Introduction
Bladder cancer is the ninth most common cancer
worldwide that ranks 13th for mortality rate, and ~430,000 cases of
bladder cancer are reported each year (1). Smoking is the highest risk factor for
bladder cancer, accounting for 50% of all cases (2). Urothelial carcinoma (UC) is a common
histological type of bladder cancer that includes
non-muscle-invasive UC (pathological stages Ta, T1 and Tis) and
muscle-invasive UC (pathological stages T2 and higher) (3). Treatment guidelines for UC recommend
the assessment of muscle invasiveness. Patients with Ta and T1
non-muscle-invasive UC undergo transurethral resection of bladder
tumor (TURBT) for diagnostic and therapeutic purposes (4). Bacillus Calmette-Guerin (BCG) therapy
is conducted for Tis UC, while a combination of cystectomy,
chemoradiotherapy and radiation therapy is used to treat
muscle-invasive UC (4).
Non-muscle-invasive UC is separated into two distinct categories
based on tumor growth as follows: Papillary and flat
non-muscle-invasive UC. Furthermore, ~70-75% of primary UC cases
are papillary carcinoma, whereas ~1-3% are the pure form of flat
carcinoma. Non-muscle-invasive papillary UC can be distinguished as
low grade (NMIPUC-L) and high grade (NMIPUC-H) based on
architectural and cytological features (3).
Peroxisome proliferator-activated receptors (PPARs)
are nuclear hormone receptors that comprise three subtypes: PPAR-α,
PPAR-γ and PPAR-δ. The PPAR-γ protein has been detected in adipose
tissue (5). The relationship
between the expression of PPAR-γ and colon cancer has attracted
increasing attention (6-8).
It was demonstrated that PPAR-γ induces cell differentiation,
arrests cell growth and reduces tumor growth rate in colon cancer.
PPAR-γ ligands are expected to become promising therapeutic agents
for chemoprevention and treatment. However, previous studies on
PPAR-γ activation using several agonists have not provided
consistent findings, such as the tumor suppressive or oncogenic
role of PPAR-γ, in a heterogeneous nature in bladder cancer
(9,10). Furthermore, limited information is
currently available on the expression of PPAR-γ in carcinoma in
situ (CIS), such as flat carcinoma. Therefore, the relationship
between PPAR-γ expression and the histological proliferation type
in UC remains unclear. The present study aimed to investigate the
expression of PPAR-γ in non-muscle-invasive UC, including CIS, and
to compare it with that in normal urinary epithelial cells to
clarify whether PPAR-γ may be used as an immunobiomarker in
urothelial carcinoma.
Materials and methods
Subjects
Tissue samples, including TURBT and biopsy samples,
were collected from 30 non-malignant cases and 79
non-muscle-invasive UC cases (NMIPUC-L 30 cases, NMIPUC-H 30 cases,
and CIS 19 cases) at the Shikoku Cancer Center between April 2013
and March 2018. Samples from patients with inflammation in the
bladder urothelium or those who recovered from UC following TURBT
and BCG therapy were included as non-malignant cases. A
histological diagnosis was based on the World Health Organization
(WHO) classification of specimens stained using hematoxylin and
eosin (3). The present study was
approved by the Institutional Research Ethics Committee of the
Shikoku Cancer Center (Ehime, Japan) and Kagawa Prefectural
University of Health Sciences (Kagawa, Japan).
Immunohistochemical staining
Formalin-fixed paraffin-embedded tissue samples were
cut into 4-µm-thick sections. All samples were rehydrated and
deparaffinized using EZ buffer (Roche Diagnostics). Antigen masking
was removed using pH 8.5 CC1 buffer (Roche Diagnostics) at 95˚C for
64 min. All samples were then incubated with
H2O2 (Roche Diagnostics) to block endogenous
peroxidase activity. Sections were incubated with primary antibody
against PPAR-γ (mouse monoclonal; Santa Cruz Biotechnology, Inc;
cat. no. sc-7273; 1:200) at 36˚C for 32 min. The I-VIEW DAB
Universal Kit (Roche Diagnostics) was used for the secondary
antibody reaction, followed by section staining with
diaminobenzidine according to the manufacturer's instructions.
Benchmark ULTRA (Roche Diagnostics) was used as an automatic
immunostainer for immunohistochemical processes. To demonstrate the
diagnostic utility of PPAR-γ in non-muscle-invasive UC cases,
staining for p53 (mouse monoclonal; Agilent Technologies, Inc.;
cat. no. M7001; Ready-to-use) and Ki-67 (mouse monoclonal; Agilent
Technologies, Inc.; cat. no. M7240; 1:100) was performed on the
same non-muscle-invasive UC and non-malignant samples. The p53 and
Ki-67 immunohistochemical protocols were the same as the PPAR-γ
immunohistochemical protocol. The expression of all
immunobiomarkers was observed under a microscope (BX53; Olympus
Corporation; magnification, x400) and images were taken using a
microscope camera (DS-Fi3; Nikon Corporation). PPAR-γ-stained
specimens were assessed by four experienced observers as follows:
Nuclear-positive type, nuclear and cytoplasmic-positive type,
cytoplasmic-positive type and no signal type. In previous studies
(11,12), specimens positive for PPAR-γ were
evaluated based on the extent and staining intensity of PPAR-γ
expression. In the present study, the extent of positive staining
was classified into six categories as follows: 0 (≤10% staining), 1
(11-25% staining), 2 (26-50% staining), 3 (51-75% staining), 4
(76-90% staining) and 5 (≥91% staining). The PPAR-γ staining
intensity was classified into four categories as follows: 0 (no
signal), 1 (weak), 2 (moderate) and 3 (strong). The extent and
intensity for nuclear and cytoplasmic PPAR-γ expression were
scored. Based on the combinations of the extent and intensity
scores of PPAR-γ staining in nuclei, a combined score ≥4
corresponded to PPAR-γ positive, while that <4 corresponded to
PPAR-γ negative. Furthermore, PPAR-γ was considered to be negative
when PPAR-γ was locally expressed in the cytoplasm or was not
expressed at all. The expression of p53 was evaluated as an
aberrant type when positive cells accounted for ≥50% of tumor cells
and as a wild type when positive cells accounted for <50%. The
expression of Ki-67 was determined as high or low based on
published cut-offs (20% positive staining) (13,14).
cBioPortal
The cBioPortal for Cancer Genomics (http://www.cbioportal.org) is an open-access web
resource for exploring and analyzing multidimensional cancer
genomic data (15). The present
study analyzed the genomic data of PPAR-γ extracted from The Cancer
Genome Atlas (TCGA) datasets of bladder cancer, namely ‘TCGA Cell
2017’ (412 cases) (16), ‘TCGA,
Nature 2014’ (131 cases) (17) and
‘TCGA, PanCancer Atlas’ (411 cases) (18-26).
