Introduction
Gastrectomy for benign disease has decreased over
the past four decades as a result of the development of proton pump
inhibitors, and hence, cancer in the remnant stomach after this
type of gastrectomy, named ‘gastric stump cancer’, is on the
decline. Meanwhile, remnant gastric cancer after partial
gastrectomy for gastric cancer, referred to as ‘metachronous
multiple gastric cancer’ (MMGC), has become relatively common.
Several studies have addressed and reported
correlations of the time until detection of remnant stomach cancer
and factors associated with initial gastrectomy. Researchers have
consistently reported that the interval between initial distal
gastrectomy (DG) and the diagnosis of stump carcinoma was
significantly longer in patients who underwent initial gastrectomy
for benign disease than in those who underwent initial gastrectomy
for cancer (1-4).
The probable reason for the shorter interval for MMGC is that
patients with gastric cancer already have precancerous lesions,
such as atrophic gastritis and intestinal metaplasia, and they are
followed up closely by endoscopic examination. Regarding the
correlation of the time interval until the detection of remnant
stomach cancer with the initial surgical procedure, most published
studies have reported remnant stomach cancer after DG, which is the
most commonly performed procedure for both benign and malignant
disease in the stomach, while information on other types of
gastrectomy is limited (5). A
characteristic correlation between the type of initial
reconstruction and the interval has been reported in patients after
DG; namely, the interval between initial DG and the diagnosis of
remnant stomach cancer is significantly longer in patients treated
with Billroth II (B-II) reconstruction than in those treated with
Billroth I (B-I) reconstruction, while most studies included small
numbers of patients with MMGC (3-5).
A Japanese nationwide survey performed by Tanigawa et al
(2) supported the findings
described above and included a sufficient number of patients with
MMGC. However, the survey was performed in 2008 and collected MMGC
patients with adenocarcinoma in the remnant stomach occurring ≥10
years after initial distal gastrectomy reconstructed with B-I or
B-II, excluding Roux-en Y (R-Y) reconstruction for cancer, which
suggests that the result may not be representative of MMGC in
Japan.
Other items of interest are the procedure performed
for MMGC and the factors associated with initial gastrectomy. The
Japanese nationwide survey mentioned above reported that completion
total gastrectomy (CTG) was performed in >80% of patients who
underwent initial partial gastrectomy for stomach cancer,
irrespective of the reconstruction method, which may be due to the
small size of the remnant stomach after gastrectomy for stomach
cancer (2). Although this previous
survey included a sufficient number of patients with MMGC, it
included only patients with MMGC who underwent distal gastrectomy
with B-I or B-II reconstruction and who were diagnosed ≥10 years
after initial gastrectomy. Therefore, it also may not reflect the
procedures currently performed during surgery for MMGC in
Japan.
As mentioned above, though it is assumed that the
type of initial gastrectomy or reconstruction method correlates
with the interval between the initial gastrectomy and detection of
MMGC or the required treatment for MMGC, the reported evidence thus
far is limited to MMGC after distal gastrectomy.
The Japanese Society for Gastro-Surgical
Pathophysiology (JSGSP) performed a questionnaire survey on remnant
stomach cancer among Japanese centers that specialize in treating
gastric cancer in 2018. This report sought to evaluate the
correlation of the type of initial gastrectomy or reconstruction
procedure with the interval between initial gastrectomy for stomach
cancer and the detection of MMGC as part of the survey. In
addition, the correlation between the type of initial gastrectomy
or reconstruction procedure and the performed treatment for MMGC
was also analyzed.
Materials and methods
Questionnaire
A nationwide questionnaire survey was planned by the
president of the JSGSP 48th Annual Meeting (TK) and conducted as a
part of the meeting. The questionnaire only collected the number of
cases for each questionnaire item and did not collect any
individual patient data. The JSGSP members accessed the web-based
questionnaire between May 2018 and October 2018 and answered via
e-mail. The data were sent to Convention Linkage, Inc. and
compiled.
The study protocol was approved by the institutional
review board of Kanazawa Medical University (trial no. I267), was
performed in accordance with the Ethical Guidelines of the Japan
Ministry of Health, Labour and Welfare for Medical and Health
Research Involving Human Subjects (6) and conformed to the provisions of the
Declaration of Helsinki (7). All
data were anonymized and assembled for each facility. Only the
statistical numbers of patients were submitted by the doctors and
this does not require ethics approval from their own affiliated
local review board prior to sharing the data in the questionnaire
or consent from the individual patients, as none of their
personal/specific data were used.
The questionnaire consisted of three parts; an
English translation of the questionnaire sheet is provided as
Supplemental data. In the first part, participating facilities were
requested to indicate the number of patients who underwent radical
surgery for remnant stomach cancer between 2003 and 2017, as well
as the number of cases with MMGC among these patients. The
questionnaire also requested that the facilities indicate the
number of patients with MMGC in accordance with the time interval
from the initial gastrectomy until treatment for MMGC by the type
of initial gastrectomy or reconstruction procedure. The number of
cases for each treatment procedure (CTG or partial gastrectomy) was
also queried. The second part was a cohort study that followed up
gastrectomized patients between 2003 and 2012, which required the
reporting of the number of cases in which MMGC was observed until
the time of observation (8). The
third part was regarding the correlation between Helicobacter
pylori infection and the occurrence of MMGC. Participating
institutions were asked to provide information on the institutional
policy for eradication after gastrectomy and the occurrence of MMGC
after eradication. The current study summarizes the data from the
first part of the questionnaire.
Statistical analysis
The χ2 test was performed with Excel 2016
(Microsoft Corporation) to compare the distribution of the time
interval from the initial gastrectomy until the detection of MMGC
among different types of initial gastrectomy or reconstruction
procedures.
