According to global cancer statistics, colorectal
cancer (CRC) is the third most common type of cancer diagnosed
worldwide, accounting for just over 10% of all diagnosed cancers.
This is surpassed only by cancers of the lung and breast (the
latter in women). CRC is the second leading cause of cancer-related
mortality, accounting for just over 9% of cancer-related deaths
(1). Approximately 20% of patients
with CRC demonstrate metastases at initial diagnosis (metastatic
CRC; mCRC), whether detected on imaging or confirmed during biopsy,
with up to 80% of such patients deemed unresectable at initial
diagnosis (2,3).
Systemic therapy is the mainstay of treatment for
patients with inoperable CRC. Localized therapy in the palliative
setting (namely surgical resection and irradiation of the primary
tumour) is commonly limited to patients suffering from primary
tumour complications. These can include bowel obstruction,
perforation, localized pain and bleeding from the tumour (4,5).
Chemotherapeutic agents typically employed in mCRC
consist of the antimetabolite 5-fluorouracil (5FU) (6,7), the
pyrimidine analogue capecitabine (8), the topoisomerase inhibitor irinotecan
(9) and the alkylating agent
oxaliplatin (10). EGFR-targeting
monoclonal antibodies include cetuximab (11) and panitumumab (12) for confirmed KRAS/NRAS wild-type
tumours and VEGF-targeting bevacizumab (13) and ramucirumab (14), the latter typically in combination
with chemotherapy using the FOLFIRI regimen (15).
This may vary in patients for whom either
oxaliplatin or irinotecan are contraindicated as first-line therapy
due to their varying toxicity profiles. In patients deemed
unsuitable for additional oxaliplatin and irinotecan with 5FU or
capecitabine (such as patients of advanced age, with co-morbidities
or poor performance status), single-agent 5FU or capecitabine may
be preferred. Such single-agent 5FU regimens include the Roswell
Park (30,31) and QUASAR (32,33)
regimens and capecitabine (34,35)
in either the adjuvant (36) or
palliative (37) setting.
Combined nucleic acid analogue/thymidine
phosphorylase inhibitor TAS-102 (tipiracil hydrochloride) (38) and the multikinase inhibitor
regorafenib (39) are typically
reserved for patients in whom 5FU-based chemotherapy in combination
with oxaliplatin or irinotecan has failed (or if the patient is
deemed clinically unsuitable to receive these treatments).
Depending on the patient, irinotecan- or oxaliplatin-based
chemotherapy may be rechallenged in the palliative setting if a
significant interval has elapsed since completing a previous course
of treatment, provided cumulative toxicity allows for this approach
(40,41).
The targeted tyrosine kinase inhibitors encorafenib
and binimetinib may be considered in BRAF V600E mutant tumours in
combination with other systemic therapies (42). Immune checkpoint inhibitors are
reserved for microsatellite instability-high (MSI-H) or deficient
mismatch repair (dMMR) tumours (43); these include the monoclonal
antibody inhibitors nivolumab (44,45)
and pembrolizumab (46), which
inhibit programmed death receptor 1, and ipilimumab (47), which inhibits cytotoxic
T-lymphocyte-associated protein 4.
Tumour heterogeneity exists in mCRC, whether within
the primary tumour (intra-tumoural heterogeneity) or between the
primary and metastatic tumours (inter-tumoural heterogeneity)
(48-50).
Intra- or inter-tumoural heterogeneity has been implicated in the
mechanisms underlying resistance to systemic therapy (51). Tumour heterogeneity in mCRC can
vary during the course of the disease (52).
Inter-tumoural heterogeneity accounts for
identifiable discordances in scientifically validated tests of
advanced CRC. These discordances may include differentiation of
adenocarcinoma (53,54), in which cancer stem cells play a
role (55), mutation status
(56-59),
including KRAS/NRAS (60-62)
and BRAF (63,64) status, MSI status (65) and dMMR status (66,67).
This may lead to discordances in biomarker profiles between the
primary tumour and metastases. Inter-tumoural heterogeneity appears
to be more prevalent in mCRC with certain pathological and
molecular features, such as confirmed MSI (68,69).
Discordant responses between the primary tumour and
metastatic sites in mCRC may arise from this underlying
heterogeneity between tumour sites. Tumour cells at the metastatic
sites may harbour clones that have gained (or lost) mutations
advantageous to their survival compared to those residing at the
primary site, or vice versa (81,82).
These molecular discrepancies in mCRC are not yet fully understood;
clonal evolution, cancer stem cells and ‘The Big Bang’ model have
all been hypothesized to play a role (83-85).
The incidence of these biomarker discordances in
mCRC varies depending on the resources consulted. Part of the
literature, including meta-analysis studies, suggests that the rate
of biomarker concordance is high between primary tumours and
metastases in mCRC (86,87). These studies further suggest that
tissues from either the primary tumour or metastatic site are
sufficient for confirming the biomarker status of mCRC to help
guide the systemic therapy approach (88). However, more recent studies suggest
that the rate of these discordances increases if next-generation
sequencing is used (89,90). There is high concordance (>90%)
between immunohistochemical analysis and molecular testing of dMMR
(91).
The rates of molecular discordances between the
primary tumour and metastatic sites in mCRC may be as high as
10-15%, depending on the study (86,92).
The rate of discrepancies in MSI or MMR status tends to be low
(<5%) upon comparison (93,94).
The expression of other specific biomarkers, such as programmed
death ligand 1 (PD-L1), may vary more markedly between primary
tumour and metastatic sites in up to one-third of patients
(95).
Evaluating molecular characteristics between primary
and metastatic tumours separately in a single patient with mCRC can
demonstrate variable biological behaviour and response to systemic
therapy due to these identified subclones (96). Non-genetic factors, such as
post-translational modification, epigenetics and the tumour
microenvironment, also contribute to this phenomenon. Comparing
such predictive or prognostic molecular signatures between the
primary and metastatic tumours in mCRC has yielded different
results. For example, mutant KRAS status exhibits concordance
between the primary tumour and distant organ metastases in up to
90% of patients with mCRC (97).
Conversely, comparisons between the primary tumour and lymph node
metastases demonstrate lower concordance rates with a KRAS mutant
status of ~37% (98).
The role of pre-emptive localized therapies in
patients with relatively asymptomatic primary tumours remains
controversial and has demonstrated an inconsistent clinical benefit
(99-101).
