Introduction
Prostate cancer is the second most commonly
diagnosed cancer in men, accounting for 15% of all types of cancer,
with an estimated 1.1 million individuals worldwide diagnosed with
prostate cancer in 2012(1). Serum
prostate-specific antigen (PSA) level is a useful tumor marker in
the diagnosis and follow-up of prostate cancer (2) and PSA is widely used in primary
screening measures such as mass screening (3). A prospective observational study in
Tyrol, Austria, notes that PSA exposure was 86.6% during the
20-year study period and mortality was 64% lower than expected
(4). Various randomized controlled
trials (RCTs) have verified the efficacy of prostate cancer
screening based on serum PSA screening. Results from the European
Randomized Study of Screening for Prostate Cancer (ERSPC) showed
that in the 55-69 years age group with a median observation period
of 13 years, the screening group had a 21% lower mortality rate
than the control group (5). An RCT
in Gothenburg, Sweden, demonstrated not only significantly reduced
mortality from PSA screening, but also lower incidence of advanced
cancer (6). In addition, the
Rotterdam section of the ERSPC showed that screening every 4 years
significantly reduced the risk of developing metastatic cancer
(7).
Some discrepancy has been seen in the outcomes of
screening, however. In the Prostate, Lung, Colorectal and Ovarian
(PLCO) Cancer Screening Study conducted in the United States, the
metastatic cancer rate was low in the control group and the
prostate cancer mortality rate was not significantly different from
the screening group, although this was because of high
contamination of PSA screening in the control group (8-10).
The Cluster Randomized Trial of PSA Testing for Prostate Cancer
(CAP) in the United Kingdom involving a single invitation to
PSA-based screening showed no significant difference in prostate
cancer mortality between the invited and control groups after a
median follow-up of 10 years (11). These discrepant outcomes create
some confusion around PSA screening, but it is now considered to be
beneficial in improving cancer-specific survival based on the
findings of a meta-analysis reported in 2018 (12,13).
This accumulation of evidence from large RCTs has led to a number
of prostate cancer guidelines recommending PSA-based screening for
prostate cancer.
The aforementioned large RCTs include men aged
between 55 and 69 or 74 years, with little data available on the
effectiveness of PSA-based screening for men >70 years of age
(7). Moreover, none of these RCTs
demonstrate the efficacy of PSA screening for older men, >75
years of age, so routine PSA-based screening for all elderly men
has not been recommended. According to the European Association of
Urology (EAU) guidelines on prostate cancer, men who have life
expectancy within 15 years are unlikely to benefit (14). The Prostate Cancer Early Detection
Panel of the US National Comprehensive Cancer Network recommends
that men >75 years of age be considered for screening only if in
very good health (15).
Conversely, the US Preventive Service Task Force recommends that
PSA-based screening not be performed in men over 70 years of age
(16).
Life expectancy and characteristics of prostate
cancer vary by region and race (1), so it is necessary to verify the
validity of screening for older adults among Asian populations.
Although some studies from China or Korean investigated huge
database about PSA screening, their analyses were undergone with
single aim, in which comparisons between screened and non-screened
men were not revealed (17,18).
In Japan, some reports show the effectiveness of PSA-based
screening (19-21).
However, no studies refer to the upper age limit for PSA screening.
In particular, no study demonstrates the usefulness of PSA
screening alone at >75 years.
In the city of Yokosuka, Japan, mass screening for
prostate cancer based on PSA has been conducted since 2001. Tabei
et al (22) reported on the
overall results in 2020. The study database contains 3,094 patients
diagnosed by needle biopsy from 2001 to 2015 in four hospitals
(Yokosuka Kyosai Hospital, Yokosuka City Uwamachi Hospital,
Kinugasa Hospital and Yokosuka City Hospital) and two urology
clinics (Satomi Jin-Hinyokika Clinic and Furuhata Hinyokika Clinic)
in the city. Using this database, the present study sought to
verify the significance of population screening for elderly men
>75 years of age.
Patients and methods
The institutional review boards of all four
participating hospitals approved the present study and agreed to
provide patient data for the study database. Patients' consent was
sought by giving them the choice to opt out of the study through
the websites and notice boards of the participating institutions.
Patient data was obtained from all institutions.