Results
Data collection
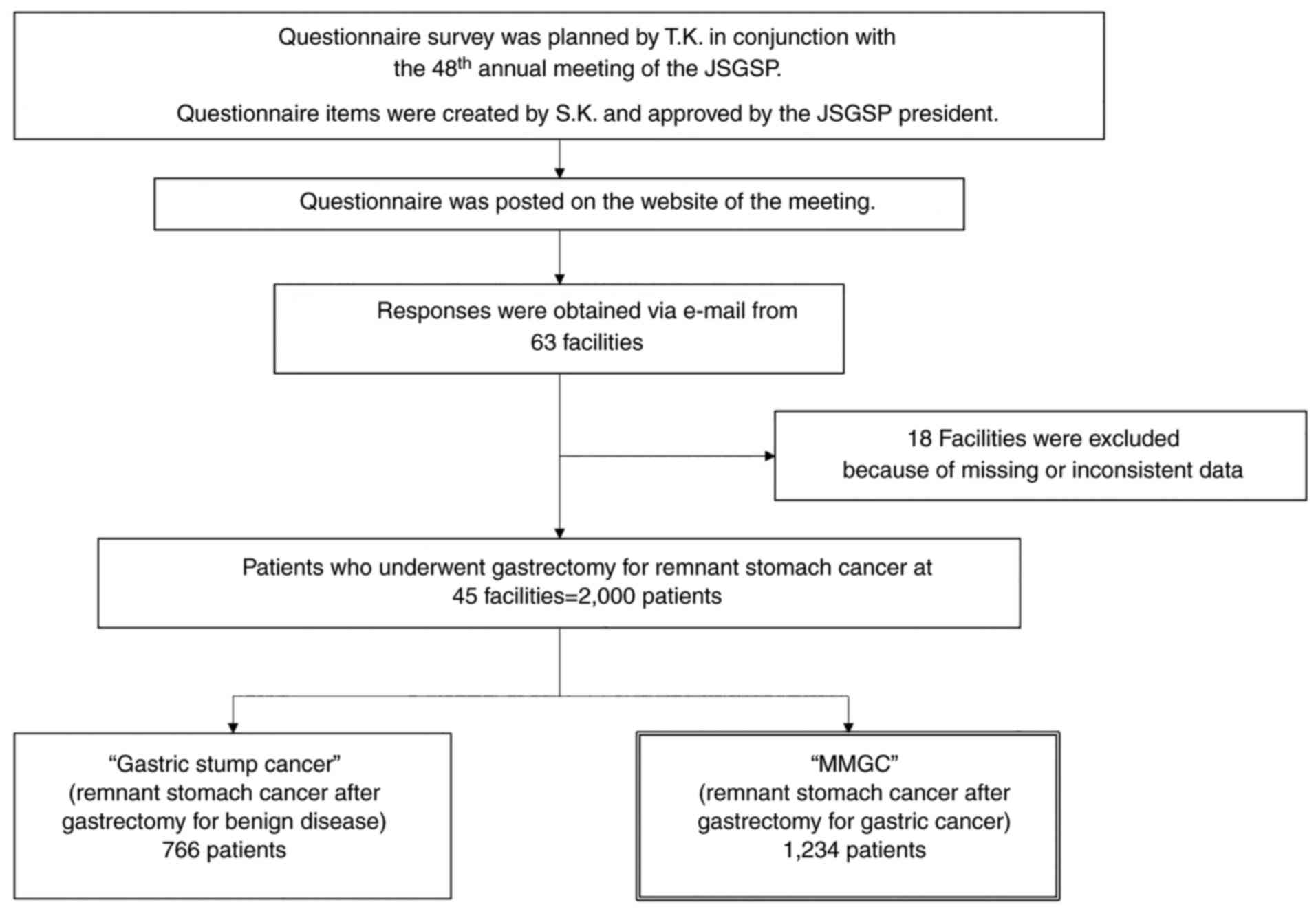
At the time of posting of the questionnaire on the
website, 204 facilities belonged to the JSGSP. Questionnaire
responses were obtained from 63 institutions; responses from 18
institutions were excluded due to missing or inconsistent data.
Thus, subsequent analyses were performed using the data from 45
institutions, which are provided in the Acknowledgements section
with the names of the responsible contributors (Fig. 1).
Between 2003 and 2017, gastrectomy for remnant
stomach cancer was performed in 2,000 patients, with MMGC
accounting for 61.7% (1,234 patients) (Fig. 1). Table I summarizes the number of each type
of gastrectomy and reconstruction procedure performed for the
initial gastrectomy among patients with MMGC. DG was the most
frequent procedure, accounting for 76.4% (943 cases), followed by
proximal gastrectomy (PG) (12.2%, 150 cases) and pylorus-preserving
gastrectomy (PPG) (6.4%, 79 cases). B-I was most frequently
performed after DG (78.5%, 712/943 cases), followed by B-II (14.6%,
138/943 cases) and R-Y (8.8%, 83/943 cases). In terms of PG for
MMGC, most patients underwent jejunal interposition (JI) (45.3%,
68/150 cases) or esophagogastric anastomosis (EG) (42.7%, 64/150
cases) (Table I).
 | Table INumber of each type of gastrectomy
performed in the initial gastrectomy among patients with
metachronous multiple gastric cancers (n=1,234). |
Table I
Number of each type of gastrectomy
performed in the initial gastrectomy among patients with
metachronous multiple gastric cancers (n=1,234).
| Type of
surgery/reconstruction or variant | n |
|---|
| DG | 943 |
|
B-I | 712 |
|
B-II | 138 |
|
R-Y | 83 |
|
Others | 10 |
| PG | 150 |
|
JI | 68 |
|
EG | 64 |
|
DT | 13 |
|
Others | 5 |
| PPG | 79 |
| SG | 10 |
| LR | 5 |
Type of gastrectomy or reconstruction
procedure and the time interval between the initial gastrectomy and
treatment for MMGC
Fig. 2A summarizes
the type of initial gastrectomy according to the time interval
between the initial gastrectomy and treatment for MMGC. PPG
accounted for only 3.6% (20/557) of the patients who underwent
surgery for MMGC ≥10 years from initial gastrectomy, while PPG
accounted for 10.1% (40/396) of the patients who underwent surgery
for MMGC within 5 years after initial gastrectomy.