Statistical analysis
Univariate analysis was performed using
χ2 test for categorical data. Statistical analyses were
conducted using JMP 15.0 software (SAS Institute, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression pattern of PPAR-γ in the
urinary bladder
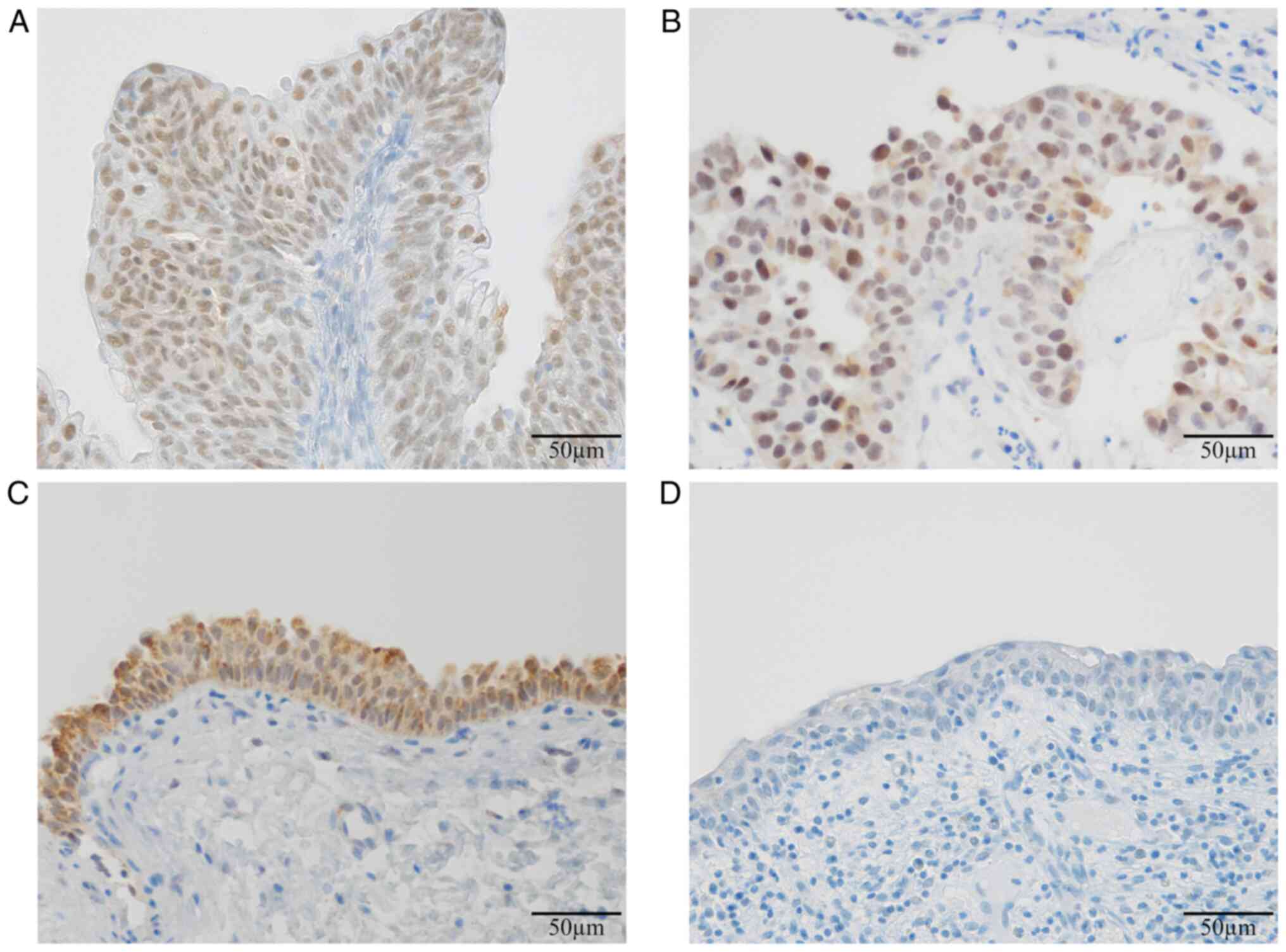
The following PPAR-γ expression patterns were
observed in the urinary bladder: Nuclear expression (Fig. 1A), nuclear-cytoplasmic expression
(Fig. 1B), cytoplasmic expression
(Fig. 1C) and no expression
(Fig. 1D). The localization of
PPAR-γ protein was significantly different between
non-muscle-invasive UC cases and non-malignant cases (P<0.0001;
Table I). PPAR-γ was mainly
expressed in the nuclei of tumor cells in non-muscle-invasive UC,
including NMIPUC-L (Fig. 2A),
NMIPUC-H (Fig. 2B) and CIS
(Fig. 2C). Conversely, PPAR-γ was
partly expressed in the cytoplasm of urinary epithelial cells in
non-malignant cases (Fig. 2D).
Statistical analyses demonstrated that the nuclear overexpression
of PPAR-γ was significantly higher in non-muscle-invasive UC cases
compared with non-malignant cases (P<0.0001; Table II). The PPAR-γ-positive expression
was significantly lower in CIS cases as flat carcinoma compared
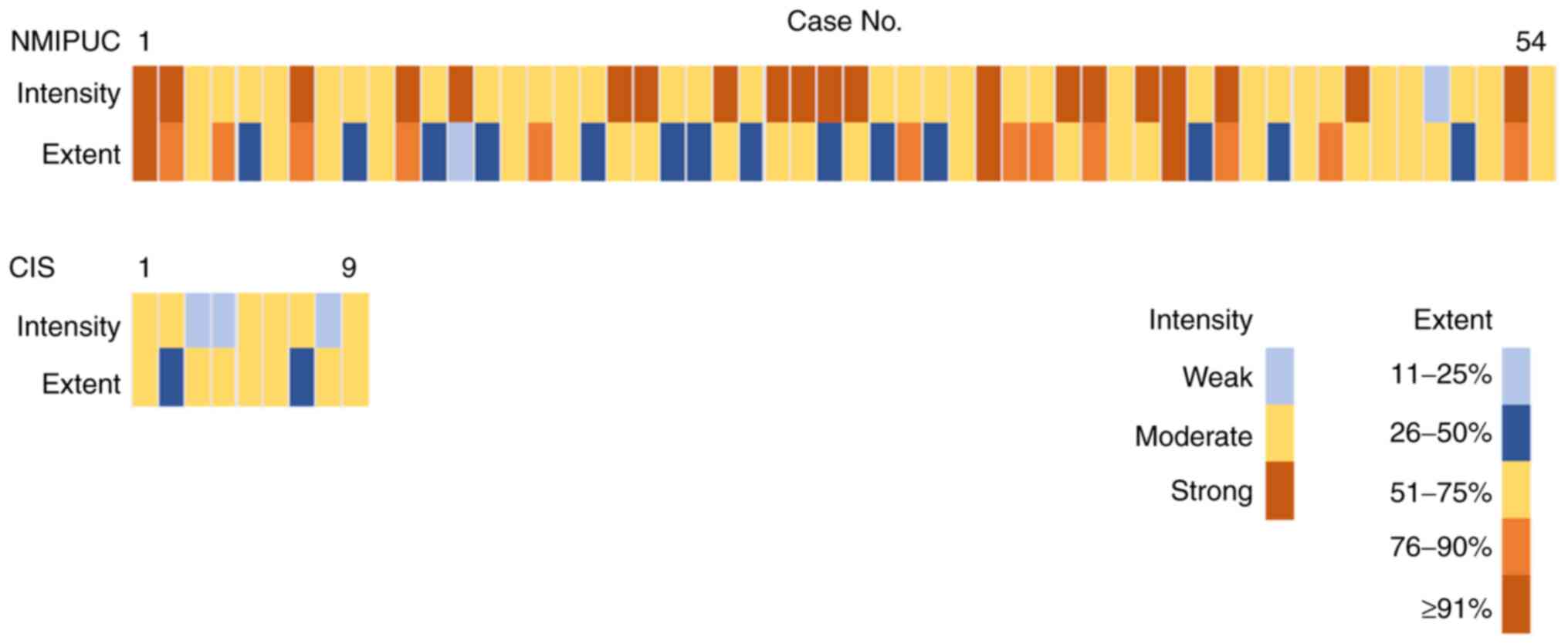
with NMIPUC-L and -H as papillary carcinoma (P=0.0002; Table III). In addition, PPAR-γ
intensity in CIS with PPAR-γ-positive tended to be weak (Fig. 3). The PPAR-γ-positive expression
was associated with the pathological stage (P=0.0003), but not with
age, sex or histological grades (Table III).
