Introduction
At present, there are 3 major chemotherapeutic
regimens for epithelial ovarian cancer: Paclitaxel plus carboplatin
every 3 weeks (TW-PC) (1-3),
paclitaxel plus carboplatin with bevacizumab followed by
bevacizumab maintenance therapy every 3 weeks (PC+BEV) (4,5) and
dose-dense paclitaxel (80 mg/m2) administered on days 1, 8 and 15
plus carboplatin [area under the curve (AUC) 6 mg/ml/min]
administered on day 1 of a 21-day cycle (dd-PC) (6). Recently, a number of therapeutic
regimens using a poly(adenosine diphosphate-ribose) polymerase
(PARP) inhibitor were also reported (7-9).
One of the above regimens is selected, and whether a PARP inhibitor
is used is decided according to the BRCA mutation status and
homologous recombination status.
However, it remains elusive whether the above
standard regimens may be administered to patients with poor
performance status (PS), elderly patients or those with severe
complications/underlying diseases. In addition, in the case of
patients who receive best supportive care (BSC) due to poor general
condition, it remains to be elucidated whether chemotherapy should
be administered.
In the present study, a regimen was used including
paclitaxel (60 mg/m2) plus carboplatin (AUC 2 mg/ml/min)
every week (W-PC) for patients who may be judged as being unable to
receive a standard regimen due to poor general condition, i.e.,
those who may select BSC. The purpose of the present study was to
retrospectively assess the response to and toxicity of the W-PC
regimen in patients with ovarian cancer and poor general
condition.
Materials and methods
Patients
The medical records of patients who were treated
using W-PC for epithelial ovarian cancer, tubal cancer or primary
peritoneal cancer at Jichi Medical University Hospital (Shimotsuke,
Japan) between January 2008 and December 2016 were reviewed.
Patients who were ≥80 years old and/or had a PS ≥3 and/or severe
complications/underlying diseases were selected. Not only patients
who were ≥80 years old but also patients younger than 80 years with
PS ≥3 and/or severe complications/underlying diseases were
selected. Severe complications and underlying diseases included
ileus, pulmonary embolism and pulmonary hypertension. All patients
received W-PC as the first-line chemotherapy regimen. Patients who
received W-PC as neoadjuvant chemotherapy (NAC) and those who did
not receive interval debulking surgery (IDS) after NAC due to poor
general condition or chemotherapy resistance were included. All
patients had evaluable lesions. Patients who had active double
cancer were excluded. W-PC was administered to the above patients
with poor general condition. As it was difficult to determine
whether the patients were able to receive chemotherapy, W-PC was
administered after sufficient informed consent from the patients
and their families. The following data were obtained from the
medical records: Age, complications, underlying diseases, Eastern
Cooperative Oncology Group PS (10), International Federation of
Gynecology and Obstetrics (FIGO) stage (11), operative procedure, timing of
chemotherapy administration, toxicities, tumor response,
progression-free survival (PFS) and overall survival (OS).
The present study was approved by the institutional
review board of Jichi Medical University (Shimotsuke, Japan).
Treatment plan
Patients received paclitaxel (60 mg/m2)
and carboplatin (AUC 2 mg/ml/min) intravenously on days 1, 8 and 15
of a 28-day cycle. Although a total of 6-9 cycles of W-PC was
planned prior to and after surgery, treatments were discontinued
when either the disease progressed, unacceptable toxicity developed
or the patient refused treatment. If W-PC was effective but cancer
lesions remained, up to 12 cycles were allowed. Bevacizumab was not
used because the patients in the present study had poor general
condition. Granulocyte colony-stimulating factor (G-CSF) was used
only if patients had grade 4 neutropenia or grade 3 febrile
neutropenia.
Safety assessment
Laboratory tests were performed on each treatment
day. Both hematological and non-hematological toxicities were
assessed through review of medical records. Toxicities were
assessed using the Common Terminology Criteria for Adverse Events
v4.0(12).
Response and survival assessment
In principle, response to treatment was evaluated by
computed tomography (CT). When the lesions did not appear on CT but
were clearly observed on pelvic examination, this was also used for
evaluating the response. The tumor response was assessed based on
the Response Evaluation Criteria in Solid Tumors version
1.1(13). OS was defined as the
time that elapsed between the start of treatment and the date of
death or last follow-up. PFS was defined as the time that elapsed
between the start of treatment and the date of progression or last
follow-up. The day of the start of treatment was defined as the
operative day in primary debulking surgery (PDS) cases and as the
first administration day of W-PC in NAC cases.
