
discontinuation of nivolumab.
Evaluation
Clinicopathological data, including the treatment
profiles, were retrospectively obtained from the medical records of
each patient. Prior to the administration of nivolumab, standard
laboratory data were obtained, and radiological examinations by
computed tomography (CT) of the brain, chest and abdomen, and/or
radionuclide bone scintigraphy were performed as routine procedures
on all patients. In addition, immune inflammation-related markers,
including NLR, the platelet-lymphocyte ratio (PLR) and systemic
immune inflammation index (SII), were evaluated based on previously
described calculations (13,18).
As a rule, tumor measurements were performed by CT every 2-3
courses after the introduction of nivolumab and disease progression
was evaluated using the Response Evaluation Criteria in Solid
Tumors, version 1.1(19).
Progression-free survival (PFS) was defined as the time from the
start of nivolumab to disease progression or death, whereas OS was
defined as the time from the start of nivolumab therapy to death
from any cause or the last follow-up.
Statistical analysis
All statistical analyses were performed using R
version 4.0.0 (r-project.org) (20) and P<0.05 was considered to
indicate a statistically significant difference. PFS and OS rates
were calculated using the Kaplan-Meier method, and the prognostic
significance of factors were analyzed employing univariate and
multivariate Cox proportional hazards models. The factors with