 | Table ILocalization of PPAR-γ expression in
non-muscle-invasive urothelial carcinoma and non-malignant
cases. |
Table I
Localization of PPAR-γ expression in
non-muscle-invasive urothelial carcinoma and non-malignant
cases.
| | Localization of
PPAR-γ expression |
|---|
| | Nuclear |
Nuclear-cytoplasmic, complex | Cytoplasmic | No signal | P-value |
|---|
| Non-muscle-invasive
urothelial carcinoma cases (n=79) | 46 | 32 | 1 | - | <0.0001 |
| Non-malignant cases
(n=30) | 1 | 13 | 15 | 1 | |
 | Table IINuclear expression and cytoplasmic
expression of PPAR-γ in non-muscle-invasive papillary urothelial
carcinoma (low and high-grades), flat carcinoma and non-malignant
cases. |
Table II
Nuclear expression and cytoplasmic
expression of PPAR-γ in non-muscle-invasive papillary urothelial
carcinoma (low and high-grades), flat carcinoma and non-malignant
cases.
| | PPAR-γ
expression |
|---|
| | Nuclear score | Cytoplasmic
score |
|---|
| | ≥4 | <4 | P-value | ≥4 | <4 | P-value |
|---|
| Non-muscle-invasive
urothelial carcinoma cases (n=79) | 63 | 16 | <0.0001 | 6 | 73 | 0.0007 |
|
NMIPUC
low-grade and high-grade cases (n=60) | 54 | 6 | <0.0001 | 2 | 58 | <0.0001 |
|
Flat
carcinomaa cases
(n=19) | 9 | 10 | 0.0009 | 4 | 15 | 0.3575 |
| Non-malignant cases
(n=30) | 2 | 28 | | 10 | 20 | |
 | Table IIIAssociation between patient
clinicopathological characteristics and the nuclear expression of
PPAR-γ in non-muscle-invasive urothelial carcinoma. |
Table III
Association between patient
clinicopathological characteristics and the nuclear expression of
PPAR-γ in non-muscle-invasive urothelial carcinoma.
| | PPAR-γ
expression | |
|---|
| | Cases no. | Positive | Negative | P-value |
|---|
| Mean age ± standard
deviation, years | | 74.0±8.11 | 70.9±8.33 | 0.3926 |
| Sex | | | | |
|
Male | 63 | 50 | 13 | 0.866 |
|
Female | 16 | 13 | 3 | |
| Histological
grade | | | | |
|
Non-muscle-invasive
urothelial carcinoma, low-grade | 30 | 26 | 4 | 0.2204 |
|
Non-muscle-invasive
urothelial carcinoma, high-gradea | 49 | 37 | 12 | |
| Histological
proliferation type | | | | |
|
Papillary
carcinomab | 60 | 54 | 6 | 0.0002 |
|
Flat
carcinomac | 19 | 9 | 10 | |
| Pathological
stage | | | | |
|
Tis | 19 | 9 | 10 | 0.0003 |
|
Ta | 45 | 41 | 4 | 0.619 |
|
T1 | 15 | 13 | 2 | |
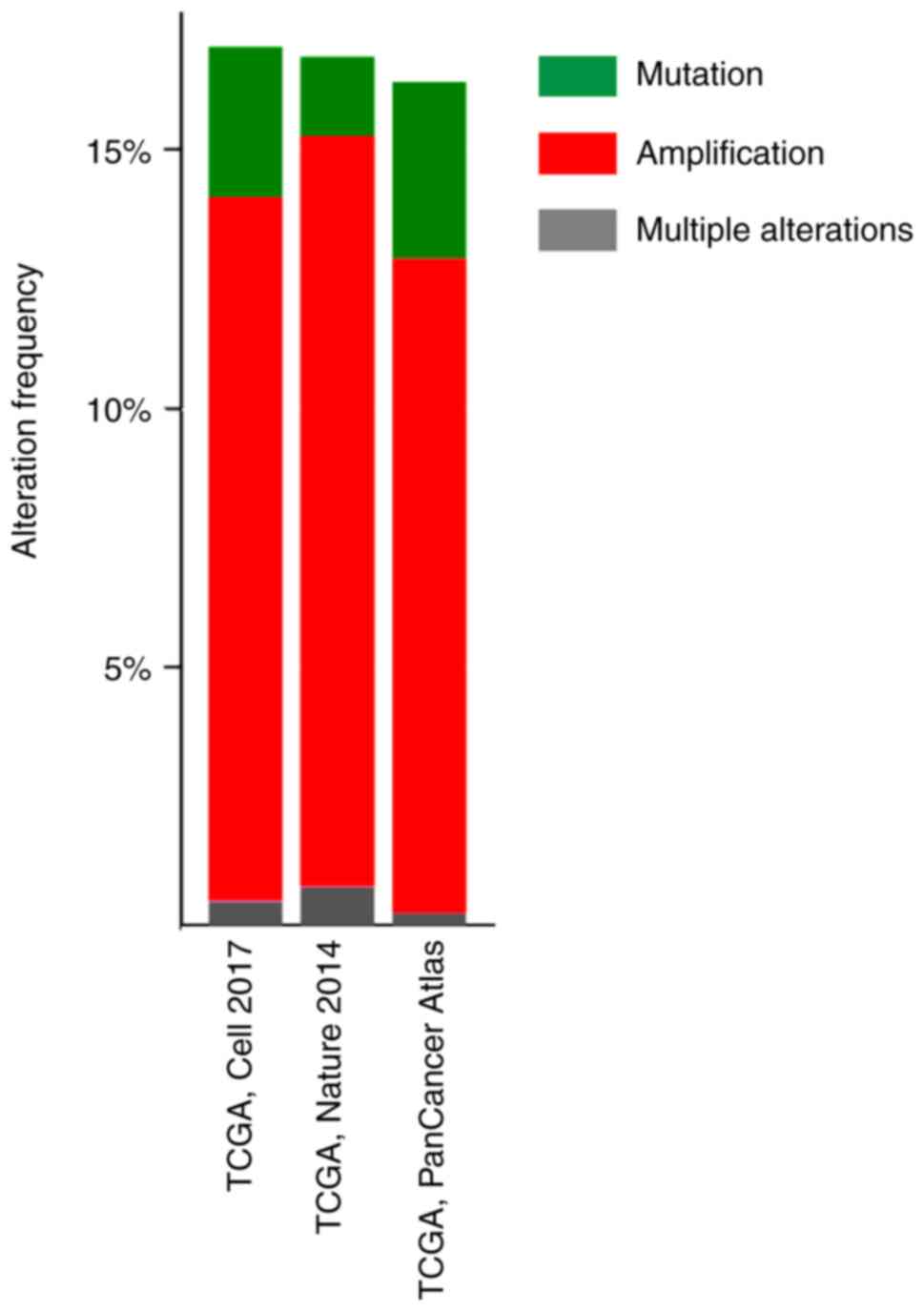
Genomic alteration of PPAR-γ in
bladder cancer
Using the TCGA datasets of bladder cancer and
cBioPortal online tool to analyze PPAR-γ gene mutations or copy
number alterations, alteration rates were 16.99% (70/412 cases;
TCGA, Cell 2017), 16.79% (22/131 cases; TCGA, Nature 2014) and
16.3% (67/411 cases; TCGA, PanCancer Atlas; Fig. 4). In addition, PPAR-γ gene
amplification accounted for most changes, with amplification rates
of 13.59% (56/412 cases; TCGA, Cell 2017), 14.50% (19/131 cases;
TCGA, Nature 2014), and 12.65% (52/411 cases; TCGA, PanCancer
Atlas; Fig. 4).