Statistical analysis
Statistical analyses were performed using EZR
version 1.54 (Saitama Medical Center, Jichi Medical University)
(14), which is a graphical user
interface for R (The R Foundation for Statistical Computing). More
precisely, it is a modified version of R commander designed to add
statistical functions frequently used in biostatistics. The
Kaplan-Meier curves were drawn to evaluate PFS and OS. The
significance of the difference in survival distribution between
subgroups was evaluated using the log-rank test. Variables with
P<0.05 in the log-rank test were subsequently entered into the
multivariate analysis. Multivariate analysis was performed using
Cox's proportional hazards model. P<0.05 was considered to
indicate statistical significance.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. A total of 31 patients
were included in this study. The median age was 73 (range, 30-82)
years. The numbers of patients who had FIGO stage IIIC, IVA and IVB
were 13 (42%), 6 (19%) and 12 (39%), respectively. Furthermore, one
(3%), 9 (29%) and 21 (68%) patients had a PS of 1, 2 and 3,
respectively. A total of 21 patients (68%) underwent surgery. Among
them, 12 underwent PDS and 9 underwent IDS. Of all patients, 19
(61%) received NAC and 12 (39%) received adjuvant chemotherapy; 10
of the 19 patients who received NAC did not undergo IDS due to poor
general condition or chemotherapy resistance. The major
complications and underlying diseases were as follows: Deep venous
thrombosis and/or pulmonary embolism, 7 (23%); ileus, 6 (19%);
hypertension, 11 (35%); and diabetes mellitus, 5 (16%). A total of
11 patients had severe complications and underlying diseases, which
were defined as ileus, pulmonary embolism and pulmonary
hypertension. The median number of chemotherapy cycles was 5
(range, 1-12) and the median number of chemotherapy doses was 11
(range, 2-30). In the NAC cases, these were the numbers after
adding up pre- and post-operative values.
 | Table ICharacteristics of the patients
(n=31). |
Table I
Characteristics of the patients
(n=31).
| Characteristic | Value |
|---|
| Age, years | 73 (30-82) |
| Stage | |
|
IIIC | 13(42) |
|
IVA | 6(19) |
|
IVB | 12(39) |
| Performance
status | |
|
1 | 1(3) |
|
2 | 9(29) |
|
3 | 21(68) |
| Operation | |
|
Performed | |
|
Primary
debulking surgery | 12(39) |
|
Interval
debulking surgery | 9(29) |
|
None | 10(32) |
| Chemotherapy | |
|
Neoadjuvant | 19(61) |
|
Adjuvant | 12(39) |
| Complications | |
|
DVT, PE | 7(23) |
|
Ileus | 6(19) |
| Underlying
diseases | |
|
Hypertension | 11(35) |
|
Diabetes
mellitus | 5(16) |
|
Dementia | 2(6) |
|
Renal
dysfunction | 2(6) |
|
PH | 1(3) |
| Number of
chemotherapy cycles | 5 (1-12) |
| Number of
chemotherapy doses | 11 (2-30) |
Toxicity
Hematological toxicities are presented in Table II. Grade 3/4 leucopenia,
neutropenia, anemia and thrombocytopenia developed in 13 (42%), 18
(58%), 5 (16%), and 1 (3%) patient(s), respectively. G-CSF was used
in 4 patients.
 | Table IIHematological toxicities by grade. |
Table II
Hematological toxicities by grade.
| Toxicity | G1 | G2 | G3 | G4 | G3+G4 |
|---|
| Leucopenia | 3(10) | 10(32) | 11(35) | 2(6) | 13(42) |
| Neutropenia | 0 (0) | 8(25) | 13(42) | 5(16) | 18(58) |
| Anemia | 0 (0) | 21(68) | 5(16) | 0 (0) | 5(16) |
| Thrombocytopenia | 0 (0) | 2(6) | 1(3) | 0 (0) | 1(3) |
Non-hematological toxicities are indicated in
Table III. Grade 2 or higher
liver dysfunction, renal dysfunction, neuropathy, nausea and
diarrhea developed in 2 (6%), 7 (23%), 1 (3%), 4 (13%) and 3 (10%)
of patients, respectively. Grade 2 or higher alopecia, which was
possible to evaluate in only 15 patients based on their medical
records, developed in 7 (47%) patients. There were no allergic
reactions. A total of 163 cycles were performed, equaling a total
of 489 (163 x 3) dose chances, as W-PC involved 3 doses per 1 cycle
in principle. Of the 489 dose chances, 392 (80%) doses were
administered without skipping. Skipping meant that a dose chance
was omitted due to toxicity.