P-values <0.15 in the univariate analysis were included in the
multivariate analysis using backward stepwise selection, as
previously reported (8). In the
assessment of prognostic factors, reference values at each
institution were used as cut-off values for laboratory data,
whereas for those without reference values, cut-off values were set
according to the Youden index obtained from receiver operating
characteristic curves plotted for the value of each parameter to
predict OS. Patients were then categorized according to the
positive number of independent risk factors for OS identified by
multivariate analysis as follows: Group A, no risk factors; group
B, single risk factor; and group C, multiple risk factors.
Results
The clinicopathological characteristics of the 77
patients included in this study at the initiation of nivolumab
treatment are summarized in Table
I. Of the 77 patients, 60 patients were males (77.9%) and 17
were females (22.1%), with a median age of 72 years (range, 44-83
years). The median number of cycles of nivolumab therapy was 12
(range, 1-67) and the median duration of treatment was 6 months
(range, 1-35 months). The best responses to nivolumab were as
follows: Complete response in 3 patients, partial response in 27,
stable disease in 33 and progressive disease in 14; therefore, the
objective response rate (ORR) in the 77 patients was 39.0%. During
the follow-up period after the introduction of nivolumab (median,
11 months; range, 1-38 months), 14 (18.2%) patients exhibited
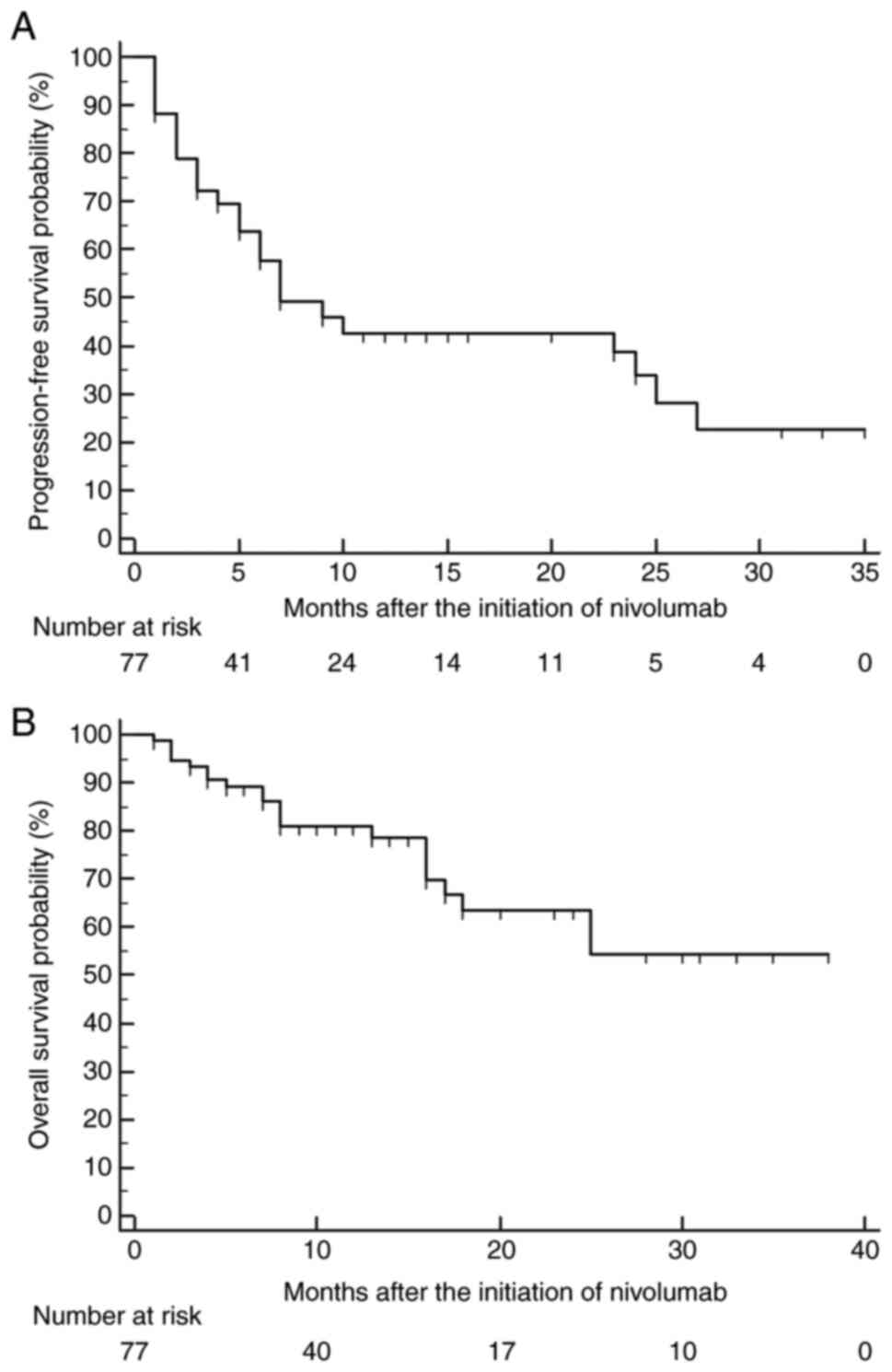