Patients collection
The present study investigated retrospectively 1,117
patients aged >75 years of age with pathologically diagnosed
prostate cancer by needle biopsy at four hospitals and two clinics
in Yokosuka city between April 2001 and March 2015. Patients
diagnosed accidentally by transurethral resection of the prostate
or total cystectomy for bladder cancer were excluded from this
study. Patients were followed until prostate cancer-specific
mortality, mortality from other causes, or final follow-up on 31
December 2019. Patients without metastasis were classified into
four disease risk categories according to the EAU guidelines: Low
(T1-T2a and Gleason Score (GS)≤6 and PSA<10 ng/ml, not N1/M1);
intermediate (T2b or GS=7 or 10≤PSA ≤20 ng/ml, not N1/M1); high
(T2c or GS =8-10 or 20<PSA, not N1/M1); and locally advanced
(T3-4 or N1, not M1). Patients with metastasis were classified into
the advanced (M1) group.
Definition of ‘screened’ or
‘non-screened’ patients
Patients were divided into two groups according to
the mode of detection. The screened group included those diagnosed
either by PSA-based population screening in the city, other
municipalities, or by workplace screening and regular PSA follow-up
patients at urology clinics or internal medicine clinics (e.g.,
benign prostatic hypertrophy, lower urinary tract symptoms or
positive on previous screening). The population screening and
workplace screening measures used a PSA cut-off of 4.0 ng/ml in
serum. The non-screened group consisted of those who had been
diagnosed pathologically due to high PSA value in serum examined
for other reasons than the above, including pathological fracture,
cancers of unknown primary or gross hematuria and lower urinary
tract symptoms.
The final decision whether biopsy would be performed
is based on consultation with patients exhibiting PSA >4.0 ng/ml
about its potential benefits and harms.
Statistical analysis
Age at diagnosis, initial PSA status, tumor stage,
risk category, GS, primary treatment, secondary treatment and
Charlson Comorbidity score (CS) (23) were compared between the two groups
using a two-sided Student's t-test and χ2 test. Clinical
and pathological factors associated with clinical outcomes were
assessed using univariate and multivariate analyses with Cox
regression analyses to calculate hazard ratios and 95% confidence
intervals. Overall mortality was defined as any cause of mortality
and cancer specific mortality was defined as mortality from
prostate cancer. Cancer-specific and overall survival (OS) rates
were calculated using Kaplan-Meier analysis with a log-rank test to
compare survival curves between the two groups. Overall mortality
was counted as an event in Kaplan-Meier curve for OS and Cancer
specific mortality was counted as an event and mortalities from
other causes were censored in the curve for CSS. All analyses were
carried out using IBM SPSS Statistics for Windows, version 19.0.
(IBM Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients' background
The backgrounds of patients >75 years of age are
shown in Table I. A total of 537
were classified in the screened group. Age, initial PSA, GS and CS
were not significantly different between the two groups. The
screened group showed less advanced cancer with significantly lower
N and M stage and risk category (P<0.001), and more T1 and T2
stage compared with the non-screened group (P<0.001).
Significantly more patients received androgen deprivation therapy
in the non-screened group compared with the screened group
(P=0.003).
 | Table IBackground of prostate cancer patients
>75 years of age who did or did not undergo PSA-based
screening. |
Table I
Background of prostate cancer patients
>75 years of age who did or did not undergo PSA-based
screening.