 | Table IIINon-hematological toxicities by
grade. |
Table III
Non-hematological toxicities by
grade.
| Toxicity | G1 | G2 | G3 | G4 | G2+G3+G4 |
|---|
| AST/ALT | 0 (0) | 1(3) | 1(3) | 0 (0) | 2(6) |
| Creatinine | 0 (0) | 5(16) | 2(6) | 0 (0) | 7(23) |
| Neuropathy | 7(23) | 1(3) | 0 (0) | 0 (0) | 1(3) |
| Nausea | 12(39) | 4(13) | 0 (0) | - | 4(13) |
| Diarrhea | 3(10) | 2(6) | 1(3) | 0 (0) | 3(10) |
| Alopeciaa | 7(47) | 7(47) | - | - | 7(47) |
Response and survival
Tumor responses are presented in Table IV. A total of 3 (10%) patients had
a complete response (CR), 12 (39%) had a partial response (PR), 5
(16%) had stable disease and 11 (35%) had progressive disease (PD).
Patients with PD included 2 who discontinued chemotherapy of their
own volition after one cycle. The overall response rate was 48%
(15/31) and the disease control rate was 65% (20/31). The median
follow-up period was 16 (1-122) months. The median PFS period was
12 months and the 5-year PFS rate was 15%. The median OS period was
18 months and the 5-year OS rate was 15%. A total of 9 patients
survived for >40 months, 3 of whom had no recurrence. The
patient who survived for the longest period survived with no
recurrence for 122 months.
 | Table IVTumor response in the cohort. |
Table IV
Tumor response in the cohort.
| Tumor response | Value |
|---|
| Complete
response | 3(10) |
| Partial response | 12(39) |
| Stable disease | 5(16) |
| Progressive
diseasea | 11(35) |
| Response rate,
% | 48 |
| Disease control
rate, % | 65 |
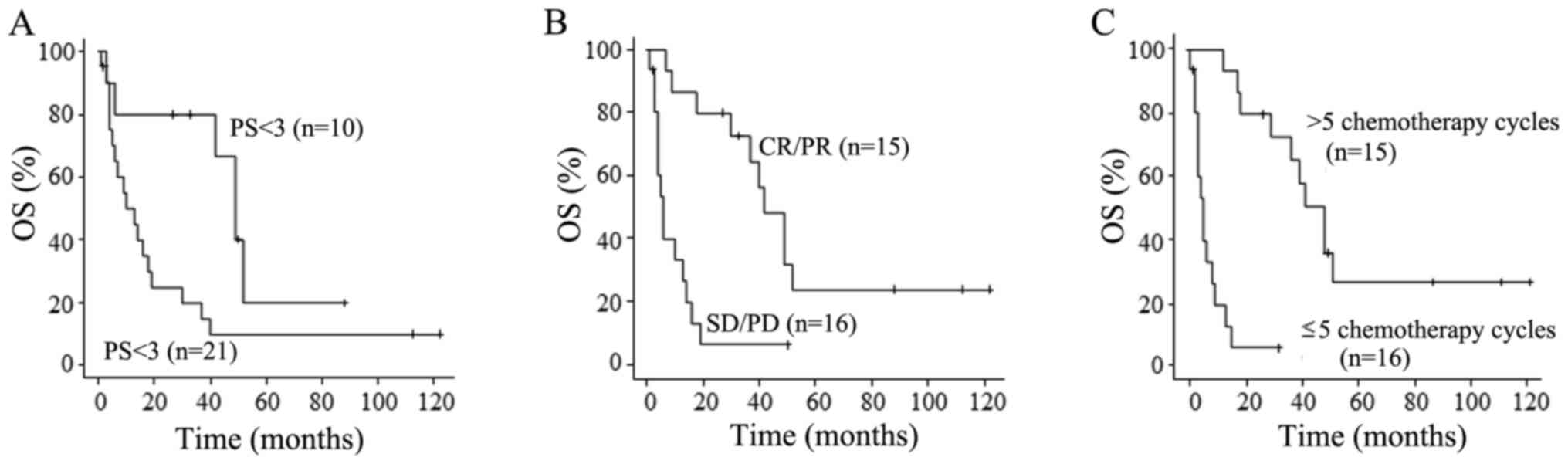
The results of the univariate analyses of prognostic
factors are provided in Table V.