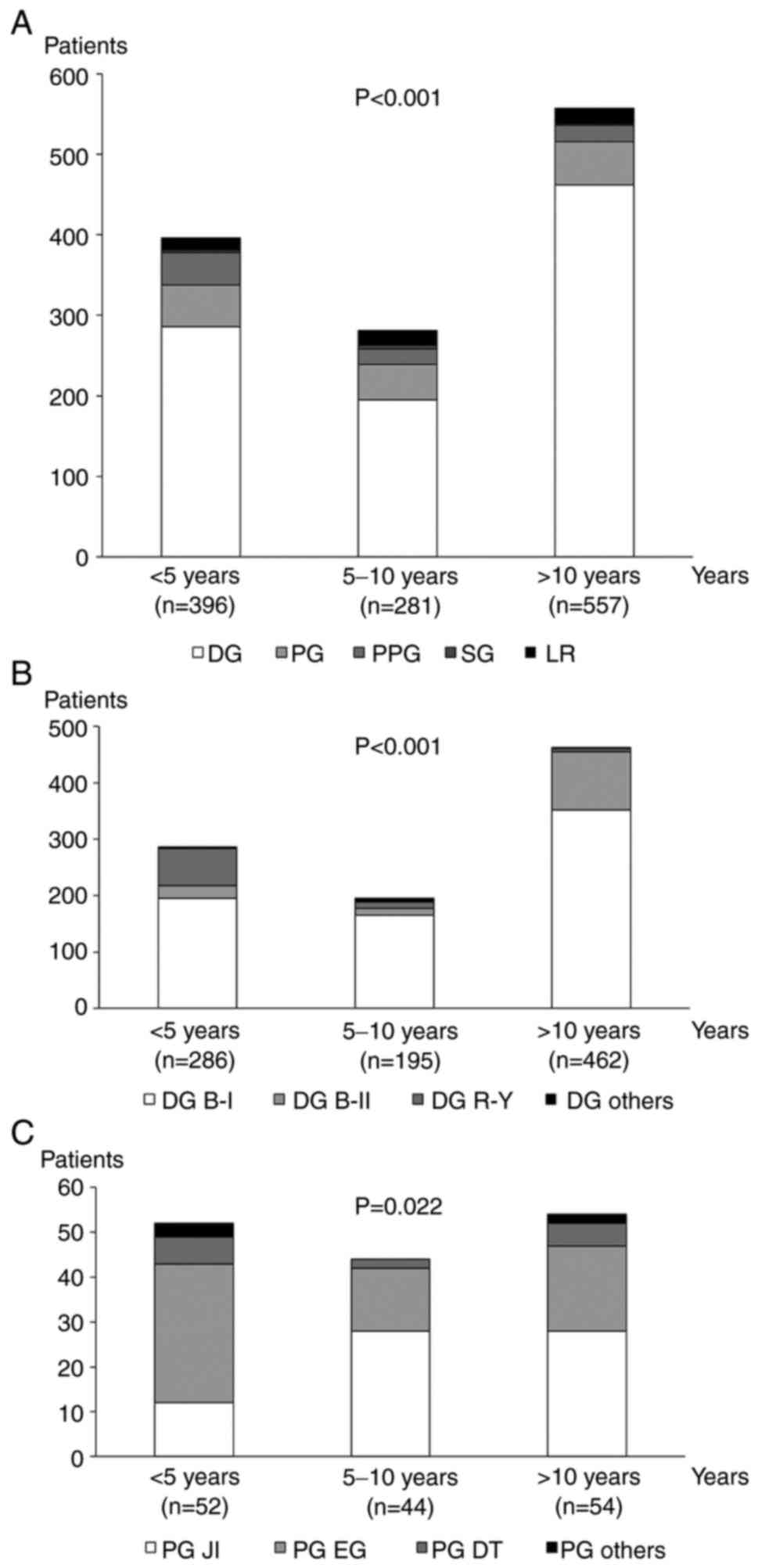 | Figure 2Type of initial gastrectomy and
reconstruction procedures after DG and PG according to the time
interval between the initial gastrectomy and treatment of MMGC. (A)
Types of initial gastrectomy differed depending on the interval
between the initial gastrectomy and treatment for MMGC.