Radiation to rectal and rectosigmoid cancer primary tumours has
been associated with a reduced risk of death in one retrospective
study (102). Previous findings
have demonstrated no additional benefit, reduced risk of
complications or death when radiation treatment is administered
before systemic therapy (103),
while other findings suggest that prior resection of the colorectal
primary in mCRC offers benefit in selected patients (104). However, this should not be
routinely considered in asymptomatic patients, as it offers no
additional benefit (105), taking
into account the currently available systemic therapies (106).
Ethics approval was obtained from the local hospital
Medical Research Ethics Committee prior to data collection (REC
Ref: 113/2020). Patient data were anonymized during data collection
and analysis. Informed consent (written or oral) was not required,
since this was a retrospective chart review (as outlined per Health
Research Consent Declaration Committee Guidelines, Ireland). All
data collection procedures followed the General Data Protection
Regulation and the Data Protection Act, 2018.
The analysis included patients with a radiologically
and pathologically confirmed diagnosis of metastatic de novo
mCRC, with the primary tumour in situ treated with up-front
palliative systemic therapy. Non-curative status was confirmed
through multidisciplinary meeting discussion (in applicable cases
requiring discussion with surgical specialists based on imaging
findings). Patients considered to have operable/potentially curable
mCRC at initial diagnosis per multidisciplinary meeting discussion
were excluded from the analysis. Patients with a prior history of
early-stage CRC treated with radical management strategies
(including surgery or high-dose radiotherapy) were excluded.
Patients diagnosed with de novo mCRC requiring up-front
localized management strategies for primary tumours (radiotherapy,
endoscopy or surgery) prior to palliative systemic therapy were
excluded.
KRAS, NRAS and BRAF status were confirmed through
next-generation sequencing CRC mutation panel test (107). MSI and MMR status were confirmed
using a multiplex PCR approach followed by DNA fragment analysis
and immunohistochemistry (using BenchMarckULTRA IHC/ISH by Roche
Diagnostics).
The responses of primary tumours and metastases to
up-front systemic chemotherapy were observed separately and
discordant responses to therapy were documented based on routine
interval radiological assessments. Molecular characteristics
possibly associated with these discordant responses were also
analysed and the incidence of complications from the primary
tumours and subsequent interventions were evaluated.
Factors including patient age, sex, primary tumour
location, molecular panel status and interval of response to
first-line systemic therapy were recorded. Responses to systemic
therapy in primary tumour and metastatic sites were recorded
separately using Response Evaluation Criteria In Solid Tumours,
v1.1(108). Furthermore, the
incidence of complications, types of complications arising from
primary tumours and subsequent management strategies for such
complications were also recorded, whether this involved
conservative management (such as endoscopy, surgery, or
radiotherapy, or a combination of these interventions).
Primary endpoints included documented response rates
to first-line up-front chemotherapy (in both primary and metastatic
sites) and the rates of discordance between these responses.
Primary endpoints also included evaluation of molecular and
pathological factors that may be associated with the discordant
radiological responses. Secondary endpoints included documenting
the rate of complications arising from the primary tumour (during
up-front palliative systemic therapy), the types of complications
encountered and the management strategies employed.
Non-parametric tests were used to compare groups,
investigate the statistical significance of the associations and
analyse survival (McNamara, Friedman's and Kaplan-Meier analyses).
P<0.05 was considered to indicate statistically significant
differences.
A total of 50 patients were identified and included
in the analysis (median age, 62 years; interquartile range, 55-69
years). A total of 30 patients (60%) were male. Primary tumours
confined to the right colon (including the caecum, ascending and
transverse colon), left colon (including the descending and sigmoid
colon) and rectum were observed in 34% (n=17), 44% (n=22) and 22%
(n=11) of the patients, respectively (Table I).
The most common site of metastasis at diagnosis of
mCRC was the liver (n=44, 88%), followed by the lung (n=20, 40%)
and peritoneum (n=10, 20%). Only 1 patient had bone metastasis. In
18 (36%) and 2 (4%) patients, the liver and lung were the only
sites of metastasis, respectively. Finally, 10 patients (20%) had
both liver and lung metastases at diagnosis (Table I).
Metastasis involving one, two and three or more
organ sites were present in 23 (46%), 20 (40%) and 7 (14%)
patients, respectively, at the time of diagnosis of non-curative
mCRC. KRAS, NRAS and BRAF were found to be mutated in 24 (48%), 2
(4%) and 2 (4%) cases, respectively. Furthermore, 2 patients (4%)
had synchronous KRAS and BRAF mutations. Only 1 patient (2%)
harboured KRAS mutation with MSI, and 5 patients (10%) could not
have their mutation panels performed due to insufficient tissue
available for diagnosis (Table
I).
All patients received 5FU-based chemotherapy. A
total of 28 patients (56%) received concurrent oxaliplatin (FOLFOX
regimen) and 17 (34%) received concurrent irinotecan (FOLFIRI
regimen) as first-line treatment, with an observed median treatment
duration of 17 and 19 weeks, respectively. Furthermore, 3 patients
(6%) received concurrent capecitabine with irinotecan (XELIRI
regimen) with a median treatment duration of 6 weeks, while 1
patient received single-agent 5FU (QUASAR regimen) for up to 30
weeks (Table I).
VEGR-targeted monoclonal antibody therapy
(bevacizumab) was used in 9 patients (18%), whereas EGFR-targeted
monoclonal antibody therapy (cetuximab and panitumumab) was used in
12 patients (24%), concurrently with first-line chemotherapy
(Table I). Over half of the
patients had received one line of systemic therapy for mCRC (n=27,
54%), 11 (22%) had received up to two lines of chemotherapy, and 11
patients (22%) had received three or more lines of chemotherapy (by
data cut-off).
Radiological assessment of response to palliative
systemic therapy demonstrated significant discordant responses
between the primary tumour and metastatic sites. Primary tumours
demonstrated disease response (DR), stable disease (SD) and
progressive disease (PD) in 24, 62 and 12% of patients on
first-line palliative systemic therapy, respectively.
By contrast, metastatic lesions demonstrated DR, SD
and PD on first-line chemotherapy in 36, 18 and 44% of patients,
respectively (Table II). Only 18
(36%) of the patients demonstrated concordant responses in both the
primary tumour and metastatic sites on first-line palliative
systemic therapy. A total of 11 patients (22%) demonstrated
discordant responses consisting of SD with DR, n=2 (4%) had PD with
SD, and n=18 (36%) had either DR or SD with PD in the primary
tumour and metastatic sites respectively (Table III). Discordant responses between
the primary tumour and metastatic sites did not vary significantly
according to the KRAS/NRAS/BRAF mutant (P>0.05).