PS<3, tumor response of CR or PR and >5 chemotherapy cycles
were favorable prognostic factors. Only >5 chemotherapy cycles
was an independent factor for favorable prognosis according to
multivariate analysis (Table VI).
However, age, stage, timing of chemotherapy administration, severe
complications/underlying diseases and toxicities did not have any
prognostic value. OS curves according to PS, response to
chemotherapy and number of chemotherapy cycles are presented in
Fig. 1.
 | Table VUnivariate analyses of prognostic
factors. |
Table V
Univariate analyses of prognostic
factors.
| Variable | Number of
patients | Median OS
(months) | P-value |
|---|
| Age, years | | | 0.874 |
|
<73 | 15 | 10 | |
|
≥73 | 16 | 30 | |
| Stage | | | 0.705 |
|
III | 13 | 7 | |
|
IV | 18 | 19 | |
| PS | | | 0.020 |
|
1, 2 | 10 | 49 | |
|
3 | 21 | 13 | |
| Chemotherapy | | | 0.813 |
|
Neoadjuvant | 19 | 30 | |
|
Adjuvant | 12 | 12 | |
|
Complications/underlying diseases | | | 0.126 |
|
Non-severe | 20 | 34 | |
|
Severe | 11 | 6 | |
| Neutropenia,
grade | | | 0.775 |
|
<3 | 13 | 11 | |
|
≥3 | 18 | 19 | |
| Tumor response | | | <0.001 |
|
CR/PR | 15 | 42 | |
|
SD/PD | 16 | 6 | |
| Number of
chemotherapy cycles | | | <0.001 |
|
>5 | 15 | 49 | |
|
≤5 | 16 | 6 | |
 | Table VIMultivariate analysis of prognostic
factors. |
Table VI
Multivariate analysis of prognostic
factors.
| Variable | Number of
patients | Hazard ratio | 95% CI | P-value |
|---|
| PS | | | 0.85-6.98 | 0.098 |
|
1, 2 | 10 | 1 | | |
|
3 | 21 | 2.43 | | |
| Tumor response | | | 0.89-8.03 | 0.080 |
|
CR/PR | 15 | 1 | | |
|
SD/PD | 16 | 2.67 | | |
| Number of
chemotherapy cycles | | | 2.26-36.6 | 0.002 |
|
>5 | 15 | 1 | | |
|
≤5 | 16 | 9.09 | | |
Discussion
In the present study, W-PC was selected for patients
with ovarian cancer who were unable to receive a standard
chemotherapy regimen due to having poor general condition. The
efficacy and safety of W-PC for patients with ovarian cancer and
poor general condition was retrospectively examined. The results
demonstrated that W-PC exhibited tolerable toxicity and was
slightly effective in patients with ovarian cancer and poor general
condition.
W-PC had tolerable toxicity in patients with ovarian
cancer who were unable to receive a standard regimen such as TW-PC,
PC-BEV or dd-PC due to having poor general condition. These
standard regimens are likely to be markedly toxic to patients with
poor general condition, preventing them from continuing
chemotherapy or worsening their general condition. In the TW-PC
group of the Multicenter Italian Trials in Ovarian cancer (MITO) 7
trial (15) and Japanese
Gynecologic Oncology Group (JGOG) 3016 trial (6), grade 3-4 neutropenia rates were 50
and 88% and grade 3-4 thrombocytopenia rates were 7 and 38%,
respectively. The patients in the JGOG 3016 trial exhibited more
severe myelosuppression than those in the MITO 7 trial, suggesting
that the Japanese patients exhibited slightly more severe
myelosuppression. The reason may be polymorphisms in genes that
function in taxane metabolism (16). Therefore, it is difficult to
administer TW-PC safely to Japanese patients with poor general
condition. However, W-PC may be safe for Japanese patients with
poor general condition. In the MITO 7 trial, patients assigned to
W-PC had milder toxicities than patients assigned to TW-PC
(15).