| | Screened group
(n=537) | Non-screened group
(n=580) | |
|---|
| | Mean/number | Range/(%) | Mean/number | Range/(%) | P-value |
|---|
| Age (years) | 78 | 75-97 | 79 | 75-93 | 0.581 |
| Initial PSA | 10.7 | 3.6-2759 | 16.1 | 1.0-13470 | 0.207 |
| T stage | | | | | <0.001 |
|
T1 | 207 | (38.5) | 173 | (29.8) | |
|
T2 | 173 | (32.2) | 176 | (30.3) | |
|
T3 | 139 | (25.9) | 155 | (26.7) | |
|
T4 | 9 | (1.8) | 4 | (0.7) | |
| N1 | 25 | (4.7) | 76 | (13.1) | <0.001 |
| M1 | 44 | (8.2) | 114 | (19.7) | <0.001 |
| Risk category | | | | | <0.001 |
|
Low | 75 | (14.0) | 56 | (9.7) | |
|
Intermediate | 143 | (26.6) | 117 | (20.2) | |
|
High | 145 | (27.0) | 130 | (22.4) | |
|
Locally
advanced | 120 | (22.3) | 129 | (22.2) | |
|
Advanced | 44 | (8.2) | 114 | (19.7) | |
| Gleason score | | | | | 0.209 |
|
≤ 6 | 151 | (28.1) | 129 | (22.2) | |
|
7 | 183 | (34.1) | 193 | (33.3) | |
|
≥8 | 186 | (34.6) | 208 | (35.9) | |
| Charlson
Comorbidity score (≥3) | 267 | (49.7) | 278 | (47.9) | 0.550 |
| Primary
treatment | | | | | 0.003 |
|
Watchful
waiting/active surveillance | 41 | (7.6) | 24 | (4.1) | |
|
Radiation | 27 | (5.0) | 15 | (2.6) | |
|
Operation | 48 | (8.9) | 33 | (5.7) | |
|
Androgen
deprivation therapy | 415 | (77.3) | 489 | (84.3) | |
|
Other | 6 | (1.1) | 19 | (3.2) | |
| Cause of
mortality | n=133 | | n=170 | | 0.643 |
|
Prostate
cancer | 29 | (21.8) | 49 | (28.8) | |
|
Other
malignancies | 28 | (21.1) | 33 | (19.4) | |
|
Pneumonia | 14 | (10.5) | 13 | (7.6) | |
|
Stroke | 5 | (4.4) | 8 | (4.7) | |
|
Heart
failure | 4 | (3.8) | 12 | (7.1) | |
|
Chronic
respiratory failure | 3 | (2.3) | 2 | (1.2) | |
|
Aortic
dissection | 2 | (1.5) | 2 | (1.2) | |
|
Myocardial
infarction | 2 | (1.5) | 1 | (0.6) | |
|
Chronic
renal failure | 2 | (1.5) | 1 | (0.6) | |
|
Other | 9 | (6.8) | 5 | (2.9) | |
|
Unknown | 35 | (26.3) | 46 | (27.1) | |
Overall and cancer-specific
survival
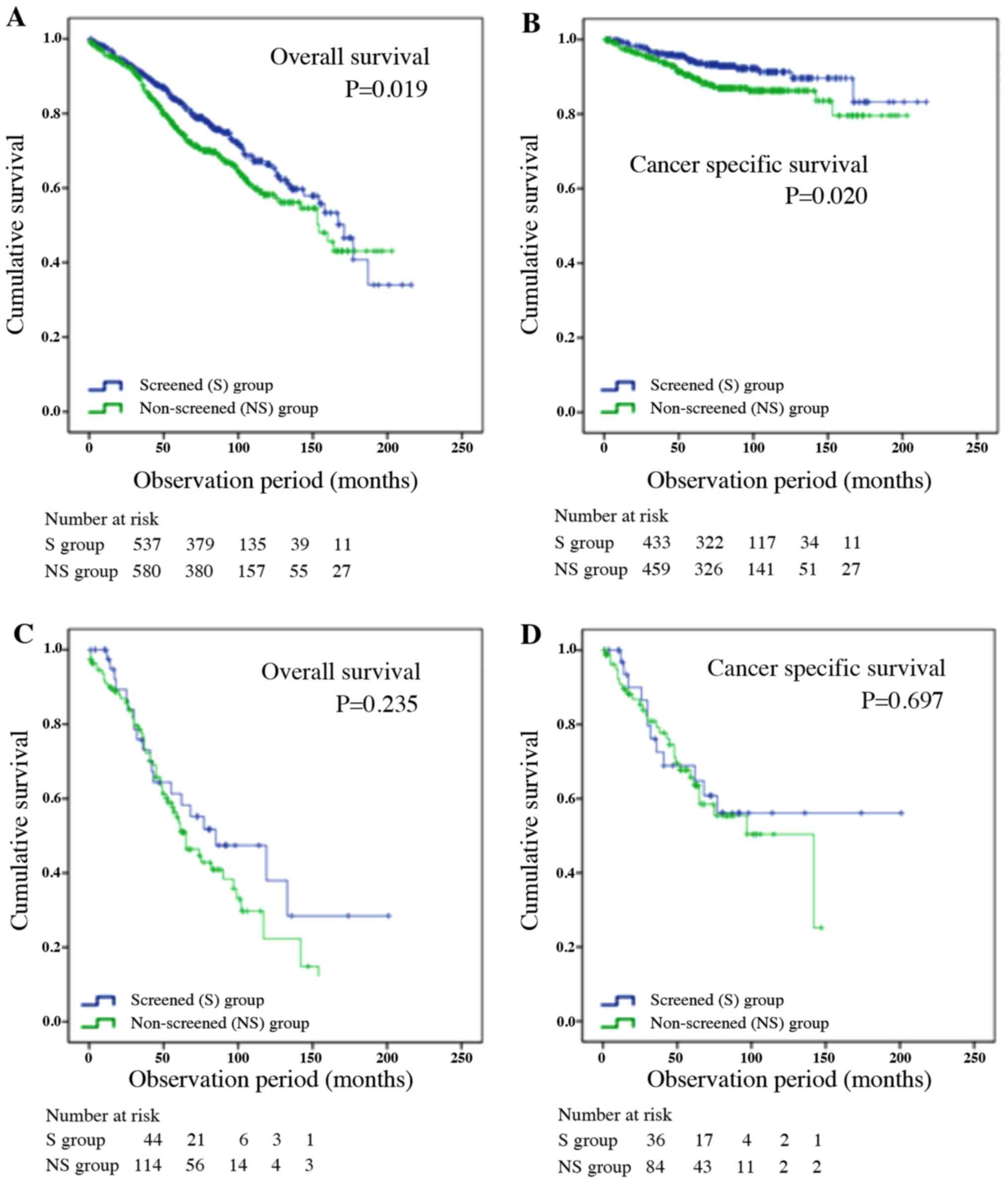
OS and CSS in the patients >75 years of age were
analyzed by the Kaplan-Meier method (Fig. 1A and B). OS and CSS were significantly longer
in the screened group (171 vs. 154 months; P=0.019; P=0.020). OS or
CSS among patients with metastasis between the screened and
non-screened groups were shown in Fig.