disease progression and 21 (27.3%) died. As shown in Fig. 1, the median PFS and OS were 7
months and not reached, respectively; there were no significant
differences in PFS or OS amongst the four institutions (data not
shown). As shown in Table II, the
univariate analysis revealed that OS was significantly associated
with age, KPS, neutrophil count, albumin levels and NLR. Of these
significant factors, only three of them, age (≥71 years), KPS
(<80%) and neutrophil count (≥ upper limit of normal detection),
independently affected OS based on the multivariate analysis. To
further clarify the effects of these three factors on OS, the 77
patients were stratified into 3 groups based as follows: Group A
(n=20), no risk factors; group B (n=48), single risk factor; and
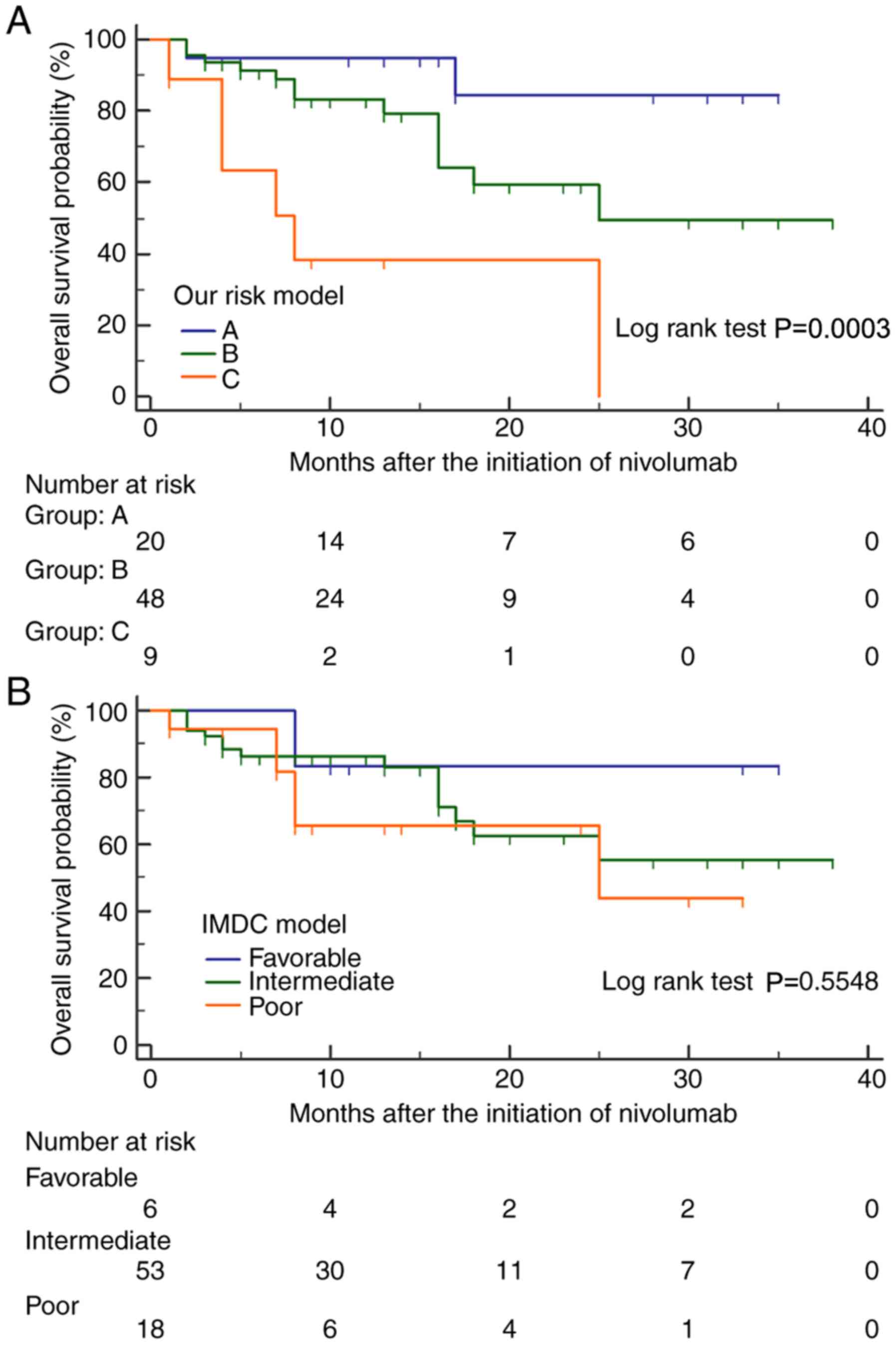
group C (n=9), multiple risk factors. The median OS in groups A, B
and C was not reached; 25 and 8 months, respectively, and there
were significant differences in OS amongst these 3 risk groups
(Fig. 2A). However, the IMDC
system was unable to significantly stratify OS after the initiation
of nivolumab in these 77 patients (Fig. 2B). Prognostic outcomes according to
the 3 risk groups stratified by the IMDC and present model systems
are summarized in Table III.
 | Table IPatient characteristics at the
initiation of nivolumab in patients with clear cell renal carcinoma
(n=77). |
Table I
Patient characteristics at the
initiation of nivolumab in patients with clear cell renal carcinoma
(n=77).