Comparison of PPAR-γ, p53 and Ki-67
values as immunobiomarkers for non-muscle-invasive UC
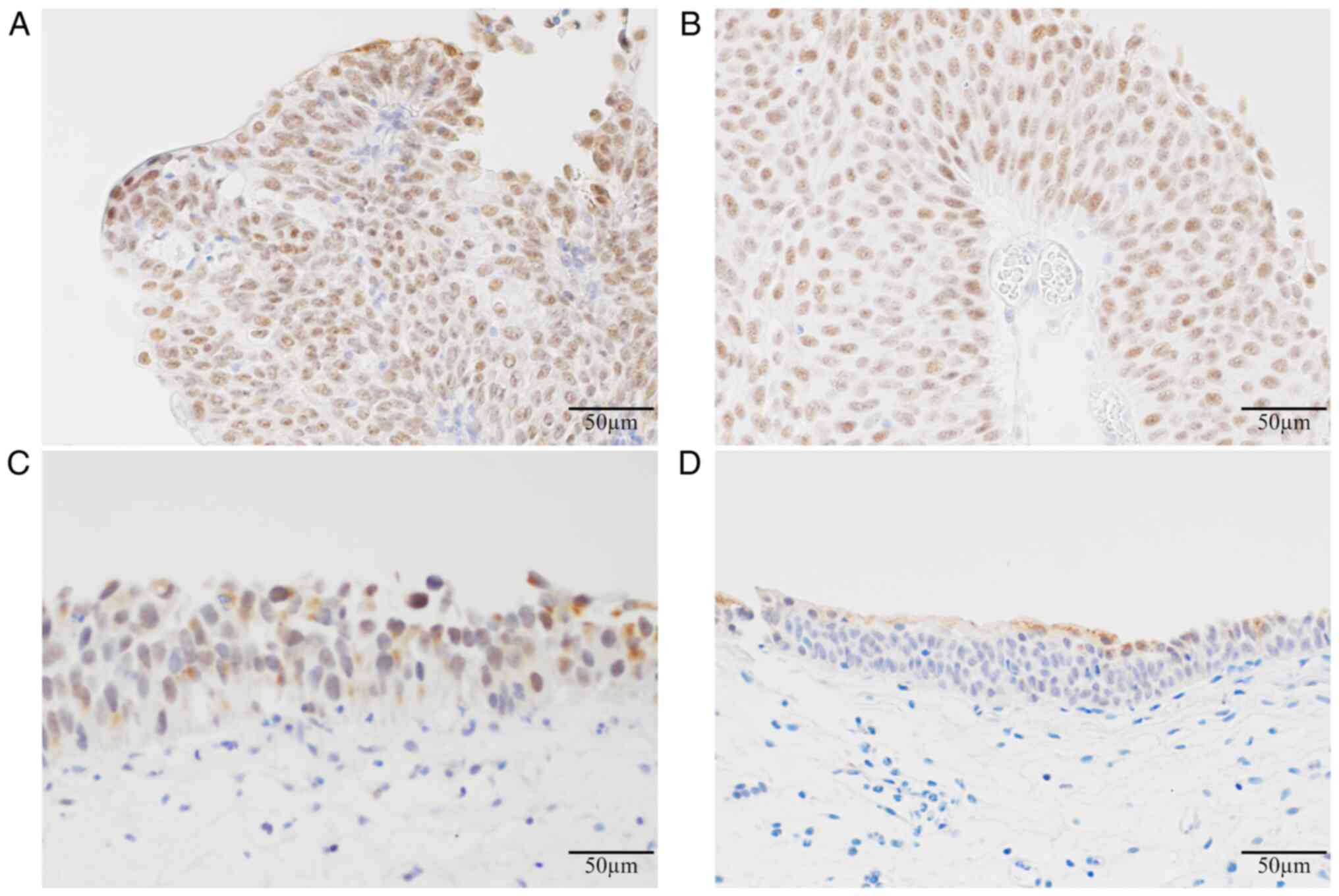
Fig. 5 shows
representative immunohistochemical staining for PPAR-γ (Fig. 5A, D, G and
J), p53 (Fig. 5B, E, H and
K) and Ki-67 (Fig. 5C, F, I and
L). Immunohistochemical staining
for PPAR-γ showed the highest sensitivity (79.7%). The diagnostic
odds ratio (DOR) was 55.13 (Table
IV). The expression of these immunobiomarkers was significantly
higher in non-muscle-invasive UC cases compared with non-malignant
cases. However, the PPAR-γ-positive expression did not
significantly differ among the histological grades of
non-muscle-invasive papillary UC (NMIPUC-L; 86.7%, NMIPUC-H; 93.3%.
P=0.3980; Table V). Conversely,
the aberrant p53 and high Ki-67 expression were significantly lower
in NMIPUC-L than in NMIPUC-H. Furthermore, only the PPAR-γ
positivity clearly distinguished NMIPUC-L from non-malignant cases
(P<0.0001; Table V). Five out
of 19 CIS cases were positive for PPAR-γ or had wild-type p53,
whereas 17 were positive for PPAR-γ or had aberrant p53 (Table VI).