A total of 15 patients (30%) developed complications
arising from the primary tumours during the course of up-front
first-line systemic therapy. As regards complications arising from
the primary tumour requiring intervention, 6 patients (12%)
developed bowel obstruction and 3 patients (6%) developed bowel
perforation; an additional 8 patients (16%) developed either pain
or bleeding from the primary tumour, necessitating local
intervention. Only 1 patient developed a primary tumour-associated
abscess requiring drainage and surgical resection. An outline of
complications from the primary tumour and the management strategies
employed is outlined in Table
IV.
Of the 50 patients, 38 (76%) did not develop any
complications from their primary tumour requiring intervention
while receiving palliative systemic therapy or by the time of data
cut-off. A total of 3 patients (6%) initially deemed
inoperable/non-curable at diagnosis ultimately proceeded to undergo
surgery with curative intent with resection of the primary tumour,
metastasectomy, or other local therapies (e.g., radiation) to
metastatic sites. These management strategies were undertaken
considering marked radiological treatment response following repeat
multidisciplinary team meeting discussions.
A total of 5 patients (10%) required emergent
defunctioning stoma creation (colostomy or ileostomy) for bowel
obstruction, or perforation. A total of 6 patients (12%) required
local radiotherapy for primary tumour in situ, most often
for rectal bleeding or localized pain. Only 1 patient underwent
both stoma creation and local irradiation (Table IV).
Left-sided primary tumours were associated with a
significantly higher rate of complications requiring local
intervention compared with right-sided tumours (P<0.001), with
complications arising in 17 (34%) and 9 (18%) cases, respectively.
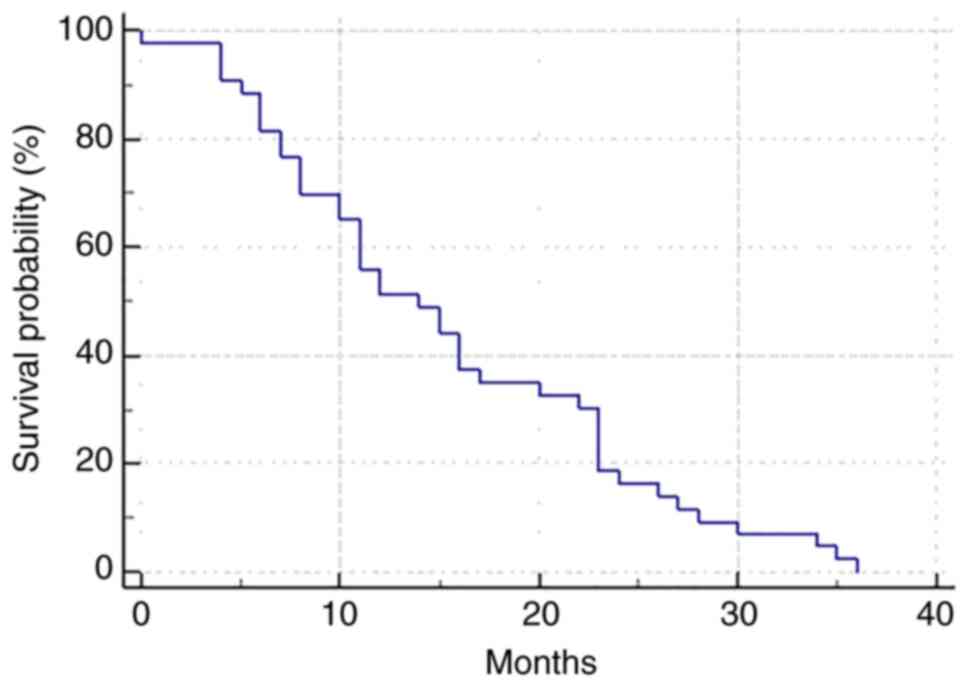
The median overall survival was 14.0 months (95% CI: 10.0-36.0;
Fig. 1). At the time of data
cut-off (December 31st, 2019), 7 patients (14%) remained alive, 3
of whom were receiving their third line of systemic therapy, 2 were
receiving their fourth line and 2 were off treatment (undergoing
active clinical follow-up with radiological surveillance).
The present study demonstrated that up-front
palliative systemic therapy can effectively control primary tumours
in patients with de novo mCRC with primary tumours in
situ that are deemed inoperable/non-curable at initial
diagnosis. However, while up-front systemic therapy with palliative
intent is predominantly effective in mCRC with primary tumour in
situ, 24% of patients in our study required localized
intervention due to complications arising from the primary tumour
(on first-line systemic therapy).
Other studies have demonstrated a reduced risk of
primary tumour-related complications and the need for emergent
surgical intervention with up-front localized interventions prior
to undertaking palliative systemic therapy (3,101).
Most international guidelines currently recommend combination
chemotherapy as the initial treatment for unresectable mCRC with
primary in situ (109,110). Local interventions for primary
tumours are typically reserved for when complications arise from
the primary tumour after palliative systemic therapy has already
been employed (including bowel obstruction, perforation,
significant pain, or bleeding from the primary tumour) (111,112).
In the present study, higher rates of radiological
response were observed in primary tumours compared with metastatic
tumour sites. Nonetheless, the rate of complications arising from
the primary tumour requiring intervention during systemic therapy
remained high (up to 25% of cases in this patient cohort).
In a retrospective study involving 233 patients with
mCRC receiving combined chemotherapy with or without bevacizumab as
up-front first-line systemic therapy (113), only 7% of these patients required
emergent surgical intervention, and 4% required emergent
non-surgical intervention (radiation and endoscopic stenting) while
on systemic therapy. On the other hand, the remaining 213 patients
(89%) never required local intervention for their primary tumour.
Another study observed that, among 83 asymptomatic patients with
non-curable mCRC treated with first-line chemotherapy (114), only 5% required surgery, while 4%
required colonic stenting to manage complications arising from the
primary tumour.
Conversely, other studies support prophylactic
surgical resection of the primary tumours in non-curable mCRC
before undertaking palliative-intent systemic therapy to reduce the
future risk of primary tumour complications (2,3,101).
One meta-analysis reviewed eight retrospective studies including
1,062 patients (101) and
observed that up-front primary tumour resection was associated with
reduced rates of primary tumour-associated complications requiring
emergent localized intervention and increased overall survival
rate. This was compared to patients receiving up-front palliative
systemic therapy alone, who were 7.3 times more likely to suffer
acute complications requiring localized interventions while on
palliative systemic therapy.