1C and D. There were no
significant differences between the two groups (P=0.235,
P=0.697).
Cause of mortality
The rate of cancer-specific mortality compared with
overall mortality for each risk category was investigated and there
were no mortalities in the low risk category in either group. In
even locally-advanced risk category, at least two thirds of
patients succumbed to another disease. Half of patients in the
advanced disease category in the non-screened group succumbed.
There were 133 mortalities from other causes in the screening
group, of which two succumbed to myocardial infarction (Table I). The most common cause of
mortality was prostate cancer, followed by other malignancies in 28
patients and pneumonia in 14 patients. There were 170 mortalities
from other causes in the non-examination group, of which one
succumbed to myocardial infarction. The most common cause of
mortality was prostate cancer in 49 patients, followed by other
malignancies in 33 patients and pneumonia in 13 patients. A
Chi-square test of the cause of mortality in patients receiving
androgen deprivation therapy showed no significant difference
(P=0.534) between the screened group and the non-screened group
(Table SI) and there was no
significant difference (P=0.127) between those who succumbed to
prostate cancer and non-prostate cancer. The increase in
cardiovascular events associated with androgen deprivation therapy
was unclear.
Multivariate analysis
The factors contributing to shortening OS and CSS
(Table II) were revealed by
multivariate analysis. For OS, these were age [odds ratio: 1.048
(1.018-1.907), P=0.002], PSA≥20 ng/ml [odds ratio: 1.551
(1.134-2.015), P=0.005), ≥T3 (odds ratio: 1.436 [1.081-1.907],
P=0.012)], M1 [odds ratio: 1.676 (1.205-2.331), P=0.002], GS≥8
[odds ratio: 1.364 (1.052-1.769), P=0.019] and CS≥3 [odds ratio:
1.884 (1.422-2.390), P<0.001]. For CSS, these were ≥T3 [odds
ratio: 3.301 (1.704-6.396), P<0.001], M1 [odds ratio: 4.856
(2.809-8.393), P<0.001] and GS≥8 [odds ratio: 4.691
(2.479-8.876), P<0.001].
 | Table IIResults of multivariate analysis of
overall survival in prostate cancer patients >75 years of
age. |
Table II
Results of multivariate analysis of
overall survival in prostate cancer patients >75 years of
age.