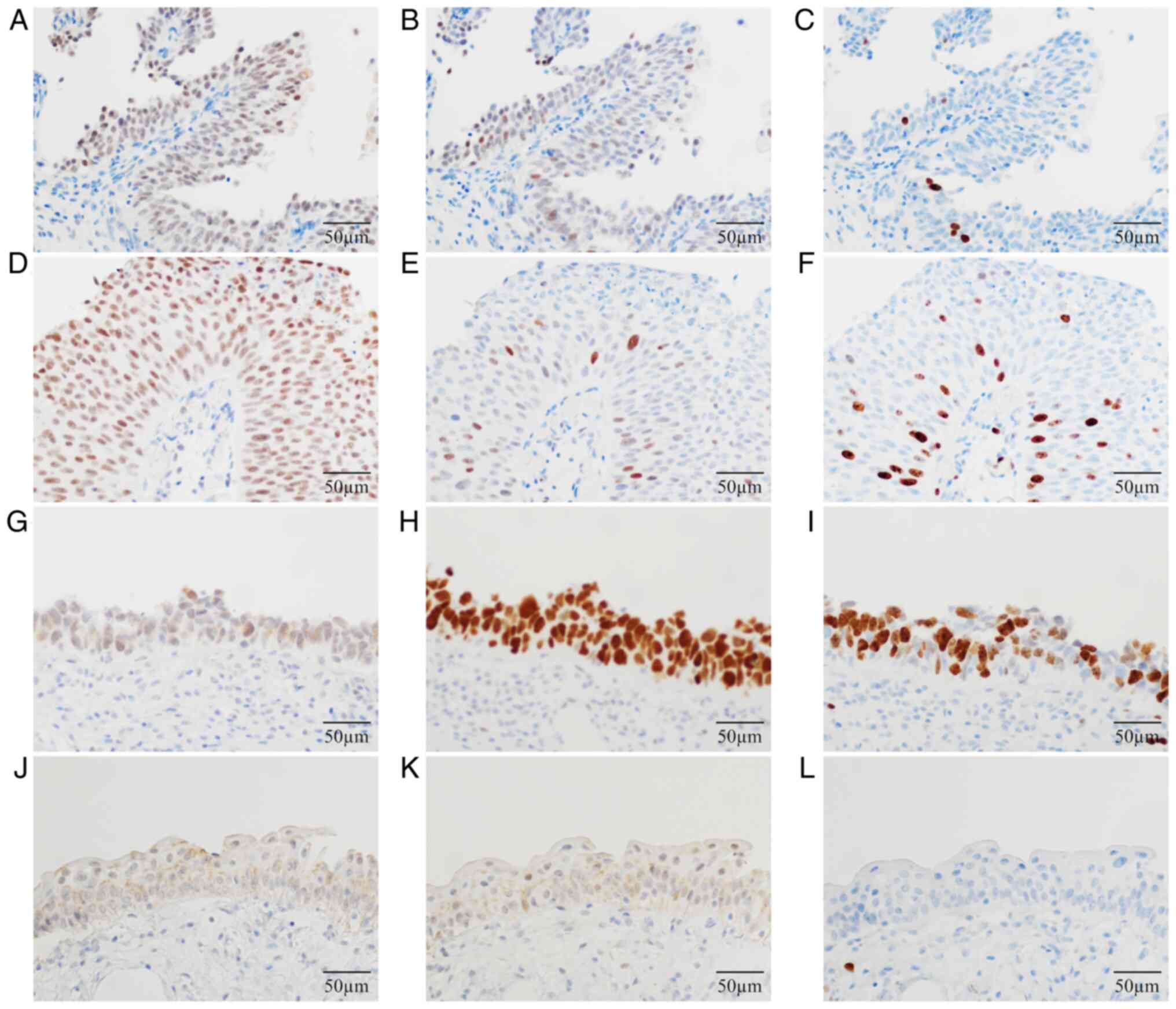 | Figure 5Representative immunohistochemical
staining for PPAR-γ, p53 and Ki-67. Immunohistochemical staining
showing (A) PPAR-γ, (B) p53 and (C) Ki-67 in non-muscle-invasive
papillary urothelial carcinoma (low-grade), (D) PPAR-γ, (E) p53 and
(F) Ki-67 in non-muscle-invasive papillary urothelial carcinoma
(high-grade), (G) PPAR-γ, (H) p53 and (I) Ki-67 in carcinoma in
situ, and (J) PPAR-γ, (K) p53 and (L) Ki-67 in non-malignant
cases. Magnification, x400. Scale bar, 50 µm. PPAR-γ, peroxisome
proliferator-activated receptor-γ. |
 | Table IVComparisons of detection accuracy for
PPAR-γ, p53 and Ki-67 immunobiomarkers. |
Table IV
Comparisons of detection accuracy for
PPAR-γ, p53 and Ki-67 immunobiomarkers.
| | Non-muscle-invasive
urothelial carcinoma (n=79) (%) | Non-malignant
(n=30) (%) | P-value | Sensitivity, % | Specificity, % | PPV, % | NPV, % | DOR, 95%CI |
|---|
| PPAR-γ | | | | | | | | |
|
Positive, n
(%) | 63 (79.7) | 2 (6.7) | <0.0001 | 79.7 | 93.3 | 96.9 | 63.6 | 55.13,
11.866-256.091 |
|
Negative, n
(%) | 16 (20.3) | 28 (93.3) | | | | | | |
| p53 | | | | | | | | |
|
Aberrant, n
(%) | 29 (36.7) | 1 (3.3) | 0.0005 | 36.7 | 96.7 | 96.7 | 36.7 | 16.82,
2.175-130.047 |
|
Wild, n
(%) | 50 (63.3) | 29 (96.7) | | | | | | |
| Ki-67 | | | | | | | | |
|
High, n
(%) | 35 (44.3) | 2 (6.7) | 0.0002 | 44.3 | 93.3 | 94.6 | 38.9 | 11.13,
2.481-49.994 |
|
Low, n
(%) | 44 (55.7) | 28 (93.3) | | | | | | |
 | Table VRelationships between PPAR-γ, p53 and
Ki-67 immunohistochemical staining and non-muscle-invasive
urothelial carcinoma and non-malignant cases. |
Table V
Relationships between PPAR-γ, p53 and
Ki-67 immunohistochemical staining and non-muscle-invasive
urothelial carcinoma and non-malignant cases.
| | PPAR-γ
expression | p53 expression | Ki-67
expression |
|---|
| | Positive (%) | vs. non-malignant,
P-value | Histological grade,
P-value | Aberrant (%) | vs. non-malignant,
P-value | Histological grade,
P-value | High (%) | vs. non-malignant,
P-value | Histological grade,
P-value |
|---|
| NMIPUC | | | | | | | | | |
|
Low grade,
n=30 (%) | 26 (86.7) | <0.0001 | 0.3980 | 5 (16.7) | 0.085 | 0.045 | 4 (13.3) | 0.3894 | <0.0001 |
|
High grade,
n=30 (%) | 28 (93.3) | <0.0001 | | 12(40) | 0.0006 | | 21(70) | <0.0001 | |
| CIS, n=19 (%) | 9 (47.4) | 0.0009 | | 12 (63.2) | <0.0001 | | 10 (52.6) | 0.0003 | |
| Non-malignant, n=30
(%) | 2 (6.7) | | | 1 (3.3) | | | 2 (6.7) | | |
 | Table VIStaining results for each
immunobiomarker in carcinoma in situ cases. |
Table VI
Staining results for each
immunobiomarker in carcinoma in situ cases.