Current randomized control trials, such as the
SYNCHRONOUS trial (ISRCTN30964555) and the iPACS study (JCOC1007)
are comparing up-front palliative chemotherapy alone with up-front
primary tumour resection followed by palliative chemotherapy in
patients with non-curative mCRC with asymptomatic primary tumours
at diagnosis (115,116).
Multiple studies have observed conflicting results
when addressing the concordance rates of KRAS, NRAS and BRAF
mutation status between the primary colorectal tumour and
metastatic sites. While some results showed no significant
difference in mutation status (namely KRAS) between the primary
tumours and corresponding metastases, others showed discordant
results in 4-32% of the patients (16). One study including 305 patients
demonstrated a high concordance rate of KRAS mutation status
(96.4%) between primary colorectal tumours and corresponding liver
metastases (23,24). Mutation status discordance rates of
≤25% between the primary tumour and the lymph node metastases were
also observed (117).
There were certain limitations to the present study.
Certain patient variables (such as past medical history, ethnicity,
dietary history, smoking history, and whether patients did or did
not attend a colorectal screening program) were not assessed as
part of the analysis, as they were considered to be outside the
scope of this study, and due to relatively small patient number.
This is further taking into account the small number of patients
accrued in this data analysis, which limits the validity of
statistical associations observed. Further research (ideally a
meta-analysis) is required to assess and, ultimately, validate the
associations observed in this study.
In the present study, statistically appreciable
rates of discordant radiological responses to up-front palliative
systemic therapy were observed between the primary tumour and
metastatic tumour sites in patients with inoperable/non-curative
de novo mCRC (in up to 60% of our patient cohort).
Approximately one-third of the patients demonstrating radiological
control of the primary tumour otherwise demonstrated progression at
metastatic sites while on first-line up-front single-modality
palliative systemic therapy at the first interval restaging
imaging.
This has implications for molecular analyses of the
tissues obtained from patients diagnosed with mCRC. Our analysis
suggests that standard molecular panels performed in mCRC
(including KRAS, NRAS and BRAF status with MMR and MSI analyses)
should preferentially be performed on tissue from metastatic sites
rather than on tissue from the primary tumour.
Up-front localized management strategies, such as
palliative radiation to the primary tumour, surgical interventions
(including stoma formation) and endoscopic procedures (such as
colonic stenting) should be considered in certain patients with
inoperable mCRC.
Not applicable.
Funding: No funding was received.
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
RAH: Conception and design of the study, data
collection and analysis, writing and drafting the manuscript. SM:
Participation in manuscript writing and collection of references.
HM and HI: Critical revision of the manuscript for important
intellectual content. SA: Assessment of radiological responses
according to RECIST criteria. RK and GK: Critical revision and
editing of the manuscript. NO: Conception and design of the study,
manuscript editing and critically revising the work for important
intellectual content. All authors have read and approved the final
manuscript. All authors agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval was obtained from the local hospital
Medical Research Ethics Committee prior to data collection (REC
Ref:113/2020). Patient data were anonymized during data collection
and analysis. Informed consent (written or oral) was not required,
since this was a retrospective chart review (as outlined per Health
Research Consent Declaration Committee Guidelines, Ireland). All
data collection procedures followed the General Data Protection
Regulation and the Data Protection Act, 2018.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomatarum I, Siegal
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Clancy C, Burke JP, Barry M, Kalady MF and
Calvin Coffey J: A meta-analysis to determine the effect of primary
tumour resection for stage IV colorectal cancer with unresectable
metastases on patient survival. Annals Surg Oncol. 21:3900–3908.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim CW, Baek JH, Choi GS, Yu CS, Kang SB,
Park WC, Lee BH, Kim HR, Oh JH, Kim JH, et al: The role of primary
tumour resection in the colorectal cancer patients with
asymptomatic, synchronous unresectable metastasis: Study protocol
for a randomized control trial. Trials. 17(34)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tan WJ, Patil S, Guillem JG, Paty PB,
Weiser MR, Nash GM, Smith JJ, Pappou EP, Wei IH and Garcia-Aguilar
J: Primary Tumour-related complications and salvage outcomes in
patients with metastatic rectal cancer and an untreated primary
tumour. Dis Colon Rectum. 64:45–52. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
de Mestier L, Manceau G, Neuzillet C,
Bachet JB, Spano JP, Kianmanesh R, Vaillant JC, Bouché O, Hannoun L
and Karoui M: Primary tumour resection in colorectal cancer with
unresectable synchronous metastases: A review. World J Gastrointest
Oncol. 6:156–169. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Machover D: A comprehensive review of
5-fluorouracil and leucovorin in patients with metastatic
colorectal carcinoma. Cancer. 8:1179–1187. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kurkjian C and Kummar S: Advances in the
treatment of metastatic colorectal cancer. Am J Ther. 16:412–420.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leicher LW, de Graaf JC, Coers W, Tascilar
M and de Groot JWB: Tolerability of capecitabine monotherapy in
metastatic colorectal cancer: A real-world study. Drugs R D.