Reconstruction procedures after (B) DG and (C) PG also differed
depending on the interval between the initial gastrectomy and
treatment for MMGC. MMGC, metachronous multiple gastric cancer; DG,
distal gastrectomy; PG, proximal gastrectomy; B-I, Billroth-I;
B-II, Billroth-II; R-Y, Roux-en Y; JI, jejunal interposition; EG,
esophagogastrostomy; DT, double tract; PG others, PG with other
reconstruction; PPG, pylorus-preserving gastrectomy; SG, segmental
gastrectomy; LR, local resection. |
The types of reconstruction procedure in the initial
DG and PG according to the time interval between the initial
gastrectomy and treatment for MMGC are presented in Fig. 2B and C, respectively. B-II accounted for 22.3%
(103/462) of the patients who underwent surgery for MMGC ≥10 years
from initial DG, while B-II accounted for only 8.0% (23/286) of the
patients who underwent surgery for MMGC within 5 years after
initial DG. Conversely, R-Y accounted for only 1.3% (6/462) of the
patients who underwent surgery for MMGC ≥10 years from initial DG
and 21.7% (65/286) of patients who underwent surgery for MMGC
within 5 years after initial DG. Similarly, the proportion of each
reconstruction procedure differed according to the time interval
between initial PG and treatment for MMGC (Fig. 2C). The distribution of the types of
gastrectomy (P<0.001; Fig. 2A)
or reconstruction procedures (P<0.001; Fig. 2B and P=0.022; Fig. 2C) differed significantly according
to the time interval between the initial gastrectomy and treatment
for MMGC.
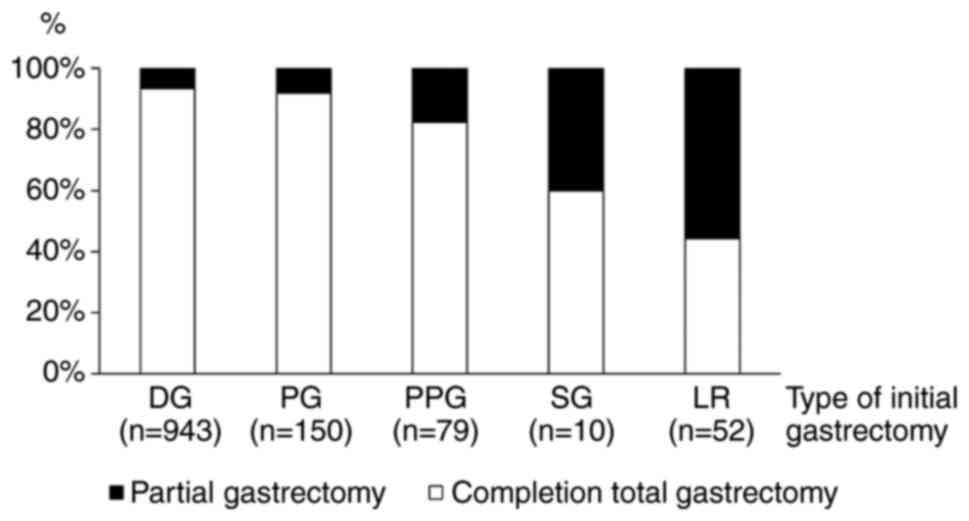
Surgical procedures for MMGC
Fig. 3 summarizes
the proportion of performed procedures for MMGC after each type of
initial gastrectomy. The proportion of partial gastrectomy
increased in accordance with the size of the remnant stomach after
the initial gastrectomy (Fig.
3).
Discussion
The present multi-institutional questionnaire survey
successfully collected data from >1,000 patients with MMGC and
is thus far the largest survey on MMGC. Facility members of the
JSGSP are dedicated to gastric cancer surgery and perform a certain
number of gastrectomies with strict follow-up. Therefore, the data
obtained from these facilities are reliable and may be regarded as
representing the actual status of MMGC in Japan. This survey
included a much larger number of MMGC patients compared with
another cohort study performed concurrently. In the present study,
1,234 patients with MMGC who underwent gastrectomy were
retrospectively analyzed, while another prospective cohort study
comprised 718 patients who developed MMGC and received any
treatment during the follow-up period, including 386 patients who
underwent surgery (8). In other
words, the present study included more than three times the number
of patients with MMGC who underwent surgery.
The present study provides the following novel
findings: i) A current overview of MMGC in Japan; ii) information
on MMGC after PG and PPG, which are relatively new
function-preserving surgeries; and iii) a definite correlation
between the time interval from the initial gastrectomy until the
detection of MMGC and the types of gastrectomy or reconstruction
applied in the initial surgery. In addition, regarding the
procedure performed for MMGC, it was indicated that the proportion
of CTGs decreased as the size of the remnant stomach increased. As
part of the survey, the present study revealed that the type of
gastrectomy and reconstruction procedure used in the initial
gastrectomy differed in accordance with the interval length between
the initial gastrectomy and the detection of MMGC.
PPG was first described by Maki et al
(9) in 1967 and was developed as a
surgical approach for benign peptic ulcer that aimed to prevent
dumping syndrome by preserving the pyloric ring. The feasibility of
PPG as a function-preserving gastrectomy for early gastric cancer
was first reported by Kodama and Koyama (10) in 1991 and became prevalent in
combination with the generalization of the concept of minimally
invasive surgery. A report from The Japanese Gastric Cancer
Association Registration Committee, which summarized the treatment
results of 8,308 gastric cancer patients treated at 113 major
Japanese hospitals in 1991, did not report any PPG cases, while PPG
accounted for 3.4% (4,026/118,367) of gastrectomies performed
between 2001 and 2007 (11,12).
As patients who underwent surgery for MMGC between 2003 and 2017
were reported in the present survey, the observed low proportion of
patients with MMGC whose interval between the initial PPG and
detection of MMGC was ≥10 years appeared reasonable.
Several epidemiological studies have reported that
B-II in gastrectomy for benign peptic ulcer is more highly
correlated with gastric stump carcinoma than B-I and the incidence
increases ≥20 years from initial gastrectomy (13,14).