| Case no. | PPAR-γ
expression | p53 expression | Ki-67
expression |
|---|
| 1 | - | Aberrant | High |
| 2 | - | Aberrant | High |
| 3 | Positive | Aberrant | High |
| 4 | - | Aberrant | - |
| 5 | Positive | Aberrant | High |
| 6 | - | Aberrant | High |
| 7 | - | Aberrant | High |
| 8 | - | - | - |
| 9 | Positive | - | - |
| 10 | Positive | - | - |
| 11 | - | Aberrant | High |
| 12 | - | Aberrant | High |
| 13 | Positive | Aberrant | - |
| 14 | Positive | - | - |
| 15 | Positive | Aberrant | High |
| 16 | Positive | - | - |
| 17 | - | Aberrant | High |
| 18 | Positive | - | - |
| 19 | - | - | - |
Discussion
The present study demonstrated that the
localization, intensity, extent and genomic alteration of PPAR-γ
expression significantly differed between non-muscle-invasive UC
and non-malignant cases. The expression pattern of PPAR-γ in CIS
suggested a relationship with the histological proliferative type
but not the histological grade. Furthermore, the present study
evaluated the usefulness of PPAR-γ, p53 and Ki-67 as
immunobiomarkers. The nuclear expression of PPAR-γ was
significantly higher in non-muscle-invasive UC compared with
non-malignant cases. In addition, PPAR-γ was more efficient for the
detection of non-muscle-invasive UC than p53 and Ki-67. These
results provided evidence for the potential role of PPAR-γ as an
immunobiomarker in non-muscle-invasive UC.
In the present study, PPAR-γ showed different
expression patterns in non-muscle-invasive UC and non-malignant
cases. We considered PPAR-γ protein as being overexpressed in
nuclei of urinary bladder tissues with malignant transformation. A
previous study reported the nuclear PPAR-γ positive staining in
colorectal tissues regardless of whether the tissue was malignant
or not (27). In ovarian tumors,
Zhang et al (11) reported
a significant difference in the expression of PPAR-γ between the
normal epithelium and malignant tumors and demonstrated that PPAR-γ
was overexpressed in nuclei along with disease progression. In the
present study, tumor cells of NMIPUC cases showed moderate
cytoplasmic expression and high nuclear expression. The cytoplasmic
expression of PPAR-γ was inversely associated with nuclear
expression, with that in non-malignant cases being significantly
higher than that in non-muscle-invasive UC. These results indicated
that PPAR-γ was overexpressed in nuclei with malignant
transformation. These differences in the expression patterns of
PPAR-γ between normal urinary epithelial cells and tumor cells
reflected the malignant transformation. PPAR-γ is a nuclear
receptor that is activated in the nucleus to regulate several
transcription factors. PPAR-γ ligands induce apoptosis in various
carcinomas (28,29). In colon cancer, the PPAR-γ ligand
15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) was shown to
inhibit the activity of nuclear factor-κB (NF-κB) and reduce the
expression of the Bcl-2 protein, ultimately leading to apoptosis
(30-32).
Furthermore, 15d-PGJ2, which is one of the natural
ligands for PPAR-γ, can inhibit the growth of neoplastic urothelial
cells (33). Bcl-2 and its
transcription factor, NF-κB, have been suggested to inhibit tumor
proliferation in UC by activating PPAR-γ. Previous studies
demonstrated that PPAR-γ activation can arrest the cell cycle;
however, most of the study materials examined were muscle-invasive
UC (9,34). The relationships between the
expression of PPAR-γ, apoptosis, cell cycle and malignant
transformation remain controversial. Further investigation is
therefore required to elucidate the PPAR-γ pathway in UC.
It is widely known that abnormal gene expression
differs with the histological proliferation type in bladder cancer
(35,36). According to the results from gene
expression analysis using cBioPortal, PPAR-γ gene alterations were
associated with bladder cancer. However, the histological
proliferation type has not yet been examined using a PPAR-γ
expression assay. Regarding the relationship between PPAR-γ
expression and cancer histological grades, Mylona et al
(37) and Nakashiro et al
(33) indicated that the nuclear
expression of PPAR-γ was inversely correlated with histological
grades in UC. However, as presented in Table III, a relationship was not
observed between the nuclear overexpression of PPAR-γ in
non-muscle-invasive UC and histological grades. Histological grades
are diagnosed from morphology based on architectural and
cytological features. Furthermore, the WHO classification defines
low-grade UC as papillary carcinoma, whereas high-grade UC includes
papillary, flat and infiltrating types. Since flat carcinoma was
not sufficiently examined in previous studies, the relationship
between PPAR-γ and histological proliferation types was not
investigated in detail. The results from the present demonstrated
that PPAR-γ expression significantly differed among pathological
stages. CIS corresponded to Tis pathological stage while papillary
carcinoma corresponded to Ta or T1 pathological stages. Therefore,
the results from statistical analyses appeared to be dependent on
the histological proliferation type and not on the pathological
stage. Statistical analyses of PPAR-γ expression in Ta and T1 did
not reveal any significant differences. Regarding the PPAR-γ
expression in muscle-invasive UC, a previous study reported that
PPAR-γ expression levels are lower in muscle-invasive UC cases than
in non-muscle-invasive UC cases (37). This finding supports our results
showing that PPAR-γ expression might be associated with the
proliferation type.
An immunohistochemical method to detect UC has not
yet been established. Previous studies reported a relationship
between UC and certain immunobiomarkers, such as p53, a tumor
suppressor protein, and Ki-67, a cell proliferation marker.
However, the sensitivity of p53 as an immunobiomarker was 26-59%,
while that of Ki-67 was 16-58% for non-invasive papillary UC,
including low and high grades, with the former grade not being
detected by these immunobiomarkers (38-42).
The sensitivities of p53 and Ki-67 in the present study were
consistent with previous findings; however, the sensitivity and DOR
of p53 and Ki-67 were dependent on the low frequency of the
aberrant type/high expression in NMIPUC-L. Therefore, PPAR-γ as an
immunobiomarker may be useful for detecting non-muscle-invasive UC
despite the histological grade. In the present study, p53 showed
the highest sensitivity as an immunobiomarker for CIS (63.2%,
12/19). The aberrant type of p53 has been widely investigated and
used as an immunobiomarker to detect CIS (43). Based on the data shown in the
present study, we considered CIS to have been comprehensively
detected using a combination of PPAR-γ and p53 as immunobiomarkers
rather than using p53 alone.