17:117–124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fujita K, Kubota Y, Ishida H and Sasaki Y:
Irinotecan, a key chemotherapeutic drug for metastatic colorectal
cancer. World J Gastroenterol. 21:12234–12248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Comella P, Casaretti R, Sandomenico C,
Avallone A and Franco L: Role of oxaliplatin in the treatment of
colorectal cancer. Ther Clin Risk Manag. 5:229–238. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fornasier G, Francescon S and Baldo P: An
update of efficacy and safety of cetuximab in metastatic colorectal
cancer: A narrative review. Adv Ther. 35:1497–1509. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Battagin F, Puccini A, Djaballah SA and
Lenz HJ: The impact of panitumumab treatment on survival and
quality of life in patients with RAS wild-type metastatic
colorectal cancer. Cancer Manag Res. 11:5911–5924. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rosen LS, Jacobs IA and Burkes RL:
Bevacizumab in colorectal cancer: Current role in treatment and the
potential of biosmiliars. Target Oncol. 12:599–610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Verdaguer H, Tebernero J and Macarulla T:
Ramucirumab in metastatic colorectal cancer: Evidence to date and
place in therapy. Ther Adv Med Oncol. 8:230–242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yoshihiro T, Kusaba H, Makiyama A,
Kobayashi K, Uenomachi M, Ito M, Doi Y, Mitsugi K, Aikawa T,
Takayoshi K, et al: Efficacy and safety of ramucirumab plus
modified FOLFIRI for metastatic colorectal cancer. Int J Clin
Oncol. 24:508–515. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Braun MS, Adab F, Bradley C, McAdam K,
Thomas G, Wadd NJ, Rea D, Philips R, Twelves C, Bozzino J, et al:
Modified de Gramont with oxaliplatin in the first-line treatment of
advanced colorectal cancer. Br J Cancer. 89:1155–1158.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo Y, Xiong BH, Zhang T, Cheng Y and Ma
L: XELOX vs. FOLFOX in metastatic colorectal cancer: An updated
meta-analysis. Cancer Invest. 34:94–104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Rittweger K, Gilberg F and
Saltz L: XELOX vs FOLFOX-4 as first-line therapy for metastatic
colorectal cancer: NO16966 updated results. Br J Cancer. 105:58–64.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Saltz LB, Douillard JY, Pirotta N, Alakl
M, Gruia G, Awad L, Elfring GL, Locker PK and Miller LL: Irinotecan
plus fluorouracil/leucovorin for metastatic colorectal cancer: A
new survival standard. Oncologist. 6:81–91. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tournigard C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Price TJ: Modified XELIRI (capecitabine
plus irinotecan) for metastatic colorectal cancer. Lancet Oncol.
19:587–589. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yaffee P, Osipov A, Tan C, Tuli R and
Hendifar A: Review of systemic therapies for locally advanced and
metastatic rectal cancer. J Gastrointest Oncol. 6:185–200.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Suzuki K, Takaharu K, Muto Y, Ichida K,
Fukui T, Takayama Y, Tsujinaka S, Sasaki J, Horie H, Kawamura YJ,
et al: XELIRI regimen plus continuous treatment with bevacizumab is
well-tolerated and effective in metastatic colorectal cancer
patients in a second-line setting involving the sequential
administration of XELOX and XELIRI. Mol Clin Oncol. 2:827–832.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kawai S, Takeshima N, Hayasaka Y, Notsu A,
Yamazaki M, Kawabata T, Yamazaki K, Mori K and Yasui H: Comparison
of irinotecan and oxaliplatin as the first-line therapies for
metastatic colorectal cancer: A meta-analysis. BMC Cancer.
21(116)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Marschner N, Arnold D, Engel E,
Hutzschenreuter U, Rauh J, Freier W, Hartmann H, Frank M and
Jänicke M: Oxaliplatin-based first-line chemotherapy is associated
with improved overall survival compared to first-line treatment
with irinotecan-based chemotherapy in patients with metastatic
colorectal cancers-Results from a prospective cohort study. Clin
Epidemiol. 7:295–303. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Colucci G, Gebbia V, Paoletti G, Giuliani
F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione
L, et al: Phase III randomized trial of FOLFIRI versus FOLFOX4 in
the treatment of advanced colorectal cancer: A multicenter study of
the Gruppo Oncologico Dell'Italia meridionale. J Clin Oncol.
23:4866–4875. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aparicio T, Desramé J, Lecomte T, Mitry E,
Belloc J, Etienney I, Montembault S, Vayre L, Locher C, Ezenfis J,
et al: Oxaliplatin- or irinotecan-based chemotherapy for metastatic
colorectal cancer in the elderly. Br J Cancer. 89:1439–1444.
2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
André T, Afchain P, Barrier A, Blanchard
P, Larsen AK, Tournigand C, Louvet C and de Gramont A: Current
status of adjuvant therapy for colon cancer. Gastrointest Cancer
Res. 1:90–97. 2007.PubMed/NCBI
|
|
31
|
Patel K, Anthoney DA, Crellin AM,
Sebag-Montefiore D, Messruther J and Seymour MT: Weekly
5-fluorouracil and leucovorin: Achieving lower toxicity with higher
dose-intensity in adjuvant chemotherapy after colorectal cancer
resection. Ann Oncol. 15:568–573. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Schippinger W, Samonigg H, Schaberl-Moser
R, Greil R, Thödtmann R, Tschmelitsch J, Jagoditsch M, Steger GG,
Jakesz R, Herbst F, et al: A prospective randomised phase III trial
of adjuvant chemotherapy with 5-fluorouracil and leucovorin in
patients with stage II colon cancer. Br J Cancer. 97:1021–1027.
2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fotheringham S, Mozolowski GA, Murray EMA
and Kerr DJ: Challenges and solutions in patient treatment
strategies for stage II colon cancer. Gastroenterol Rep (Oxf).
7:151–161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Twelves C, Wong A, Nowacki MP, Abt M,
Burris H III, Carrato A, Cassidy J, Cervantes A, Fagerberg J,
Georgoulias V, et al: Capecitabine as adjuvant treatment for stage
III colon cancer. N Engl J Med. 352:2696–2704. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cassidy J, Twelves C, Van Cutsem E, Hoff
P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, et
al: First-line oral capecitabine therapy in metastatic colorectal
cancer: A favorable safety profile compared with intravenous
5-fluorouracil/leucovorin. Ann Oncol. 13:566–575. 2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rizzo A, Nannini M, Astolfi A, Indio V, De
Iaco P, Perrone AM, De Leo A, Incorvaia L, Di Scioscio V and
Pantaleo MA: Impact of chemotherapy in the adjuvant setting of
early stage uterine leiomyosarcoma: A systematic review and updated
meta-analysis. Cancers (Basel). 12(1899)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rosales J and Leong LA: Chemotherapy for
metastatic colorectal cancer. J Natl Compr Canc Netw. 3:525–529.
2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tonini G, Imperatori M, Vincenzi B, Frezza
AM and Santini D: Rechallenge therapy and treatment holiday:
Different strategies in management of metastatic colorectal cancer.
J Exp Clin Cancer Res. 32(92)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bekaii-Saab T, Kim R, Kim TW, O'Connor JM,
Strickler JH, Malka D, Sartore-Bianchi A, Bi F, Yamaguchi K,
Yoshino T and Prager GW: Third- or Later-line therapy for
metastatic colorectal cancer: Reviewing best practice. Clin
Colorectal Cancer. 18:e117–e129. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kopetz S, Grothey A, Yaeger R, Van Cutsem
E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS,
et al: Encorafenib, Binimetinib, and cetuximab in BRAF
V600E-mutated colorectal cancer. N Engl J Med. 381:1632–1643.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Smith KM and Desai J: Nivolumab for the
treatment of colorectal cancer. Expert Rev Anticancer Ther.