| Characteristic | Value |
|---|
| Age at nivolumab
initiation, yearsa | 72 (44-83) |
| Sex
(male)b | |
|
Female | 17 (22.1) |
|
Male | 60 (77.9) |
| Prior
immunotherapyb | 13 (16.9) |
| Prior
nephrectomyb | 69 (89.6) |
| <1 year from
diagnosis to systemic therapyb | 49 (63.6) |
| Karnofsky Performance
Status <80%b | 20 (26.0) |
| IMDC classification
at nivolumab initiationb | |
|
Favorable | 6 (7.8) |
|
Intermediate | 53 (68.8) |
|
Poor | 18 (23.4) |
| Metastatic
lesionb | |
|
Brain | 6 (8.0) |
|
Lung | 61 (81.3) |
|
Bone | 32 (42.7) |
| Number of metastatic
organs (≥2)b | 45 (58.4) |
| Laboratory
dataa | |
|
Hemoglobin,
g/dl | 11.9 (7.8-16.8) |
|
Serum-corrected
calcium, mg/dl | 9.6 (8.2-11.4) |
|
Neutrophils,
x109/l | 3.62 (0.90-24.1) |
|
Platelets,
x109/l | 219 (28-664) |
|
Albumin,
g/dl | 3.6 (1.7-4.4) |
|
C-reactive
protein, mg/dl | 0.43 (0.02-27.0) |
|
Lactate
dehydrogenase, U/l | 191 (123-3,490) |
|
Neutrophil
to lymphocyte ratio | 3.1 (0.6-19.6) |
|
Platelet to
lymphocyte ratio | 185.8
(6.8-961.0) |
|
Systemic
immune inflammation index, x109/l | 637.7
(50.5-7,806.3) |
| First-line targeted
agentb | |
|
Sunitinib | 42 (54.5) |
|
Sorafenib | 4 (5.2) |
|
Axitinib | 7 (9.1) |
|
Pazopanib | 20(26) |
|
Temsirolimus | 3 (3.9) |
| Second-line targeted
agentb | |
|
Axitinib | 36 (87.8) |
|
Everolimus | 1 (2.4) |
|
Temsirolimus | 2 (4.9) |
|
Pazopanib | 2 (4.9) |
| Duration from
first-line therapy to nivolumab initiation, monthsa | 15 (1-134) |
| Cycles of nivolumab
administration, cyclesa | 12 (1-67) |
| Duration of nivolumab
administration, monthsa | 6 (1-35) |
| Follow-up period
after nivolumab initiation, monthsa | 11 (1-38) |
 | Table IIUnivariate and multivariate analyses
of prognostic factors for overall survival after the initiation of
nivolumab. |
Table II
Univariate and multivariate analyses
of prognostic factors for overall survival after the initiation of
nivolumab.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex, male | 0.62 | 0.25-1.53 | 0.30 | | | |
| Age, ≥71 years | 2.04 | 0.84-4.97 | 0.11a | 3.8 | 1.40-10.33 | 0.0087c |
| <1 year from
diagnosis-systemic therapy | 1.04 | 0.42-2.58 | 0.93 | | | |
| Karnofsky
Performance Status <80% | 2.56 | 1.08-6.08 | 0.03b | 4.98 | 1.83-13.59 | 0.0017c |
| IMDC model at
nivolumab initiation | | | | | | |
|
Favorable | 1 | | | | | |
|
Intermediate | 1.92 | 0.25-14.48 | 0.53 | | | |
|
Poor | 2.76 | 0.34-22.71 | 0.35 | | | |
| Metastatic
lesion | | | | | | |
|
Brain | 1.69 | 0.39-7.42 | 0.49 | | | |
|
Lung | 2.08 | 0.48-9.00 | 0.33 | | | |
|
Bone | 0.86 | 0.36-2.08 | 0.74 | | | |
| Number of
metastatic organs (≥2) | 1.13 | 0.47-2.74 | 0.79 | | | |
| Laboratory
data | | | | | | |
|
Low
hemoglobin, ≤LLN | 1.32 | 0.51-3.38 | 0.57 | | | |
|
High
calcium, ≥ULN | 1.93 | 0.65-5.73 | 0.24 | | | |
|
High
neutrophils, ≥ULN | 2.69 | 0.90-8.05 | 0.07a | 3.75 | 1.22-11.50 | 0.021b |
|
High
platelets, ≥ULN | 0.9 | 0.21-3.89 | 0.89 | | | |
|
Low albumin
(≤3.5 g/dl | 1.9 | 0.79-4.67 | 0.15a | - | - | - |
|
High
C-reactive protein, ≥0.5 mg/dl | 1.63 | 0.68-3.91 | 0.28 | | | |
|
High lactate
dehydrogenase, ≥1.5xULN | 1.73 | 0.51-5.87 | 0.38 | | | |
|
Neutrophil-lymphocyte
ratio, ≥6.1 | 3.02 | 1.11-8.25 | 0.03b | - | - | - |
|
Platelet-lymphocyte
ratio, ≥249 | 1.84 | 0.76-4.43 | 0.18 | | | |
|
Systemic
immune inflammation index, ≥456x109/l | 2.52 | 0.75-8.51 | 0.14 | | | |
| Duration from
first-line therapy-nivolumab initiation (≥16 months) | 1.86 | 0.75-4.60 | 0.18 | | | |
 | Table IIIRisk stratification using each
prognostic model after the initiation of nivolumab. |
Table III
Risk stratification using each
prognostic model after the initiation of nivolumab.