Previous studies reported that reflux of bile and pancreatic juice
may be important factors for cancer development in the remnant
stomach. Studies also assumed that atypical hyperplasia, called
‘gastritis cystica polyposa’, proximal to the gastrojejunal
anastomosis in B-II reconstruction caused by reflux, was associated
with gastric stump carcinoma (15-18),
although a consensus has not been reached. However, several animal
model experiments provided evidence supporting that pancreatic
juice alone or in combination with bile acids, the main component
duodenal juice, may cause carcinogenesis in the remnant stomach
(19). These characteristics, such
as the higher incidence of remnant stomach cancer after B-II and
the longer interval from initial gastrectomy to detecting remnant
stomach cancer, may be applicable to MMGC after B-II. However, it
was not possible to prove this in the present study, as the number
of patients who underwent B-II in each period at participating
facilities was unknown and because the most probable cause of the
observed difference in the interval is the trend in the use of each
reconstruction procedure, as with that for the type of gastrectomy.
R-Y reconstruction in DG is a relatively new method compared with
B-I or B-II (2). Although R-Y was
invented and reported by Roux (20) in 1893, the use of R-Y in DG became
prevalent and its feasibility was published in the 2010s in Japan
(21-25).
Therefore, it is also reasonable that the proportion of R-Y
procedures increased over time. The Japanese Society for the Study
of Postoperative Morbidity after Gastrectomy performed a
questionnaire survey in 2010 to reveal the status of reconstruction
after gastrectomy. The results supported an increase in R-Y in
recent years, as the most common reconstruction method after DG was
B-I in 112 (77%) of the 145 responding institutions, R-Y in 30
(21%) and B-II in one (0.7%), in 2010(26).
Another advantage of the present survey is that a
considerable number of patients with MMGC after PG were reported.
Significant differences in the proportion of each reconstruction
procedure according to the time interval between initial PG and
treatment for MMGC were found and the reason for the difference may
be explained as follows: JI is applicable to the relatively small
remnant stomach and may theoretically prevent reflux esophagitis,
but it requires procedures that are relatively complex (27,28).
EG has also been attempted in PG, as the procedure is much simpler
than JI (29,30). A major drawback of EG is
gastroesophageal reflux after surgery. To compensate for this, the
hand-sewn double-flap technique and other techniques using linear
staples have been introduced and a better postoperative quality of
life was increasingly reported in the late 2010s (31-34).
Double-tract reconstruction (DT) after PG is also an emerging
procedure, with which surgeons are more familiar than JI, as only
jejuno-gastric anastomosis is supposed to be added to R-Y
reconstruction (35-38).
A Japanese nationwide survey performed in 2010 indicated that the
most preferred reconstruction approach after PG was EG (48% of the
responding institutions) followed by JI (28%) and DT (13%)
(26). The small proportion of
MMGC detected within 5 years after JI in the present survey may be
explained by the assumption that JI became common in the 1990s but
has been replaced by DT in the current century.
Although CTG is the standard surgery for advanced
cancer in the remnant stomach, partial gastrectomy may be applied
to early cancer (39). The current
survey clearly demonstrated that the possibility of avoiding CTG
depends on the size of the remnant stomach.
There are several limitations to the current survey
that should be addressed. First, the retrospective nature of the
data collection is an issue, and individual patient data, including
the detailed surgical treatments of the initial gastrectomy and
MMGC, were not collected to protect patient privacy. In the present
survey, each institution was requested to provide the number of
patients who underwent surgery for MMGC between 2003 and 2017.
There are potential risks of selection bias due to the
retrospective nature of the data collection, although the
participating facilities were requested to report all MMGC patients
who underwent surgery during this period. The 15-year inclusion
period and the lack of published literature on the time trend of
the type of gastrectomy or reconstruction procedure made it
difficult to assess the correlation between the time trend of the
type of gastrectomy or reconstruction procedure and the time
interval from the initial gastrectomy until the detection of MMGC.
Another limitation is the lack of universality of the results of
the study. R-Y is mainly performed in Western countries, while B-I
or B-II is rarely performed following DG due to the potential risk
of gastroesophageal reflux in obese individuals. In addition, PG or
PPG, which are indicated for early gastric cancer, are also
seldomly performed in the West, as most patients with gastric
cancer have advanced disease. The vast majority of the patients
included in the present study were thought to be Japanese and
information on ethnicity was not collected in this study.
Therefore, the results of the present study are not universal and
are specific to Japanese patients. Furthermore, the type of
gastrectomy depends on several clinicopathological factors that may
be confounding factors, which affect the time interval from the
initial gastrectomy to the detection of MMGC. However, the
questionnaire used in the present study only included the number of
patients subjected to each procedure and the time interval between
the initial gastrectomy and the detection of MMGC, as this survey
comprised >1,000 patients and it was necessary to simplify the
questionnaire. Hence, the retrospective nature is one of the
limitations of the current study.
A large-scale prospective study is esteemed to
elucidate factors other than the time trend of gastrectomy or
reconstruction procedure that may correlate with the time interval
from initial gastrectomy until the detection of MMGC.
Despite the limitations discussed above, the present
multi-institutional questionnaire survey study demonstrated that
the type of initial gastrectomy and reconstruction procedure
differs according to the time interval between initial gastrectomy
and the detection of MMGC. The proportion of CTG decreased as the
size of the remnant stomach increased.
Supplementary Material
Questionnaire used for the analysis
(English translation).
Acknowledgements
Data were collected from 45 facilities. The names of
these facilities and the responsible persons involved in data
collection are as follows: Mr. Koshi Kumagai (Department of
Gastroenterological Surgery, Cancer Institute Hospital of JFCR,
Tokyo Japan), Mr. Masayuki Kano (Department of Frontier Surgery,
Chiba University, Chiba, Japan), Mr. Kinro Sasaki (First Department
of Surgery, Dokkyo Medical University, Tochigi, Japan), Mr.