In the JGOG 3016 trial, the dd-PC group had more
severe anemia than the TW-PC group (6). This may have been because the
paclitaxel dose per week in the dd-PC group was higher than that in
the TW-PC group (80 vs. 60 mg/m2, respectively). The
paclitaxel dose per administration in the W-PC regimen of the
present study was 60 mg/m2, which may be safe for
patients with poor general condition. Since the patients of the
present study had a poor general condition and were not able to
receive chemotherapy itself, a lower dose (paclitaxel 60
mg/m2) was selected for safety. W-PC in the MITO 7 trial
was administered on days 1, 8 and 15 of a 21-day cycle. However, in
the present study, the W-PC regimen was administered on days 1, 8
and 15 of a 28-day cycle. A 28-day cycle regimen was considered to
be safer than a 21-day cycle regimen for patients with poor general
condition. Watanabe et al (17) used the same W-PC regimen as in the
present study; their regimen was also administered in a 28-day
cycle. Their patients had platinum-sensitive relapsed ovarian
cancer and a PS of 0-2, which was better than the condition of the
patients of the present study. They reported a rate of grade 3-4
neutropenia of 32% and a rate of grade 3-4 thrombocytopenia of 0%
(17). Grade 3-4 neutropenia was
observed more frequently in the present study (58%), as the present
cases had a poorer general condition. Therefore, the present
regimen in a 28-day cycle for patients with poor general condition
may be appropriate. If a 21-day cycle had been adopted, the
toxicities may have been more severe.
In the present study, W-PC was slightly effective in
patients with ovarian cancer and poor general condition. A similar
study recently reported that NAC with W-PC for patients with
ovarian cancer and poor PS reduced the toxicity of chemotherapy and
had the same efficacy as TW-PC (18). Their W-PC regimen was also
administered in a 28-day cycle. Response and disease control rates
in their W-PC group were 70% (14/20) and 80% (16/20), respectively,
being higher than those in the present study. One reason may be
that the patients in the present study had a poorer general
condition. For instance, the median age of patients in the present
study and their study was 73 (30-82) and 61 (36-75), respectively,
and the rate of patients with a PS of 3 was 68 and 40%,
respectively. The ICON8 trial compared 3 regimens of TW-PC, W-PC
and dd-PC. The PFS of the three groups was similar (24.4, 25.3 and
24.9 months, respectively) (19).
The W-PC regimen in the ICON8 trial was paclitaxel (80 mg/m2) and
carboplatin (AUC 2 mg/ml/min) administered on days 1, 8 and 15 of a
21-day cycle, whereas the W-PC regimen of the present study was
paclitaxel (60 mg/m2) and carboplatin (AUC 2 mg/ml/min)
administered on days 1, 8 and 15 of a 28-day cycle. Although the
W-PC regimen in the ICON8 trial differed from the W-PC regimen of
the present study, W-PC may have similar efficacy to TW-PC and
dd-PC. However, sufficient evidence as to whether the present W-PC
regimen may be applied to patients with ovarian cancer and good
general condition is lacking.
In the present study, the W-PC regimen was selected
for patients who may receive BSC due to advanced age, PS ≥3 or
severe complications/underlying diseases. Of these patients with
poor general condition, 9 survived for 40 months or longer and 3
had no recurrence. Furthermore, one patient survived with no
recurrence for 122 months. According to multivariate analysis,
>5 chemotherapy cycles was the only independent favorable
prognostic factor. The 9 patients who survived for >40 months
all received >5 cycles of W-PC. The number of chemotherapy
cycles may be a valuable prognostic factor that reflects numerous
elements such as tumor response and adverse effects. In the W-PC
regimen, unlike in TW-PC and dd-PC, both paclitaxel and carboplatin
are administered in divided doses from day 1. In patients with poor
general condition, severe adverse effects may develop or general
conditions may deteriorate after the administration of anticancer
agents. If an anticancer agent is administered in a small dose on
day 1, such as in W-PC, the development of severe adverse effects
and the deterioration of the general condition may be minimized.
Therefore, W-PC is safe for patients with poor general condition.
These methods are also performed for other types of carcinoma, such
as urothelial carcinoma (20).
The current study had certain limitations due to its
retrospective design. First, sufficient data on adverse events
other than blood test results were unavailable, but the results may
not be significantly altered as severe toxicity was confirmed. The
reason is as follows: Since almost all severe toxicities of
neuropathy, nausea, diarrhea and alopecia have apparent symptoms,
it was unlikely that any toxicities remained unnoticed.
Furthermore, it was planned to compare the present W-PC with the
standard regimens, such as TW-PC, PC-BEV and dd-PC; however, the
subjects of the present study were ineligible for standard TW-PC,
PC-BEV or dd-PC. At our hospital, these 3 regimens are not used for
patients with poor general condition, precluding comparisons
between W-PC and the above regimens.
In conclusion, W-PC exhibited tolerable toxicity and
was slightly effective in patients with ovarian cancer with poor
general condition. In addition, W-PC may lead to long-term
survival, albeit in only a small number of patients. If patients
receive >5 cycles of W-PC, their survival may be prolonged.