18:611–618. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus Ipilimumab
in DNA mismatch Repair-Deficient/Microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zheng Z, Yu T, Zhao X, Gao X, Zhao Y and
Liu G: Intratumour heterogeneity: A new perspective on colorectal
cancer research. Cancer Med. 9:7637–7645. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Losi L, Baisse B, Bouzourene H and
Benhattar J: Evolution of intratumoural genetic heterogeneity
during colorectal cancer progression. Carcinogenesis. 26:916–922.
2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Molinari C, Marisi G, Passardi A,
Matteucci L, De Maio G and Ulivi P: Heterogeneity in colorectal
cancer: A challenge for personalized medicine? Int J Mol Sci.
19(3733)2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Buikhuisen JY, Torang A and Medema JP:
Exploring and modelling colon cancer inter-tumour heterogeneity:
Opportunities and challenges. Oncogenesis. 9(66)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Del Carmen S, Sayagués JM, Bengoechea O,
Anduaga MF, Alcazar JA, Gervas R, García J, Orfao A, Bellvis LM,
Sarasquete ME and Del Mar Abad M: Spatio-temporal tumour
heterogeneity in metastatic CRC tumours: A mutational-based
approach. Oncotarget. 9:34279–34288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Blank A, Roberts DE II, Dawson H, Zlobec I
and Lugli A: Tumour heterogeneity in primary colorectal cancer and
corresponding metastases. Does the apple fall far from the tree?
Front Med (Lausanne). 5(234)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Eide PW, Moosavi SH, Eilertsen IA,
Brunsell TH, Langerud J, Berg KCG, Røsok BI, Bjørnbeth BA,
Nesbakken A, Lothe RA and Sveen A: Metastatic heterogeneity of the
consensus molecular subtypes of colorectal cancer. NPJ Genom Med.
6(59)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hirata A, Hatano Y, Niwa M, Hara A and
Tomita H: Heterogeneity of colon cancer stem cells. Adv Exp Med
Biol. 1139:115–126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Arakawa K, Hata K, Nozawa H, Kawai K,
Tanaka T, Nishikawa T, Sasaki K, Shuno Y, Kaneko M, Hiyoshi M, et
al: Molecular subtypes are frequently discordant between lesions in
patients with synchronous colorectal cancer: Molecular analysis of
59 patients. Anticancer Res. 39:1425–1432. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li ZN, Zhao L, Yu LF and Wei MJ: BRAF and
KRAS mutations in metastatic colorectal cancer: Future perspectives
for personalized therapy. Gastroenterol Rep (Oxf). 8:192–205.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Christensen TD, Palshof JA, Larsen FO,
Poulsen TS, Høgdall E, Pfeiffer P, Jensen BV, Yilmaz MK and Nielsen
D: Associations between primary tumour RAS, BRAF and PIK3CA
mutation status and metastatic site in patients with
chemo-resistant metastatic colorectal cancer. Acta Oncol.
57:1057–1062. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Fedyanin M, Stroganova A, Senderovich A,
Dranko S, Tryakin A, Polyanskaya E, Popova A, Sekhina O, Rasulov A,
Gordeev S, et al: Factors associated with discordance of KRAS,
NRAS, BRAF, PIK3CA mutation status in the primary tumour and
metastases in patients (pts) with colorectal cancer (CRC). Annals
Oncol. 27 (Suppl 6)(VI174)2016.
|
|
60
|
Watanabe T, Kobunai T, Yamamoto Y, Matsuda
K, Ishihara S, Nozawa K, Iinuma H, Shibuya H and Eshima K:
Heterogeneity of KRAS status may explain the subset of discordant
KRAS status between primary and metastatic colorectal cancer. Dis
Colon Rectum. 54:1170–1178. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Siyar Ekinci A, Demirci U, Cakmak
Oksuzoglu B, Ozturk A, Esbah O, Ozatli T, Celik B, Budakoglu B,
Turker I, Bal O and Turan N: KRAS discordance between primary and
metastatic tumour in patients with metastatic colorectal carcinoma.
J BUON. 20:128–135. 2015.
|
|
62
|
Ardito F, Razionale F, Salvatore L, Cenci
T, Vellone M, Basso M, Panettieri E, Calegari MA, Tortora G,
Martini M and Giuliante F: Discordance of KRAS mutational status
between primary tumours and liver metastases in colorectal cancer:
Impact on long-term survival following radical resection. Cancers
(Basel). 13(2148)2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Estrella JS, Tetzlaff MT, Bassett RL Jr,
Patel KP, Williams MD, Curry JL, Rashid A, Hamilton SR and Broaddus
RR: Assessment of BRAF V600E status in colorectal carcinoma:
Tissue-specific discordances between immunohistochemistry and
sequencing. Mol Cancer Ther. 14:2887–2895. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mas L, Bachet JB, Taly V, Bouché O, Taieb
J, Cohen R, Meurisse A, Normand C, Gornet JM, Artru P, et al: BRAF
mutation status in circulating tumour DNA from patients with
metastatic colorectal cancer: Extended mutation analysis from the
AGEO RASANC study. Cancers (Basel). 11(998)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Bai H, Wang R, Cheng W, Shen Y, Li H, Xia
W, Ding Z and Zhang Y: Evaluation of concordance between deficient
mismatch repair and microsatellite instability testing and their
association with clinicopathological features in colorectal cancer.
Cancer Manag Res. 12:2863–2873. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Haraldsdottir S, Roth R, Pearlman R,
Hampel H, Arnold CA and Frankel WL: Mismatch repair deficiency
concordance between primary colorectal cancer and corresponding
metastasis. Fam Cancer. 15:253–260. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Vyas M, Firat C, Hechtman JF, Weiser MR,
Yaeger R, Vanderbilt C, Benhamida JK, Keshinro A, Zhang L, Ntiamoah
P, et al: Discordant DNA mismatch repair protein status between
synchronous or metachronous gastrointestinal carcinomas: Frequency,
patterns, and molecular etiologies. Fam Cancer. 20:201–213.
2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yaeger R: Heterogeneity in microsatellite
instability in metastatic colorectal cancer: Mechanisms and
clinical implications. J Natl Compr Canc Netw. 17:1263–1264.