Norimichi Kogure (Department of General Surgical Science, Gunma
University Graduate School of Medicine, Gunma, Japan), Mr. Takahiro
Muroya (Department of Gastrointestinal Surgery, Hirosaki University
Graduate School of Medicine, Aomori, Japan), Mr. Hidemaro Yoshiba
(Department of Surgery, Japanese Red Cross Fukui Hospital, Fukui,
Japan), Mr. Naoki Kakihara (Department of Surgery, Japanese Red
Cross Kyoto Daini Hospital, Kyoto, Japan), Mr. Tatsushi Shimokuni
(Department of Surgery, JCHO Sapporo Hokushin Medical Hospital,
Hokkaido, Japan), Mr. Takaaki Arigami (Department of
Onco-Biological Surgery, Kagoshima University Graduate School of
Medical and Dental Sciences, Kagoshima, Japan), Mr. Hiroshi
Kusanagi (Department of Gastroenterological Surgery, Kameda Medical
Center, Chiba, Japan), Professor Shinichi Kinami (Department of
Surgical Oncology, Kanazawa Medical University, Ishikawa, Japan),
Mr. Takahisa Yamaguchi (Department of Gastroenterological Surgery
and Division of Cancer Medicine, Graduate School of Medical
Science, Kanazawa University, Ishikawa, Japan), Ms. Marie Washio
(Department of Upper Gastrointestinal Surgery, Kitasato University
School of Medicine, Kanagawa, Japan), Mr. Kojiro Eto (Department of
Gastroenterological Surgery, Graduate School of Medical Sciences,
Kumamoto University, Kumamoto, Japan), Ms. Hiromi Yasuda
(Departments of Gastrointestinal and Pediatric Surgery, Division of
Reparative Medicine, Institute of Life Sciences, Mie University
Graduate School of Medicine, Mie, Japan), Mr. Hiroyuki Sagawa
(Department of Gastroenterological Surgery, Nagoya City University
Graduate School of Medical Sciences, Aichi, Japan), Ms. Chie Tanaka
(Department of Gastroenterological Surgery, Nagoya University
Graduate School of Medicine, Aichi, Japan), Mr. Sohei Matsumoto
(Department of Surgery, Nara Medical University, Nara, Japan), Mr.
Akio Kaito (Gastric Surgery Division, National Cancer Center
Hospital East, Chiba, Japan), Mr. Masaki Aizawa (Department of
Digestive Surgery, Niigata Cancer Center Hospital, Niigata, Japan),
Mr. Takuya Noguchi (Department of Gastroenterological Surgery, Oita
Kouseiren Tsurumi Hospital, Oita, Japan), Mr. Hiroshi Isozaki
(Department of Surgery, Oomoto Hospital, Okayama, Japan), Mr. Ryo
Tanaka (Department of General and Gastroenterological Surgery,
Osaka Medical College, Osaka, Japan), Mr. Yoshitaka Toyomasu
(Department of Digestive Tract and General Surgery, Saitama Medical
Center, Saitama Medical University, Saitama, Japan), Professor
Shinichi Sakuramoto (Division of Gastroenterological Surgery,
Saitama Medical University International Medical Center, Saitama,
Japan), Mr. Satoshi Kamiya (Department of Gastric Surgery, Shizuoka
Cancer Center, Shizuoka, Japan), Mr. Hirokazu Yamaguchi (Department
of Gastroenterological Surgery, Showa General Hospital, Tokyo,
Japan), Mr. Kimiyasu Yamazaki (Department of General and
Gastroenterological Surgery, Showa University School of Medicine,
Tokyo, Japan), Mr. Shinya Mikami (Division of Gastroenterological
and General Surgery, St. Marianna University School of Medicine,
Kanagawa, Japan), Mr. Takashi Kiyokawa (Department of Surgery,
Teikyo University School of Medicine, Tokyo, Japan), Mr. Muneharu
Fujisaki (Department of Surgery, Jikei University School of
Medicine, Tokyo, Japan), Professor Hiroharu Yamashita (Department
of Gastrointestinal Surgery, Graduate School of Medicine, The
University of Tokyo, Tokyo, Japan), Mr. Yoko Oshima (Department of
Surgery, School of Medicine, Toho University, Tokyo, Japan),
Professor Eiji Nomura (Department of Surgery, Tokai University
Hachioji Hospital, Tokyo, Japan), Mr. Kenta Kobayashi (Department
of Gastric Surgery, Tokyo Medical and Dental University Hospital,
Tokyo, Japan), Mr. Takeshi Suda (Department of Gastrointestinal and
Pediatric Surgery, Tokyo Medical University, Tokyo, Japan), Mr.
Haruhiko Cho (Department of Gastric Surgery, Tokyo Metropolitan
Cancer and Infectious Disease Center, Komagome Hospital, Tokyo,
Japan), Mr. Isaya Hashimoto (Department of Surgery and Science,
Faculty of Medicine, Academic Assembly, University of Toyama,
Toyama, Japan), Mr. Takeshi Kubota (Division of Digestive Surgery,
Department of Surgery, Kyoto Prefectural University of Medicine,
Kyoto, Japan), Mr. Makoto Toda (Department of Surgery, Yamagata
Prefectural Central Hospital, Yamagata, Japan), Professor Osamu
Hachiya (Department of Gastroenterology, General, Breast and
Thyroid Surgery, Faculty of Medicine, Yamagata University,
Yamagata, Japan), Mr. Takashi Kosaka (Department of
Gastroenterological Surgery, Graduate School of Medicine, Yokohama
City University, Kanagawa, Japan), Mr. Hiroshi Miyamoto (Department
of Surgery, Gastroenterological Center, Yokohama City University,
Kanagawa, Japan), Mr. Masazumi Takahashi (Department of Surgery,
Yokohama Municipal Citizen's Hospital, Kanagawa, Japan).