Thus, W-PC may be an appropriate therapeutic option for patients
for whom standard regimens are contraindicated due to advanced age,
poor PS or severe complications/underlying diseases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ST, YT, and HF designed the present study,
critically revised the manuscript and analyzed data. KT, AT, YT,
TY, TK, RN and YS retrieved the data from the medical records,
confirmed the authenticity of the raw data and revised the work
critically for important intellectual content. ST and YT wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Jichi Medical University (Shimotsuke, Japan). The ethics committee
of Jichi Medical University (Shimotsuke, Japan) judged that patient
consent was not required as this was a retrospective observational
study. The patients or their families were given the opportunity to
opt out.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Gynecologic Oncology Group. Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
Gynecologic Oncology Group study. J Clin Oncol. 21:3194–3200.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
du Bois A, Lück HJ, Meier W, Adams HP,
Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et
al: A randomized clinical trial of cisplatin/paclitaxel versus
carboplatin/paclitaxel as first-line treatment of ovarian cancer. J
Natl Cancer Inst. 95:1320–1329. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Takei Y, Suzuki M, Ohwada M, Saga Y, Kohno
T, Machida S and Sato I: A feasibility study of paclitaxel and
carboplatin therapy in Japanese patients with epithelial ovarian
cancer. Oncol Rep. 10:951–955. 2003.PubMed/NCBI
|
|
4
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al: A phase 3 trial of bevacizumab in
ovarian cancer. N Engl J Med. 365:2484–2496. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Katsumata N, Yasuda M, Takahashi F,
Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura
E, et al: Dose-dense paclitaxel once a week in combination with
carboplatin every 3 weeks for advanced ovarian cancer: A phase 3,
open-label, randomised controlled trial. Lancet. 374:1331–1338.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
González-Martín A, Pothuri B, Vergote I,
DePont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D,
Hoskins P, Freyer G, et al: Niraparib in patients with newly
diagnosed advanced ovarian cancer. N Engl J Med. 381:2391–2402.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ray-Coquard I, Pautier P, Pignata S, Pérol
D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus Bevacizumab as First-Line
Maintenance in Ovarian Cancer. N Engl J Med. 381:2416–2428.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cancer Therapy Evaluation Program: Common
Toxicity Criteria. Version 2.0, 1999. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf.
Accessed October 23, 2021.
|
|
11
|
Prat J: FIGO Committee on Gynecologic
Oncology. Staging classification for cancer of the ovary, fallopian
tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events. Version 4.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
Accessed August 15, 2021.
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pignata S, Scambia G, Katsaros D, Gallo C,
Pujade-Lauraine E, De Placido S, Bologna A, Weber B, Raspagliesi F,
Panici PB, et al: Carboplatin plus paclitaxel once a week versus
every 3 weeks in patients with advanced ovarian cancer (MITO-7): A
randomised, multicentre, open-label, phase 3 trial. Lancet Oncol.
15:396–405. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma BB, Hui EP and Mok TS: Population-based
differences in treatment outcome following anticancer drug
therapies. Lancet Oncol. 11:75–84. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Watanabe Y, Nakai H, Ueda H and Hoshiai H:
Evaluation of weekly low-dose paclitaxel and carboplatin treatment
for patients with platinum-sensitive relapsed ovarian cancer.
Gynecol Oncol. 96:323–329. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Takaya H, Nakai H, Murakami K, Tobiume T,
Suzuki A, Mandai M and Matsumura N: Efficacy of weekly
administration of paclitaxel and carboplatin for advanced ovarian
cancer patients with poor performance status. Int J Clin Oncol.
23:698–706. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Clamp AR, James EC, McNeish IA, Dean A,
Kim JW, O'Donnell DM, Hook J, Coyle C, Blagden S, Brenton JD, et
al: Weekly dose-dense chemotherapy in first-line epithelial
ovarian, fallopian tube, or primary peritoneal carcinoma treatment
(ICON8): Primary progression free survival analysis results from a
GCIG phase 3 randomised controlled trial. Lancet. 394:2084–2095.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Han JJ, Kim YJ, Kim JW, Chang H, Lee JO,
Lee KW, Jeong CW, Kim JH, Hong SK, Bang SM, et al: Salvage
treatment with low-dose weekly paclitaxel in elderly or poor
performance status patients with metastatic urothelial carcinoma.
Tumori. 100:439–445. 2014.PubMed/NCBI View Article : Google Scholar
|