| | Full model | Reduced model |
|---|
| | P-value | Odds | 95% CI | P-value | Odds | 95% CI |
|---|
| Overall
survival | | | | | | |
|
Age | 0.003 | 1.046 | 1.016-1.078 | 0.002 | 1.048 | 1.018-1.907 |
|
PSA ≥20
ng/ml | 0.006 | 1.507 | 1.128-2.013 | 0.005 | 1.511 | 1.134-2.015 |
|
Non-screened
group | 0.401 | 1.113 | 0.867-1.429 | | | |
|
≥T3 | 0.010 | 1.454 | 1.093-1.934 | 0.012 | 1.436 | 1.081-1.907 |
|
N1 | 0.456 | 0.857 | 0.571-1.287 | | | |
|
M1 | 0.003 | 1.720 | 1.208-2.449 | 0.002 | 1.676 | 1.205-2.331 |
|
Gleason
score ≥8 | 0.014 | 1.388 | 1.068-1.803 | 0.019 | 1.364 | 1.052-1.769 |
|
Charlson
Comorbidity score ≥3 | <0.001 | 1.857 | 1.432-2.408 | <0.001 | 1.844 | 1.422-2.390 |
| Cancer-specific
survival | | | | | | |
|
Age | 0.345 | 1.031 | 0.967-1.100 | | | |
|
PSA ≥20
ng/ml | 0.233 | 1.531 | 0.760-3.083 | | | |
|
Non-screened
group | 0.523 | 1.185 | 0.704-1.993 | | | |
|
≥T3 | 0.001 | 3.065 | 1.553-6.049 | <0.001 | 3.301 | 1.704-6.396 |
|
N1 | 0.258 | 0.692 | 0.365-1.311 | | | |
|
M1 | <0.001 | 4.745 | 2.530-8.898 | <0.001 | 4.856 | 2.809-8.393 |
|
Gleason
score ≥8 | <0.001 | 4.614 | 2.404-8.858 | <0.001 | 4.691 | 2.479-8.876 |
|
Charlson
Comorbidity score ≥3 | 0.690 | 0.902 | 0.544-1.496 | | | |
Discussion
In elderly patients >75 years of age, median OS
was significantly longer in the screened group compared with the
non-screened group (171 vs. 154 months; P=0.019; Fig. 1A and B). Median CSS was not reached in either
group, but CSS was significantly longer in the screened group
(P=0.020). However, screening was not an independent factor in both
OS and CSS in multivariate analyses (Table II).
In the elderly, clinically insignificant types of
cancer (e.g., low GS, low T stage and no metastasis), where there
are no clinical signs of prostate cancer, is prevalent (24) with a 1.71 increase in odds ratio
for every 10 years of age (25).
Therefore, it was hypothesized that a number of clinically
insignificant cancers were potentially present in the elderly and
it was possible that few fatal cancers would contribute to
prognosis. PSA screening for elderly men might have more risk of
over-diagnosis than that for middle-aged men. The present study
found that CS was a significant factor associated with shorter OS
(Table II). Moreover, prostate
cancer mortalities did not account for a large proportion of all
mortalities and even in patients with advanced disease ~50%
succumbed other causes. These were consistent with the results of
our previous study (22).
The presence of metastasis was the most significant
factor associated with shorter CSS in the elderly [odds ratio:
4.856 (2.809-8.393), P<0.001; Table II]. Some guidelines for prostate
cancer management are hesitant to recommend PSA screening in
elderly men. The authors of the present study expected favorable
outcome for screening even for elderly in a previous study
(22).
By contrast, the present study found no significant
difference in OS or CSS among patients with metastasis between the
screened and non-screened groups (Fig.
1C and D). The latest findings
for men >75 years of age therefore do not support our previous
study (22). It is suspected that
this discrepancy is due to the following reasons. In Japan, PSA
screening is carried out under the initiative of local governments
and not under a national policy, although there are regional
differences and consultation is also voluntary for individuals.
Median overall age in the Yokosuka City database was high (71 years
in the screened group and 73 years in the non-screened group) and
prostate cancer with metastasis was more frequent in elderly
patients in both the screened (8.2 vs. 5.2%) and non-screened (19.7
vs. 15.0%) groups than in all patients (22). Thus, it is hypothesized that PSA
screening in middle-aged men is likely to associated with poor
exposure and that cancer was not detected early, so there were a
number of cases of advanced cancer in the elderly.
The study database was created in a medical area
that covers almost the entire city of Yokosuka and nearby Miura.
Using municipal demographics and prostate cancer incidence rates
from national databases, the estimated number of prostate cancer
patients for the preceding 15 years is ~1,268 for those >75
years of age. The study database contains a number of records for
prostate cancer patients, of whom about 88% are >75 years of
age. Considering that the average life expectancy of men in Japan
was 81 years in 2017, it is estimated that the database covers most
elderly patients with prostate cancer in the area.