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SKi and TKo designed the study. KK, MA, SKa, TT,
MTo, HC, MTa and TKu performed data acquisition, data analysis and
interpretation. KK and SKi checked and approved the authenticity of
the raw data and prepared the manuscript. SKi, SWL and MO revised
the paper for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the institutional
review board of Kanazawa Medical University (Ishikawa, Japan; trial
no. I267). This study was conducted in accordance with the Ethical
Guidelines of the Japan Ministry of Health, Labour and Welfare for
Medical and Health Research Involving Human Subjects and conformed
to the provisions of the Declaration of Helsinki. The requirement
to obtain informed consent was waived by the institutional review
board of Kanazawa Medical University (Ishikawa, Japan; approval no.
I267).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahn HS, Kim JW, Yoo MW, Park DJ, Lee HJ,
Lee KU and Yang HK: Clinicopathological features and surgical
outcomes of patients with remnant gastric cancer after a distal
gastrectomy. Ann Surg Oncol. 15:1632–1639. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tanigawa N, Nomura E, Lee SW, Kaminishi M,
Sugiyama M, Aikou T and Kitajima M: Society for the Study of
Postoperative Morbidity after Gastrectomy. Current state of gastric
stump carcinoma in Japan: Based on the results of a nationwide
survey. World J Surg. 34:1540–1547. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Komatsu S, Ichikawa D, Okamoto K, Ikoma D,
Tsujiura M, Nishimura Y, Murayama Y, Shiozaki A, Ikoma H, Kuriu Y,
et al: Progression of remnant gastric cancer is associated with
duration of follow-up following distal gastrectomy. World J
Gastroenterol. 18:2832–2836. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Takeno S, Noguchi T, Kimura Y, Fujiwara S,
Kubo N and Kawahara K: Early and late gastric cancer arising in the
remnant stomach after distal gastrectomy. Eur J Surg Oncol.
32:1191–1194. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ohashi M, Katai H, Fukagawa T, Gotoda T,
Sano T and Sasako M: Cancer of the gastric stump following distal
gastrectomy for cancer. Br J Surg. 94:92–95. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Ministry of Health Labour and Welfare:
Ethical Guidelines for Medical and Health Research Involving Human
Subjects. Available from: https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf,
2015.
|
|
7
|
World Medical Association. World Medical
Association Declaration Of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kinami S, Aizawa M, Yamashita H, Kumagai
K, Kamiya S, Toda M, Takahata T, Fujisaki M, Miyamoto H, Kusanagi
H, et al: The incidences of metachronous multiple gastric cancer
after various types of gastrectomy: Analysis of data from a
nationwide Japanese survey. Gastric Cancer. 24:22–30.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Maki T, Shiratori T, Hatafuku T and
Sugawara K: Pylorus-preserving gastrectomy as an improved operation
for gastric ulcer. Surgery. 61:838–845. 1967.PubMed/NCBI
|
|
10
|
Kodama M and Koyama K: Indications for
pylorus preserving gastrectomy for early gastric cancer located in
the middle third of the stomach. World J Surg. 15:628–634.
1991.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Japanese Gastric Cancer Association
Registration Committee. Maruyama K, Kaminishi M, Hayashi K, Isobe
Y, Honda I, Katai H, Arai K, Kodera Y and Nashimoto A: Gastric
cancer treated in 1991 in Japan: Data analysis of nationwide
registry. Gastric Cancer. 9:51–66. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Katai H, Ishikawa T, Akazawa K, Fukagawa
T, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, et
al: Registration Committee of the Japanese Gastric Cancer
Association: Optimal extent of lymph node dissection for remnant
advanced gastric carcinoma after distal gastrectomy: a
retrospective analysis of more than 3000 patients from the
nationwide registry of the Japanese Gastric Cancer Association.
Gastric Cancer. 23:1091–1101. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lundegardh G, Adami HO, Helmick C, Zack M
and Meirik O: Stomach cancer after partial gastrectomy for benign
ulcer disease. N Engl J Med. 319:195–200. 1988.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Toftgaard C: Gastric cancer after peptic
ulcer surgery. A historic prospective cohort investigation. Ann
Surg. 210:159–164. 1989.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qizilbash AH: Gastritis cystica and
carcinoma arising in old gastrojejunostomy stoma. Can Med Assoc J.
112:1432–1433. 1975.PubMed/NCBI
|
|
16
|
Bogomoletz WV, Potet F, Barge J, Molas G
and Qizilbash AH: Pathological features and mucin histochemistry of
primary gastric stump carcinoma associated with gastritis cystica
polyposa. A study of six cases. Am J Surg Pathol. 9:401–410.
1985.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aoyagi K, Koufuji K, Yano S, Murakami N,
Terasaki Y, Yamasaki Y, Takeda J, Tanaka M and Shirouzu K: Two
cases of cancer in the remnant stomach derived from gastritis
cystica polyposa. Kurume Med J. 47:243–248. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matsuda I, Konno H, Maruo Y, Tanaka T,
Baba M, Nishino N, Nakamura S, Baba S and Kino I: A case of triple
early gastric cancer in the remnant stomach. Am J Gastroenterol.