| Model | Number of patients,
n (%) | Number of deaths, n
(%) | Median overall
survival, months (95% CI) | Hazard ratio (95%
CI) |
|---|
| IMDC model | | | | |
|
Favorable | 6 (7.8) | 1 (16.7) | NR | 1 (reference) |
|
Intermediate | 53 (68.8) | 14 (26.4) | NR | 1.92
(0.44-3.39) |
|
Poor | 18 (23.4) | 6 (33.3) | 25.0
(8.0-25.0) | 2.75
(0.52-14.6) |
| Risk model
developed in the present study | | | | |
|
A | 20 (26.0) | 2 (10.0) | NR | 1 (reference) |
|
B | 48 (62.3) | 13 (27.1) | 25.0
(16.0-25.0) | 3.43
(1.34-8.74) |
|
C | 9 (11.7) | 6 (66.7) | 8.0 (1.0-25.0) | 12.5
(2.23-69.9) |
Discussion
Several types of ICIs, either alone or in
combination with another agent, were demonstrated to significantly
prolong the survival of treatment-naïve or previously treated
patients with mRCC (2-6).
Considering the increasing number of therapeutic options for
patients with mRCC, including several regimens containing ICIs, it
has become important to identify parameters that can aid in the
selection of patients with mRCC who are more likely to benefit from
the use of ICIs (1,2). However, data related to the outcomes
of patients with mRCC treated using ICIs in routine clinical
practice are limited, resulting in the lack of established
prognostication tools for this cohort of patients. As such, 77
previously treated Japanese patients with clear cell mRCC who
received nivolumab were recruited to analyze their clinical data in
order to develop a system to stratify their prognosis.
Recently, Hinata et al (21) reported the real-world prognostic
outcomes of a wide range of Japanese patients with mRCC receiving
nivolumab, and they were shown to be slightly poorer compared with
those in the present study. The present study excluded patients who
were diagnosed with non-clear cell RCC and/or received >3
molecular-targeted agents prior to the introduction of nivolumab in
order to minimize the risk of bias induced by the heterogeneous
characteristics of the included patients. As a result, the ORR, PFS
and OS in this series were 39%, 7 months and not reached,
respectively, which were similar to the outcomes of a Japanese
subgroup analysis from the CheckMate 025 study, reporting 43%, 5.6
months and not reached, respectively (22). Accordingly, it may be optimal to
use the prognostic data from the 77 patients included in the
present study for the development of a prognostication system for
patients with mRCC receiving nivolumab as either a second- or
third-line agent.
To date, well-accepted models predicting the
prognosis of patients with mRCC, specifically the MSKCC and IMDC
models, have been used to classify patients with mRCC into 3
prognostic groups (7-10);
however, these models were not developed based on data from those
receiving ICIs. Therefore, it remains controversial whether these
models can be used in the era of ICI therapy. For example, Yip
et al (23) performed a
retrospective analysis using the IMDC database analyzing patients
with mRCC who received ≥1 line of ICI, and confirmed that the IMDC
criteria appropriately stratified these patients into
favorable-risk, intermediate-risk and poor-risk groups for OS,
whereas Martini et al (12)
reported no significant differences in the OS of 100 patients with
mRCC who were treated using ICIs classified according to the IMDC
model. Both of these studies included patients receiving either ICI
monotherapy or ICI combination therapy at the first-line setting;
however, the conclusion is controversial, and may be explained by
differences in patient backgrounds, such as the proportion of those
with clear cell RCC. In the present study, 6, 53 and 18 patients
were classified into favorable, intermediate and poor risk groups,
respectively, based on the IMDC model, whereas 16, 58 and 3 were
similarly classified by the MSKCC model. However, no significant
differences in OS were noted according to the classification by
either model. This suggests that a novel prognostication system
applicable to patients with mRCC treated using ICIs is
required.
In recent years, there have been a number of studies
reporting the significant impact of inflammatory biomarkers, such
as NLR, PLR, the monocyte-to-lymphocyte ratio (MLR) and SII, on the
prognosis of patients with mRCC receiving ICIs (11-15).