A limitation of the present study was that the
molecular biological analysis did not include muscle-invasive UC
cases or UC cell lines. Therefore, an association was not observed
between UC invasiveness and PPAR-γ expression. In addition, we did
not obtain clinical data on recurrence because of the limited
number of PPAR-γ-negative cases in UC presenting with recurrence
following TURBT. However, non-muscle-invasive UC frequently
relapses, and we speculated that PPAR-γ may serve an important role
in this process. Although further investigation is required, we
herein attempted to clarify the usefulness of PPAR-γ as an
immunobiomarker in samples from patients with non-muscle-invasive
UC.
A routine and less invasive method to detect UC is
urinary cytology; however, it has not yet been established as a
useful screening method for UC due to its low sensitivity. Meuleman
and Delaere (44) reported that
the diagnostic findings of urinary cytology were subject to UC
differentiation level and infiltrating stage. In cytological
samples, difficulties are associated with distinguishing NMIPUC-L
from normal urinary epithelial cells based on a morphological
diagnosis under a microscope because morphologically, NMIPUC-L
negligibly exhibits nuclear atypia and pleomorphism (45,46).
According to NCCN Clinical Practice Guidelines in Oncology
(4), ~70-75% of primary UC cases
are NMIPUC-L or NMIPUC-H. Therefore, NMIPUC-L and NMIPUC-H are
frequently encountered in urinary cytology but are not accurately
diagnosed. The results from the present study demonstrated that
PPAR-γ immunohistochemical staining could detect more cancer cells
than other immunobiomarkers for NMIPUC-L and NMIPUC-H. An ancillary
diagnostic test using PPAR-γ immunocytochemical staining may
effectively increase the accuracy of urinary cytology.
In summary, the present study demonstrated that
expression patterns of PPAR-γ were associated with histological
proliferation type and that PPAR-γ was expressed in the nuclei of
papillary carcinoma cells. Immunohistochemical staining for PPAR-γ
appeared to be more useful as an immunobiomarker for
non-muscle-invasive UC than the other biomarkers examined. Although
further investigation is needed, this study suggested that PPAR-γ
immunobiomarker may be considered as a promising tool for UC early
detection.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ST, YT and EH designed the study. ST, YT, SH, HO,
TM, TY and NT performed the experiments. ST, YT, SH and EH analyzed
all data. ST and YT wrote the manuscript. HO, NT and EH confirm the
authenticity of all raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki and was approved by the Ethics
Committee of the Shikoku Cancer Center (Ehime, Japan; approval no.
2018-95) and Kagawa Prefectural University of Health Sciences
(Kagawa, Japan; approval no. 291). In this retrospective study, the
Institutional Review Board previously granted a waiver for written
informed consent by publishing information on the study on the Home
Page and providing the option to opt-out.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cumberbatch MG, Rota M, Catto JW and La
Vecchia C: The role of tobacco smoke in bladder and kidney
carcinogenesis: A comparison of exposures and meta-analysis of
incidence and mortality risks. Eur Urol. 70:458–466.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Humphrey PA, Moch H, Cubilla AL, Ulbright
TM and Reuter VE: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part B: Prostate and bladder
tumours. Eur Urol. 70:106–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Flaig TW, Spiess PE, Agarwal N, Bangs R,
Boorjian SA, Buyyounouski MK, Chang S, Downs TM, Efstathiou JA,
Friedlander T, et al: Bladder cancer, version 3.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
18:329–354. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Braissant O, Foufelle F, Scotto C, Dauça M
and Wahli W: Differential expression of peroxisome
proliferator-activated receptors (PPARs): tissue distribution of
PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology.
137:354–366. 1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sarraf P, Mueller E, Jones D, King FJ,
DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C
and Spiegelman BM: Differentiation and reversal of malignant
changes in colon cancer through PPARgamma. Nat Med. 4:1046–1052.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Theocharis S, Giaginis C, Parasi A,
Margeli A, Kakisis J, Agapitos E and Kouraklis G: Expression of
peroxisome proliferator-activated receptor-gamma in colon cancer:
Correlation with histopathological parameters, cell cycle-related
molecules, and patients' survival. Dig Dis Sci. 52:2305–2311.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tsukahara T, Haniu H and Matsuda Y:
PTB-associated splicing factor (PSF) is a PPARγ-binding protein and
growth regulator of colon cancer cells. PLoS One.
8(e58749)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lv S, Wang W, Wang H, Zhu Y and Lei C:
PPARγ activation serves as therapeutic strategy against bladder
cancer via inhibiting PI3K-Akt signaling pathway. BMC Cancer.
19(204)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dong F, Chen L, Wang R, Yang W, Lu T and
Zhang Y: 4-nitrophenol exposure in T24 human bladder cancer cells
promotes proliferation, motilities, and epithelial-to-mesenchymal
transition. Environ Mol Mutagen. 61:316–328. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Zhang GY, Ahmed N, Riley C, Oliva K,
Barker G, Quinn MA and Rice GE: Enhanced expression of peroxisome
proliferator-activated receptor gamma in epithelial ovarian
carcinoma. Br J Cancer. 92:113–119. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Armes JE, Trute L, White D, Southey MC,
Hammet F, Tesoriero A, Hutchins AM, Dite GS, McCredie MR, Giles GG,
et al: Distinct molecular pathogeneses of early-onset breast
cancers in BRCA1 and BRCA2 mutation carriers: A population-based
study. Cancer Res. 59:2011–2017. 1999.PubMed/NCBI
|
|
13
|
Koyama Y, Morikawa T, Miyakawa J, Miyama
Y, Nakagawa T, Homma Y and Fukayama M: Diagnostic utility of Ki-67
immunohistochemistry in small endoscopic biopsies of the ureter and
renal pelvis. Pathol Res Pract. 213:737–741. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Krabbe LM, Bagrodia A, Lotan Y, Gayed BA,
Darwish OM, Youssef RF, John G, Harrow B, Jacobs C, Gaitonde M, et
al: Prospective analysis of Ki-67 as an independent predictor of
oncologic outcomes in patients with high grade upper tract
urothelial carcinoma. J Urol. 191:28–34. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Robertson AG, Kim J, Al-Ahmadie H,
Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA,
Akbani R, et al: Comprehensive molecular characterization of
muscle-invasive bladder cancer. Cell. 171:540–556.e25.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cancer Genome Atlas Research Network.
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hoadley KA, Yau C, Hinoue T, Wolf DM,
Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, et
al: Cell-of-origin patterns dominate the molecular classification
of 10,000 tumors from 33 types of cancer. Cell. 173:291–304.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ellrott K, Bailey MH, Saksena G, Covington
KR, Kandoth C, Stewart C, Hess J, Ma S, Chiotti KE, McLellan M, et
al: Scalable open science approach for mutation calling of tumor
exomes using multiple genomic pipelines. Cell Syst. 6:271–281.e7.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Taylor AM, Shih J, Ha G, Gao GF, Zhang X,
Berger AC, Schumacher SE, Wang C, Hu H, Liu J, et al: Genomic and
functional approaches to understanding cancer aneuploidy. Cancer
Cell. 33:676–689.e3. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu J, Lichtenberg T, Hoadley KA, Poisson
LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee
AV, et al: An integrated TCGA Pan-cancer clinical data resource to
drive high-quality survival outcome analytics. Cell.