The present study had some limitations. First,
patient information was obtained from the medical records only. The
design was retrospective, so not all patients were followed up
fully and some were lost during follow-up. Second, this study
included only pathologically diagnosed patients. In real-world
settings, some patients with clinically advanced prostate cancer
are diagnosed without prostate biopsy and imaging findings.
Consequently, the results probably overestimated CSS, especially in
the non-screened group. In the screened group, health awareness
might have been high and this could pose some bias. Several new
treatments for prostate cancer (e.g., abiraterone acetate,
enzalutamide and radium-223 chloride) became available during the
observation period and there might have been differences in
treatment effect depending on the time of observation. Thus, the
therapeutic effect could have been underestimated, compared with
standard treatment widely used today. Third, almost all patients
were Asian. Individual patient backgrounds were searched as much as
possible to avoid sample contamination, but it is possible that
patients previously exposed to PSA testing are classified in the
non-screened group. Moreover, in this database, >90% of cases
had undergone some initial treatment since diagnosis and therefore
the results of the present study do not represent the natural
history of prostate cancer. As s a retrospective study, the
validity of PSA screening in the elderly could not be verified.
Furthermore, assessments for cost effectivity or quality of line
are lacking. From these points of view, PSA screening might have
some benefits for elderly individuals. Yet, the data from the
present study could not reveal the survival benefit of PSA
screening for the patients >75 years old.
Supplementary Material
Cause of mortality in the patients who
received androgen deprivation therapy.
Acknowledgements
The authors thank Ms. Ayumi Yokokawa (Assistant,
Department of Urology, Yokosuka Kyosai Hospital) for exceptional
contributions to the current study.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TN was involved in project development and performed
data collection or management, data analysis and writing of the
manuscript. TT was involved in project development, data
collection, data analysis and editing the manuscript. HI was
involved in data collection, data analysis and editing the
manuscript. NS, HK, MY, AF, ST, SF and SN performed data
collection. MT performed data analysis. KK edited the manuscript
and supervised the present study.
Ethics approval and consent to
participate
The institutional review boards of all four
participating hospitals approved this study and agreed to provide
patient data for the study database (approval no. 19-70). Patients'
consent was sought by giving them the choice to opt out of the
study through the websites and notice boards of the participating
institutions.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stamey TA, Yang N, Hay AR, McNeal JE,
Freiha FS and Redwine E: Prostate-specific antigen as a serum
marker for adenocarcinoma of the prostate. N Engl J Med.
317:909–916. 1987.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mistry K and Cable G: Meta-analysis of
prostate-specific antigen and digital rectal examination as
screening tests for prostate carcinoma. J Am Board Fam Pract.
16:95–101. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Oberaigner W, Siebert U, Horninger W,
Klocker H, Bektic J, Schäfer G, Frauscher F, Schennach H and
Bartsch G: Prostate-specific antigen testing in Tyrol, Austria:
Prostate cancer mortality reduction was supported by an update with
mortality data up to 2008. Int J Public Health. 57:57–62.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schröder FH, Hugosson J, Roobol MJ,
Tammela TL, Zappa M, Nelen V, Kwiatkowski M, Lujan M, Määttänen L,
Lilja H, et al: ERSPC Investigators. Screening and prostate cancer
mortality: Results of the European Randomised Study of Screening
for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet.
384:2027–2035. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aus G, Bergdahl S, Lodding P, Lilja H and
Hugosson J: Prostate cancer screening decreases the absolute risk
of being diagnosed with advanced prostate cancer - results from a
prospective, population-based randomized controlled trial. Eur
Urol. 51:659–664. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kerkhof M, Roobol MJ, Cuzick J, Sasieni P,
Roemeling S, Schröder FH and Steyerberg EW: Effect of the
correction for noncompliance and contamination on the estimated
reduction of metastatic prostate cancer within a randomized
screening trial (ERSPC section Rotterdam). Int J Cancer.