For example, De Giorgi et al (13) reported that patients with mRCC
treated using nivolumab with a high SII and low body mass index
(BMI) had a markedly poor OS, whereas Martini et al
(12) demonstrated the value of
risk scoring using MLR, BMI and the number and sites of metastases
for prognostication of patients with mRCC receiving ICIs.
Therefore, the associations between OS and several parameters,
including inflammatory biomarkers were assessed in the present
study to identify potential prognostic factors for previously
treated patients with mRCC receiving nivolumab, and revealed that
OS was independently affected by age, KPS and the neutrophil count,
and was able to be stratified by dividing them into 3 groups
according to these 3 independent risk factors. However, all
laboratory data, except the neutrophil count, had no significant
impact on OS, which may be explained by the following: The
indispensable effects of previous systemic therapies or disease
aggressiveness at the introduction of nivolumab on laboratory data,
and the close association between the efficacies of ICIs and the
host cell-mediated immune system. Taken together, assessing the
impacts of a wide variety of parameters on the prognosis of
patients with mRCC receiving ICIs may aid in the development of
useful alternatives to conventional prognostication models for this
patient cohort.
The present study has several limitations. First,
this was a retrospective study including a small number of patients
and different populations may have different responses to
molecular-targeted agents or nivolumab, thus the present findings
must be confirmed in a prospective study with a larger sample size,
with multiple different ethnicities. Second, the present study
included all patients treated using nivolumab for at least one
cycle, resulting in the lack of consideration of usage cycles and
dosage, which may affect the prognostic outcomes. Third, a focus
was placed on only previously treated patients with mRCC receiving
nivolumab; however, considering the current therapeutic trend for
mRCC (4-6),
prognostication of patients with treatment-naïve mRCC should also
be investigated. Fourth, although the unbalanced distribution of
patients with mRCC based on the MSKCC and IMDC models is considered
a disadvantage (24), the
proportion of patients classified into the intermediate risk group
by the model used in the present study was the highest amongst the
groups. Lastly, there may be other parameters that have not been
well characterized, but are closely associated with the prognosis
of patients with mRCC receiving ICIs that were not taken into
consideration.
In conclusion, a retrospective multi-institutional
study on 77 previously treated patients with mRCC who received
nivolumab as either the second- or third-line agent, and
demonstrated a comparatively favorable prognosis. Moreover, unlike
the IMDC model, only 3 independent risk factors, age, KPS and
neutrophil count, were identified as independent risk factors of
OS, making it possible to stratify the OS of these patients into 3
groups.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK, TY, RS and HM conceived and designed the study.
TI, KM, RA and KT acquired the data. TI and HM analyzed and
interpretated the data, and drafted the manuscript. TI and HM
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in this study were done so
in accordance with the ethical standards of all the institutional
and/or national research committees (approval no. 19-101), and the
guidelines described in the 1964 Helsinki declaration and its later
amendments or comparable ethical standards. The need to obtain
informed consent for the publication of any associated data and
accompanying images from all patients included in this study was
waived due to its retrospective design after approval by the ethics
committees of all four institutions.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bedke J, Gauler T, Grünwald V, Hegele A,
Herrmann E, Hinz S, Janssen J, Schmitz S, Schostak M, Tesch H, et
al: Systemic therapy in metastatic renal cell carcinoma. World J
Urol. 35:179–188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Flippot R, Escudier B and Albiges L:
Immune checkpoint inhibitors: Toward new paradigms in renal cell
carcinoma. Drugs. 78:1443–1457. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Motzer RJ, Tannir NM, McDermott DF,
Frontera OA, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Motzer RJ, Penkov K, Haanen J, Rini B,
Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S,
Uemura M, et al: Avelumab plus axitinib versus sunitinib for
advanced renal-cell carcinoma. N Engl J Med. 380:1103–1115.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Heng DY, Xie W, Regan MM, Warren MA,
Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al:
Prognostic factors for overall survival in patients with metastatic
renal cell carcinoma treated with vascular endothelial growth
factor-targeted agents: Results from a large, multicenter study. J
Clin Oncol. 27:5794–5799. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Motzer RJ, Bacik J, Schwartz LH, Reuter V,
Russo P, Marion S and Mazumdar M: Prognostic factors for survival
in previously treated patients with metastatic renal cell
carcinoma. J Clin Oncol. 22:454–463. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI,
Knox JJ, Bjarnason GA, Srinivas S, Pal SK, Yuasa T, et al: The
international metastatic renal cell carcinoma database consortium
model as a prognostic tool in patients with metastatic renal cell
carcinoma previously treated with first-line targeted therapy: A
population-based study. Lancet Oncol. 16:293–300. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Suzuki K, Terakawa T, Furukawa J, Harada
K, Hinata N, Nakano Y and Fujisawa M: C-reactive protein and the
neutrophil-to-lymphocyte ratio are prognostic biomarkers in
metastatic renal cell carcinoma patients treated with nivolumab.