173:400–416.e11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sanchez-Vega F, Mina M, Armenia J, Chatila
WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia
S, et al: Oncogenic signaling pathways in the cancer genome atlas.
Cell. 173:321–337.e10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gao Q, Liang WW, Foltz SM, Mutharasu G,
Jayasinghe RG, Cao S, Liao WW, Reynolds SM, Wyczalkowski MA, Yao L,
et al: Driver fusions and their implications in the development and
treatment of human cancers. Cell Rep. 23:227–238.e3.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Poore GD, Kopylova E, Zhu Q, Carpenter C,
Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ,
et al: Microbiome analyses of blood and tissues suggest cancer
diagnostic approach. Nature. 579:567–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ding L, Bailey MH, Porta-Pardo E, Thorsson
V, Colaprico A, Bertrand D, Gibbs DL, Weerasinghe A, Huang KL,
Tokheim C, et al: Perspective on oncogenic processes at the end of
the beginning of cancer genomics. Cell. 173:305–320.e10.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol: Oct 3, 2017 (Epub ahead of print). doi:
10.1200/PO.17.00073.
|
|
27
|
Yun SH, Roh MS, Jeong JS and Park JI:
Peroxisome proliferator-activated receptor γ coactivator-1α is a
predictor of lymph node metastasis and poor prognosis in human
colorectal cancer. Ann Diagn Pathol. 33:11–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Michael MS, Badr MZ and Badawi AF:
Inhibition of cyclooxygenase-2 and activation of peroxisome
proliferator-activated receptor-gamma synergistically induces
apoptosis and inhibits growth of human breast cancer cells. Int J
Mol Med. 11:733–736. 2003.PubMed/NCBI
|
|
29
|
Tsubouchi Y, Sano H, Kawahito Y, Mukai S,
Yamada R, Kohno M, Inoue K, Hla T and Kondo M: Inhibition of human
lung cancer cell growth by the peroxisome proliferator-activated
receptor-gamma agonists through induction of apoptosis. Biochem
Biophys Res Commun. 270:400–405. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Grimm S, Bauer MK, Baeuerle PA and
Schulze-Osthoff K: Bcl-2 down-regulates the activity of
transcription factor NF-kappaB induced upon apoptosis. J Cell Biol.
134:13–23. 1996.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen GG, Lee JF, Wang SH, Chan UP, Ip PC
and Lau WY: Apoptosis induced by activation of
peroxisome-proliferator activated receptor-gamma is associated with
Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 70:2631–2646.
2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Catz SD and Johnson JL: Transcriptional
regulation of bcl-2 by nuclear factor kappa B and its significance
in prostate cancer. Oncogene. 20:7342–7351. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nakashiro KI, Hayashi Y, Kita A, Tamatani
T, Chlenski A, Usuda N, Hattori K, Reddy JK and Oyasu R: Role of
peroxisome proliferator-activated receptor gamma and its ligands in
non-neoplastic and neoplastic human urothelial cells. Am J Pathol.
159:591–597. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang G, Cao R, Wang Y, Qian G, Dan HC,
Jiang W, Ju L, Wu M, Xiao Y and Wang X: Simvastatin induces cell
cycle arrest and inhibits proliferation of bladder cancer cells via
PPARγ signalling pathway. Sci Rep. 6(35783)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Castillo-Martin M, Domingo-Domenech J,
Karni-Schmidt O, Matos T and Cordon-Cardo C: Molecular pathways of
urothelial development and bladder tumorigenesis. Urol Oncol.
28:401–408. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Spruck CH III, Ohneseit PF,
Gonzalez-Zulueta M, Esrig D, Miyao N, Tsai YC, Lerner SP, Schmütte
C, Yang AS, Cote R, et al: Two molecular pathways to transitional
cell carcinoma of the bladder. Cancer Res. 54:784–788.
1994.PubMed/NCBI
|
|
37
|
Mylona E, Giannopoulou I, Diamantopoulou
K, Bakarakos P, Nomikos A, Zervas A and Nakopoulou L: Peroxisome
proliferator-activated receptor gamma expression in urothelial
carcinomas of the bladder: Association with differentiation,
proliferation and clinical outcome. Eur J Surg Oncol. 35:197–201.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kim M, Ro JY, Amin MB, de
Peralta-Venturina M, Kwon GY, Park YW and Cho YM: Urothelial eddies
in papillary urothelial neoplasms: A distinct morphologic pattern
with low risk for progression. Int J Clin Exp Pathol. 6:1458–1466.
2013.PubMed/NCBI
|
|
39
|
Shim JW, Cho KS, Choi YD, Park YW, Lee DW,
Han WS, Shim SI, Kim HJ and Cho NH: Diagnostic algorithm for
papillary urothelial tumors in the urinary bladder. Virchows Arch.
452:353–362. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Goussia AC, Papoudou-Bai A, Charchanti A,
Kitsoulis P, Kanavaros P, Kalef-Ezra J, Stefanou D and Agnantis NJ:
Alterations of p53 and Rb pathways are associated with high
proliferation in bladder urothelial carcinomas. Anticancer Res.
38:3985–3988. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Quintero A, Alvarez-Kindelan J, Luque RJ,
Gonzalez-Campora R, Requena MJ, Montironi R and Lopez-Beltran A:
Ki-67 MIB1 labelling index and the prognosis of primary TaT1
urothelial cell carcinoma of the bladder. J Clin Pathol. 59:83–88.
2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ogata DC, Marcondes CA, Tuon FF, Busato WF
Jr, Cavalli G and Czeczko LE: Superficial papillary urothelial
neoplasms of the bladder (PTA E PT1): Correlation of expression of
P53, KI-67 and CK20 with histologic grade, recurrence and tumor
progression. Rev Col Bras Cir. 39:394–400. 2012.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
43
|
Sato M, Yanai H, Morito T, Oda W, Shin-no
Y, Yamadori I, Tshushima T and Yoshino T: Association between the
expression pattern of p16, pRb and p53 and the response to
intravesical bacillus Calmette-Guerin therapy in patients with
urothelial carcinoma in situ of the urinary bladder. Pathol Int.
61:456–460. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Meuleman EJ and Delaere KP: Diagnostic
efficacy of the combination of urine cytology, urine analysis and
history in the follow-up of bladder carcinoma. Br J Urol.
62:150–153. 1988.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wiener HG, Mian C, Haitel A, Pycha A,
Schatzl G and Marberger M: Can urine bound diagnostic tests replace
cystoscopy in the management of bladder cancer? J Urol.
159:1876–1880. 1998.PubMed/NCBI
|
|
46
|
Yamashiro K, Taira K, Nakajima M, Azuma M,
Koseki M, Abe T, Suzuki H, Minami K, Harabayashi T and Nagamori S:
Voided urine cytology and low-grade urothelial neoplasia of the
bladder: Factors that influence the sensitivity. J Am Soc
Cytopathol. 5:227–234. 2016.PubMed/NCBI View Article : Google Scholar
|