2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
De Smedt L, Lemahieu J, Palmans S, Govaere
O, Tousseyn T, Van Cutsem E, Prenen H, Tejpar S, Spaepen M,
Matthijs G, et al: Microsatellite instable vs stable colon
carcinomas: Analysis of tumour heterogeneity, inflammation and
angiogenesis. Br J Cancer. 113:500–509. 2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Levin-Sparenberg E, Bylsma LC, Lowe K,
Sangare L, Fryzek JP and Alexander DD: A systematic literature
review and meta-analysis describing the prevalence of KRAS, NRAS,
and BRAF Gene mutations in metastatic colorectal cancer.
Gastroenterology Res. 13:184–198. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Hu Y, Tao SY, Deng JM, Hou ZK, Liang JQ,
Huang QG, Li LH, Li HB, Chen YM, Yi H, et al: Prognostic value of
NRAS gene for survival of colorectal cancer patients: A systematic
review and meta-analysis. Asian Pac J Cancer Prev. 19:3001–3008.
2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wang J, Shen J, Huang C, Cao M and Shen L:
Clinicopathological significance of BRAFV600E mutation
in colorectal cancer: An updated meta-analysis. J Cancer.
10:2332–2341. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wensink E, Bond M, Kucukkose E, May A,
Vink G, Koopman M, Kranenburg O and Roodhart J: A review of the
sensitivity of metastatic colorectal cancer patients with deficient
mismatch repair to standard-of-care chemotherapy and monoclonal
antibodies, with recommendations for future research. Cancer Treat
Rev. 95(102174)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Wheeler JM, Bodmer WF and Mortensen NJ:
DNA mismatch repair genes and colorectal cancer. Gut. 47:148–153.
2000.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Hou JT, Zhao LN, Zhang DJ, Lv DY, He WL,
Chen B, Li HB, Li PR, Chen LZ and Chen XL: Prognostic value of
mismatch repair genes for patients with colorectal cancer:
Meta-analysis. Technol Cancer Res Treat.
17(1533033818808507)2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kang S, Na Y, Joung SY, Lee SI, Oh SC and
Min BW: The significance of microsatellite instability in
colorectal cancer after controlling for clinicopathological
factors. Medicine (Baltimore). 97(e0019)2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Nojadeh JN, Behrouz Sharif S and Sakhinia
E: Microsatellite instability in colorectal cancer. EXCLI J.
17:159–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Anele CC, Adegbola SO, Askari A, Rajendran
A, Clark SK, Latchford A and Faiz OD: Risk of metachronous
colorectal cancer following colectomy in Lynch syndrome: A
systematic review and meta-analysis. Colorectal Dis. 19:528–536.
2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Duraturo F, Liccardo R, De Rosa M and Izzo
P: Genetics, diagnosis and treatment of Lynch syndrome: Old lessons
and current challenges. Oncol Lett. 17:3048–3054. 2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Boland CR and Goel A: Microsatellite
instability in colorectal cancer. Gastroenterology.
138:2073–2087.e3. 2010.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Morelli MP, Overman MJ, Dasari A, Kazmi
SMA, Mazard T, Vilar E, Morris VK, Lee MS, Herron D, Eng C, et al:
Characterizing the patterns of clonal selection in circulating
tumour DNA from patients with colorectal cancer refractory to
anti-EGFR treatment. Ann Oncol. 26:731–736. 2015.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Van Emburgh BO, Sartore-Bianchi A, Di
Nicolantonio F, Siena S and Bardelli A: Acquired resistance to
EGFR-targeted therapies in colorectal cancer. Mol Oncol.
8:1084–1094. 2014.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Yachida S, Jones S, Bozic I, Antal T,
Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1117. 2010.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Sottoriva A, Kang H, Ma Z, Graham TA,
Salomon MP, Zhao J, Marjoram P, Siegmund K, Press MF, Shibata D and
Curtis C: A Big Bang model of human colorectal tumour growth. Nat
Genet. 47:209–216. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Fujiyoshi K, Yamamoto G, Takahashi A, Arai
Y, Yamada M, Kakuta M, Yamaguchi K, Akagi Y, Nishimura Y, Sakamoto
H and Akagi K: High concordance rate of KRAS/BRAF mutations and
MSI-H between primary colorectal cancer and corresponding
metastases. Oncol Rep. 37:785–792. 2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Bhullar DS, Barriuso J, Mullamitha S,
Saunders MP, O'Dwyer ST and Aziz O: Biomarker concordance between
primary colorectal cancer and its metastases. EBioMedicine.
40:363–374. 2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Ruiz-Bañobre J, Kandimalla R and Goel A:
Predictive biomarkers in metastatic colorectal cancer: A systematic
review. JCO Precis Oncol. 3(PO.18.00260)2019.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Zou Y, Hu X, Zheng S, Yang A, Li X, Tang
H, Kong Y and Xie X: Discordance of immunotherapy response
predictive biomarkers between primary lesions and paired metastases
in tumours: A systematic review and meta-analysis. EBioMedicine.
63(103137)2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Zou SM, Li WH, Wang WM, Li WB, Shi SS,
Ying JM and Lyu N: The gene mutational discrepancies between
primary and paired metastatic colorectal carcinoma detected by
next-generation sequencing. J Cancer Res Clin Oncol. 144:2149–2159.
2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Guyot D'Asnières De Salins A, Tachon G,
Cohen R, Karayan-Tapon L, Junca A, Frouin E, Godet J, Evrard C,
Randrian V, Duval A, et al: Discordance between immunochemistry of
mismatch repair proteins and molecular testing of microsatellite
instability in colorectal cancer. ESMO Open.
6(100120)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Mao C, Wu XY, Yang ZY, Threapleton DE,
Yuan JQ, Yu YY and Tang JL: Concordant analysis of KRAS, BRAF,
PIK3CA mutations, and PTEN expression between primary colorectal
cancer and matched metastases. Sci Rep. 5(8065)2015.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kim KP, Kim JE, Hong YS, Ahn SM, Chun SM,
Hong SM, Jang SJ, Yu CS, Kim JC and Kim TW: Paired primary and
metastatic tumour analysis of somatic mutations in synchronous and
metachronous colorectal cancer. Cancer Res Treat. 49:161–167.