Int J Clin Oncol. 25:135–144. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Martini DJ, Liu Y, Shabto JM, Carthon BC,
Hitron EE, Russler GA, Caulfield S, Kissick HT, Harris WB, Kucuk O,
et al: Novel risk scoring system for patients with metastatic renal
cell carcinoma treated with immune checkpoint inhibitors.
Oncologist. 25:e484–e491. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Giorgi U, Procopio G, Giannarelli D,
Sabbatini R, Bearz A, Buti S, Basso U, Mitterer M, Ortega C, Bidoli
P, et al: Association of systemic inflammation index and body mass
index with survival in patients with renal cell cancer treated with
nivolumab. Clin Cancer Res. 25:3839–3846. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Raimondi A, Sepe P, Zattarin E, Mennitto
A, Stellato M, Claps M, Guadalupi V, Verzoni E, de Braud F and
Procopio G: Predictive biomarkers of response to immunotherapy in
metastatic renal cell cancer. Front Oncol. 10(1644)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ishihara H, Tachibana H, Takagi T, Kondo
T, Fukuda H, Yoshida K, Iizuka J, Kobayashi H, Okumi M, Ishida H
and Tanabe K: Predictive impact of peripheral blood markers and
C-reactive protein in nivolumab therapy for metastatic renal cell
carcinoma. Target Oncol. 14:453–463. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Perrone F, Minari R, Bersanelli M, Bordi
P, Tiseo M, Favari E, Sabato R and Buti S: The prognostic role of
high blood cholesterol in advanced cancer patients treated with
immune checkpoint inhibitors. J Immunother. 43:196–203.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bruce-Chwatt LJ: Declaration of Helsinki.
Recommendations guiding doctors in clinical research. WHO Chron.
19:31–32. 1965.PubMed/NCBI
|
|
18
|
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W,
Zhang X, Wang WM, Qiu SJ, Zhou J and Fan J: Systemic
immune-inflammation index predicts prognosis of patients after
curative resection for hepatocellular carcinoma. Clin Cancer Res.
20:6212–6222. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
R Core Team (2020). R: A language and
environment for statistical computing. R Foundation for Statistical
Computing, Vienna, Austria. URL http://www.R-project.org/. Accessed June 14, 2020.
|
|
21
|
Hinata N, Yonese J, Masui S, Nakai Y,
Shirotake S, Tatsugami K, Inamoto T, Nozawa M, Ueda K, Etsunaga T,
et al: A multicenter retrospective study of nivolumab monotherapy
in previously treated metastatic renal cell carcinoma patients:
Interim analysis of Japanese real-world data. Int J Clin Oncol.
25:1533–1542. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tomita Y, Fukasawa S, Shinohara N,
Kitamura H, Oya M, Eto M, Tanabe K, Kimura G, Yonese J, Yao M, et
al: Nivolumab versus everolimus in advanced renal cell carcinoma:
Japanese subgroup analysis from the CheckMate 025 study. Jpn J Clin
Oncol. 47:639–646. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yip SM, Wells C, Moreira R, Wong A,
Srinivas S, Beuselinck B, Porta C, Sim HW, Ernst DS, Rini BI, et
al: Checkpoint inhibitors in patients with metastatic renal cell
carcinoma: Results from the international metastatic renal cell
carcinoma database consortium. Cancer. 124:3677–3683.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tamura K, Matsushita Y, Watanabe H,
Motoyama D, Ito T, Sugiyama T, Otsuka A and Miyake H: Feasibility
of the ACL (albumin, C-reactive protein and lactate dehydrogenase)
model as a novel prognostic tool in patients with metastatic renal
cell carcinoma previously receiving first-line targeted therapy.
Urol Oncol. 38:6.e9–6.e16. 2020.PubMed/NCBI View Article : Google Scholar
|