90:1016–1018. 1995.PubMed/NCBI
|
|
19
|
Kondo K: Duodenogastric reflux and gastric
stump carcinoma. Gastric Cancer. 5:16–22. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hutchison RL and Hutchison AL: Cesar Roux
and his original 1893 paper. Obes Surg. 20:953–956. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ,
Kim HH, Yang HK, Kim N and Lee WW: What is the best reconstruction
method after distal gastrectomy for gastric cancer? Surg Endosc.
26:1539–1547. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hirao M, Takiguchi S, Imamura H, Yamamoto
K, Kurokawa Y, Fujita J, Kobayashi K, Kimura Y, Mori M and Doki Y:
Osaka University Clinical Research Group for Gastroenterological
Study. Comparison of Billroth I and Roux-en-Y reconstruction after
distal gastrectomy for gastric cancer: One-year postoperative
effects assessed by a multi-institutional RCT. Ann Surg Oncol.
20:1591–1597. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nakamura M, Nakamori M, Ojima T, Iwahashi
M, Horiuchi T, Kobayashi Y, Yamade N, Shimada K, Oka M and Yamaue
H: Randomized clinical trial comparing long-term quality of life
for Billroth I versus Roux-en-Y reconstruction after distal
gastrectomy for gastric cancer. Br J Surg. 103:337–347.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang D, He L, Tong WH, Jia ZF, Su TR and
Wang Q: Randomized controlled trial of uncut Roux-en-Y vs. Billroth
II reconstruction after distal gastrectomy for gastric cancer:
Which technique is better for avoiding biliary reflux and
gastritis? World J Gastroenterol. 23:6350–6356. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang K, Zhang WH, Liu K, Chen XZ, Zhou ZG
and Hu JK: Comparison of quality of life between Billroth-capital
I, Ukrainian and Roux-en-Y anastomosis after distal gastrectomy for
gastric cancer: A randomized controlled trial. Sci Rep.
7(11245)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kumagai K, Shimizu K, Yokoyama N, Aida S,
Arima S and Aikou T: Japanese Society for the Study of
Postoperative Morbidity after Gastrectomy. Questionnaire survey
regarding the current status and controversial issues concerning
reconstruction after gastrectomy in Japan. Surg Today. 42:411–418.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Adachi Y, Aramaki M, Shiraishi N, Shimoda
K, Yasuda K and Kitano S: Long-term survival after perforation of
advanced gastric cancer: Case report and review of the literature.
Gastric Cancer. 1:80–83. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shiraishi N, Hirose R, Morimoto A, Kawano
K, Adachi Y and Kitano S: Gastric tube reconstruction prevented
esophageal reflux after proximal gastrectomy. Gastric Cancer.
1:78–79. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hiki N, Fukunaga T, Yamaguchi T, Nunobe S,
Tokunaga M, Ohyama S, Seto Y and Muto T: Laparoscopic
esophagogastric circular stapled anastomosis: A modified technique
to protect the esophagus. Gastric Cancer. 10:181–186.
2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Takeuchi H, Oyama T, Kamiya S, Nakamura R,
Takahashi T, Wada N, Saikawa Y and Kitagawa Y: Laparoscopy-assisted
proximal gastrectomy with sentinel node mapping for early gastric
cancer. World J Surg. 35:2463–2471. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kuroda S, Nishizaki M, Kikuchi S, Noma K,
Tanabe S, Kagawa S, Shirakawa Y and Fujiwara T: Double-Flap
technique as an antireflux procedure in esophagogastrostomy after
proximal gastrectomy. J Am Coll Surg. 223:e7–e13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Muraoka A, Kobayashi M and Kokudo Y:
Laparoscopy-assisted proximal gastrectomy with the hinged double
flap method. World J Surg. 40:2419–2424. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hayami M, Hiki N, Nunobe S, Mine S, Ohashi
M, Kumagai K, Ida S, Watanabe M, Sano T and Yamaguchi T: Clinical
outcomes and evaluation of laparoscopic proximal gastrectomy with
double-flap technique for early gastric cancer in the upper third
of the stomach. Ann Surg Oncol. 24:1635–1642. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yamashita Y, Yamamoto A, Tamamori Y,
Yoshii M and Nishiguchi Y: Side overlap esophagogastrostomy to
prevent reflux after proximal gastrectomy. Gastric Cancer.
20:728–735. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nakajima K, Kawano M, Kinami S, Fujimura
T, Miwa K and Tonami N: Dual-radionuclide simultaneous gastric
emptying and bile transit study after gastric surgery with
double-tract reconstruction. Ann Nucl Med. 19:185–191.
2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nomura E, Lee SW, Tokuhara T, Kawai M and
Uchiyama K: Functional outcomes according to the size of the
gastric remnant and type of reconstruction following open and
laparoscopic proximal gastrectomy for gastric cancer.
Hepatogastroenterology. 59:1677–1681. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Nomura E, Lee SW, Kawai M, Yamazaki M,
Nabeshima K, Nakamura K and Uchiyama K: Functional outcomes by
reconstruction technique following laparoscopic proximal
gastrectomy for gastric cancer: Double tract versus jejunal
interposition. World J Surg Oncol. 12(20)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ahn SH, Jung DH, Son SY, Lee CM, Park DJ
and Kim HH: Laparoscopic double-tract proximal gastrectomy for
proximal early gastric cancer. Gastric Cancer. 17:562–570.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Goto H, Kanaji S, Otsubo D, Oshikiri T,
Yamamoto M, Nakamura T, Suzuki S, Fujino Y, Tominaga M and Kakeji
Y: Comparison of total versus subtotal gastrectomy for remnant
gastric cancer. Langenbecks Arch Surg. 404:753–760. 2019.PubMed/NCBI View Article : Google Scholar
|