2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Jesinghaus M, Wolf T, Pfarr N, Muckenhuber
A, Ahadova A, Warth A, Goeppert B, Sers C, Kloor M, Endris V, et
al: Distinctive spatiotemporal stability of somatic mutations in
metastasized microsatellite-stable colorectal Cancer. Am J Surg
Pathol. 39:1140–1147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Lee CC, Soon YY, Lum JHY, Tan CL and Tey
JCS: Frequency of discordance in programmed death-ligand 1 (PD-L1)
expression between primary tumours and paired distant metastases in
advanced cancers: A systematic review and meta-analysis. Acta
Oncol. 59:696–704. 2020.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Gerlinger M, Rowan AJ, Horswell S, Math M,
Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N,
Stewart A, et al: Intratumour heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Molinari F, Martin V, Saletti P, De Dosso
S, Spitale A, Camponovo A, Bordoni A, Crippa S, Mazzucchelli L and
Frattini M: Differing deregulation of EGFR and downstream proteins
in primary colorectal cancer and related metastatic sites may be
clinically relevant. Br J Cancer. 100:1087–1094. 2009.PubMed/NCBI View Article : Google Scholar
|
|
98
|
He Q, Xu Q, Wu W, Chen L, Sun W and Ying
J: Comparison of KRAS and PIK3CA gene status between primary
tumours and paired metastases in colorectal cancer. Onco Targets
Ther. 9:2329–2335. 2016.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Niitsu H, Hinoi T, Shimomura M, Egi H,
Hattori M, Ishizaki Y, Adachi T, Saito Y, Miguchi M, Sawada H, et
al: Up-front systemic chemotherapy is a feasible option compared to
primary tumour resection followed by chemotherapy for colorectal
cancer with unresectable synchronous metastases. World J Surg
Oncol. 13(162)2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Giacchetti S, Itzhaki M, Gruia G, Adam R,
Zidani R, Kunstlinger F, Brienza S, Alafaci E, Bertheault-Cvitkovic
F, Jasmin C, et al: Long-term survival of patients with
unresectable colorectal cancer liver metastases following
infusional chemotherapy with 5-fluorouracil, leucovorin,
oxaliplatin and surgery. Ann Oncol. 10:663–669. 1999.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Stillwell AP, Buettner PG and Ho YH:
Meta-analysis of survival of patients with stage IV colorectal
cancer managed with surgical resection versus chemotherapy alone.
World J Surg. 34:797–807. 2010.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wang G, Wang W, Jin H, Dong H, Chen W, Li
X, Li G and Li L: The effect of primary tumour radiotherapy in
patients with Unresectable stage IV rectal or Rectosigmoid cancer:
A propensity score matching analysis for survival. Radiat Oncol.
15(126)2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Poultsides GA and Paty PB: Reassessing the
need for primary tumour surgery in unresectable metastatic
colorectal cancer: Overview and perspective. Ther Adv Med Oncol.
3:35–42. 2011.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Park JH, Kim TY, Lee KH, Han SW, Oh DY, Im
SA, Kang GH, Chie EK, Ha SW, Jeong SY, et al: The beneficial effect
of palliative resection in metastatic colorectal cancer. Br J
Cancer. 108:1425–1431. 2013.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Simillis C, Kalakouti E, Afxentiou T,
Kontovounisios C, Smith JJ, Cunningham D, Adamina M and Tekkis PP:
Tumour resection in patients with incurable localized or metastatic
colorectal cancer: A systematic review and meta-analysis. World J
Surg. 43:1829–1840. 2019.
|
|
106
|
Feo L, Polcino M and Nash GM: Resection of
the primary tumour in stage IV colorectal cancer: When is it
necessary? Surg Clin North Am. 97:657–669. 2017.PubMed/NCBI View Article : Google Scholar
|
|
107
|
www.beaumont.ie/media/Testing_Services_Offered1.pdf.
|
|
108
|
Radiology Assistant: RECIST 1.1-and more.
Response Evaluation Criteria In Solid Tumors. https://radiologyassistant.nl/more/recist-1-1/recist-1-1.
Accessed December 7, 2021.
|
|
109
|
Cutsem EV, Cervantes A, Nordlinger B and
Arnold D: ESMO Guidelines Working Group. Metastatic colorectal
cancer: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 25 (Suppl 3):iii1–iii9. 2014.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Chiorean EG, Nandakumar G, Fadelu T, Temin
S, Alarcon-Rozas AE, Bejarano S, Croitoru AE, Grover S, Lohar PV,
Odhiambo A, et al: Treatment of patients with late-stage colorectal
cancer: ASCO Resource-stratified guideline. JCO Glob Oncol.
6:414–438. 2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Muratore A, Zorzi D, Bouzari H, Amisano M,
Massucco P, Sperti E and Capussotti L: Asymptomatic colorectal
cancer with Un-Resectable liver metastases: Immediate colorectal
resection or up-front systemic chemotherapy? Ann Surg Oncol.
14:766–770. 2007.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Pędziwiatr M, Mizera M, Witowski J, Major
P, Torbicz G, Gajewska N and Budzyński A: Primary tumour resection
in stage IV unresectable colorectal cancer: What has changed? Med
Oncol. 34(188)2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Poultsides GA, Servais EL, Saltz LB, Patil
S, Kemeny NE, Guillem JG, Weiser M, Temple LK, Wong WD and Paty PB:
Outcome of primary tumour in patients with synchronous stage IV
colorectal cancer receiving combination chemotherapy without
surgery as initial treatment. J Clin Oncol. 27:3379–3384.
2009.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Seo GJ, Park JW, Yoo SB, Kim SY, Choi HS,
Chang HJ, Shin A, Jeong SY, Kim DY and Oh JH: Intestinal
complications after palliative treatment for asymptomatic patients
with unresectable stage IV colorectal cancer. J Surg Oncol.
102:94–99. 2010.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Rahbari NN, Lordick F, Fink C, Bork U,
Stange A, Jäger D, Luntz SP, Englert S, Rossion I, Koch M, et al:
Resection of the primary tumour versus no resection prior to
systemic therapy in patients with colon cancer and synchronous
unresectable metastases (UICC stage IV): SYNCHRONOUS-a randomised
controlled multicentre trial (ISRCTN30964555). BMC Cancer.
12(142)2012.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Moritani K, Kanemitsu Y, Shida D, Shitara
K, Mizusawa J, Katayama H, Hamaguchi T and Shimada Y: Colorectal
Cancer Study Group (CCSG) of Japan Clinical Oncology Group (JCOG).
A randomized controlled trial comparing primary tumour resection
plus chemotherapy with chemotherapy alone in incurable stage IV
colorectal cancer: JCOG1007 (iPACS study). Jpn J Clin Oncol.
50:89–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016.PubMed/NCBI View Article : Google Scholar
|