127:2639–2644. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pinsky PF, Prorok PC, Yu K, Kramer BS,
Black A, Gohagan JK, Crawford ED, Grubb RL and Andriole GL:
Extended mortality results for prostate cancer screening in the
PLCO trial with median follow-up of 15 years. Cancer. 123:592–599.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pinsky PF, Blacka A, Kramer BS, Miller A,
Prorok PC and Berg C: Assessing contamination and compliance in the
prostate component of the Prostate, Lung, Colorectal, and Ovarian
(PLCO) Cancer Screening Trial. Clin Trials. 7:303–311.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shoag JE, Mittal S and Hu JC: Reevaluating
PSA testing rates in the PLCO trial. N Engl J Med. 374:1795–1796.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Martin RM, Donovan JL, Turner EL, Metcalfe
C, Young GJ, Walsh EI, Lane JA, Noble S, Oliver SE, Evans S, et al:
CAP Trial Group: Effect of a low-intensity PSA-based screening
intervention on prostate cancer mortality: The CAP randomized
clinical trial. JAMA. 319:883–895. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fenton JJ, Weyrich MS, Durbin S, Liu Y,
Bang H and Melnikow J: Prostate-specific antigen-based screening
for prostate cancer: Evidence report and systematic review for the
US Preventive Services Task Force. JAMA. 319:1914–1931.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ilic D, Djulbegovic M, Jung JH, Hwang EC,
Zhou Q, Cleves A, Agoritsas T and Dahm P: Prostate cancer screening
with prostate-specific antigen (PSA) test: A systematic review and
meta-analysis. BMJ. 362(k3519)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mottet N, Bellmunt J, Bolla M, Briers E,
Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau
S, et al: EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1:
Screening, diagnosis, and local treatment with curative intent. Eur
Urol. 71:618–629. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Carroll PR, Parsons JK, Andriole G,
Bahnson RR, Barocas DA, Castle EP, Catalona WJ, Dahl DM, Davis JW,
Epstein JI, et al: NCCN clinical practice guidelines prostate
cancer early detection, version 2.2015. J Natl Compr Canc Netw.
13:1534–1561. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Grossman DC, Curry SJ, Owens DK,
Bibbins-Domingo K, Caughey AB, Davidson KW, Doubeni CA, Ebell M,
Epling JW Jr, Kemper AR, et al: US Preventive Services Task Force:
Screening For Prostate Cancer: US preventive services task force
recommendation statement. JAMA. 319:1901–1913. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mok Y, Kimm H, Shin SY, Jee SH and Platz
EA: Screening prostate-specific antigen concentration and prostate
cancer mortality: The Korean Heart Study. Urology. 85:1111–1116.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Y, Xiao G, Zhou JW, Yang JK, Lu L,
Bian J, Zhong L, Wei QZ, Zhou QZ, Xue KY, et al: Optimal starting
age and baseline level for repeat tests: Economic concerns of psa
screening for chinese men - 10-year experience of a single center.
Urol Int. 104:230–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sakai N, Taguri M, Kobayashi K, Noguchi S,
Ikeda S, Koh H, Satomi Y and Furuhata A: Clinical outcomes of
prostate cancer patients in Yokosuka City, Japan: A comparative
study between cases detected by prostate-specific antigen-based
screening in Yokosuka and those detected by other means. Int J
Urol. 22:747–752. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Okihara K, Kitamura K, Okada K, Mikami K,
Ukimura O and Miki T: Ten year trend in prostate cancer screening
with high prostate-specific antigen exposure rate in Japan. Int J
Urol. 15:156–161. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ito K, Kakehi Y, Naito S and Okuyama A:
Japanese Urological Association. Japanese Urological Association
guidelines on prostate-specific antigen-based screening for
prostate cancer and the ongoing cluster cohort study in Japan. Int
J Urol. 15:763–768. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tabei T, Taguri M, Sakai N, Koh H, Yosida
M, Fujikawa A, Nirei T, Tsutsumi S, Ito H, Furuhata S, et al: Does
screening for prostate cancer improve cancer-specific mortality in
Asian men? Real-world data in Yokosuka City 15 years after
introducing PSA-based population screening. Prostate. 80:824–830.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rebbeck TR and Haas GP: Temporal trends
and racial disparities in global prostate cancer prevalence. Can J
Urol. 21:7496–7506. 2014.PubMed/NCBI
|
|
25
|
Bell KJ, Del Mar C, Wright G, Dickinson J
and Glasziou P: Prevalence of incidental prostate cancer: A
systematic review of autopsy studies. Int J Cancer. 137:1749–1757.
2015.PubMed/NCBI View Article : Google Scholar
|