Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
condition in which fatty liver is diagnosed histologically or using
imaging in the absence of other liver diseases, such as alcoholic
liver disease (ALD). Considering that the onset of NAFLD is a
consequence of obesity, diabetes, dyslipidemia, hypertension or
other metabolic disorders, it is currently considered as a liver
phenotype of metabolic syndrome, and, in recent years, a concept
known as metabolic-associated fatty liver disease (MAFLD) has been
proposed (1).
NAFLD progression is histologically characterized by
large hepatic lipid droplets and is classified into non-alcoholic
fatty liver, in which the condition hardly progresses, and
non-alcoholic steatohepatitis (NASH), in which the condition
progresses and may subsequently lead to cirrhosis and liver
cancer.
The global prevalence of NAFLD increased from 20.1%
in 2000-2005 to 23.8% in 2006-2010 and 26.8% in 2011-2015(2). Changes in the prevalence of NASH are
not fully known, but it is thought to increase in parallel with an
increase in NAFLD prevalence. In Japan, there were 660,000 patients
with NAFLD and advanced fibrosis of stage 3 or higher in 2016, and
this is expected to increase to 990,000 by 2030(3).
The rate of liver carcinogenesis from NAFLD is as
low as 0.44/1,000 person-years (2); however, the risk reportedly increases
with the progression of liver pathology to 5.29/1,000 person-years
for NASH and 20/1,000 person-years for cirrhosis in Japan (4). Although this is low compared to the
carcinogenesis rate from other liver diseases, as aforementioned,
the prevalence of NAFLD is extremely high, and a Japanese
nationwide survey on patients with hepatocellular carcinoma (HCC)
revealed that the proportion of patients with non-viral etiologies,
including NAFLD, had increased from 10.0% in 1991 to 32.5% in 2015,
and is continually increasing (5).
Regarding prognosis, liver disease-related mortality
in patients with NAFLD increases with the progression of liver
fibrosis (6), with liver fibrosis
being reported as the factor most significantly associated with
prognosis among other pathological findings in NAFLD (7).
Serine palmitoyltransferase (SPT) catalyzes fatty
acid metabolism, particularly sphingolipid synthesis, and serine
palmitoyltransferase long chain base subunit 3 (SPTLC3) has
recently been identified as its catalytic subunit; however, its
association with and contribution to liver disease remain unclear.
We previously demonstrated that SPTLC3 was highly expressed in the
liver tissue in a mouse model of NASH, which frequently displays
hepatocellular carcinoma (HCC) (8).
Herein, the present study aimed to analyze the
association between SPTLC3 and NAFLD pathological progression, as
well as liver carcinogenesis, by examining SPTLC3 expression in
human liver cancer cell lines and human serum/liver tissues.
Materials and methods
Patients and sample collection
In total, 99 patients diagnosed with NAFLD (66
without HCC and 33 with HCC) and 6 healthy volunteers (HVs) were
recruited at the Digestive and Lifestyle Diseases, Kagoshima
University Graduate School of Medical and Dental Sciences between
August 2016 and June 2020. The patient population consisted of 27
men and 62 women with a median age of 58 years (range, 22-86
years). NAFLD was diagnosed based on the clinical guidelines of the
American Association for the Study of Liver Diseases (9) and The European Association for the
Study of the Liver (10). Patient
inclusion criteria were as follows: i) 5% or more of liver cells
containing lipid droplets detected by liver biopsy, or evidence of
fatty liver on using ultrasound (US) or computed tomography (CT)
imaging; and ii) daily alcohol intake of <30 g for men and
<20 g for women and negativity for hepatitis virus markers to
exclude viral liver disease and autoimmune liver diseases. Those
who met both i) and ii) were diagnosed with NAFLD (9,10).
The diagnosis of HCC was made by a radiologist and a hepatologist
using contrast-enhanced CT or contrast-enhanced MRI. Subjects were
considered as HVs if they had no history of lifestyle-related
diseases, including NAFLD. Serum was collected from both the
NAFL/NASH groups (without HCC) and the HCC group on the day of the
first visit to the Digestive and Lifestyle Diseases, in the blood
laboratory of the Kagoshima University Graduate School of Medical
and Dental Sciences, on the day before the biopsy and before the
intervention, in the period between August 2016 and June 2020, in
order to evaluate SPTLC3 concentration using ELISA, and the levels
of blood biochemical parameters [platelet count, aspartate
aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl
transferase, total bilirubin, albumin, prothrombin index,
low-density lipoprotein cholesterol (LDL-Chol), triglyceride,
glucose, HbA1c, hyaluronic acid, α-fetoprotein (AFP) and
des-γ-carboxy prothrombin (DCP)] were also analyzed. Blood
biochemical parameters were evaluated via routine laboratory tests
that were conducted at the same time as the SPTLC3 assay.
Furthermore, as a combination of clinical and routine laboratory
parameters of liver fibrosis, fibrosis index based on the four
factors (FIB-4 index) [age (year) x AST (U/l)/{platelet count
(1x109/l) x √ALT (U/l)}] (11-13)
and AST to platelet ratio index (APRI) [AST/upper limit of normal x
100)/platelet count] were calculated based on the aforementioned
blood biochemical parameters (13,14).
Non-tumor and tumor parts were collected from the surgical
specimens of patients with NAFLD and HCC.
In addition to the patients mentioned earlier, serum
SPTLC3 levels were measured in 31 patients with hepatitis B virus
(HBV), 36 patients with hepatitis C virus (HCV) and 24 patients
with alcoholic liver disease (ALD). Patients positive for serum HBs
antigen were considered to have HBV; those positive for HCV
antibody were considered to have HCV; and those with daily alcohol
intake >60 g were classed as patients with ALD. The patients
with HBV, HCV and ALD consisted of patients both with and without
HCC at the time of the SPTLC3 assay. In the present study, the
SPTLC3 values were compared between both groups.
The present study was approved by the Ethics
Committee of Kagoshima University Hospital (approval no. 28-107)
and written informed consent was obtained from all the patients.
All procedures were performed in accordance with the World Medical
Association's Declaration of Helsinki.
Reverse transcription-quantitative
(RT-q)PCR
SPTLC3 expression levels were assessed in
human liver cancer cell lines and human liver tissues. HepG2, Huh7
and Hep3B cells (obtained from Sumitomo Dainippon Pharma Co., Ltd.)
were selected as the liver cancer cell lines; HT29 and HCT116 cells
(obtained from DS Pharma Biomedical Co., Ltd.) were selected as the
colorectal cancer cell lines; and Panc1 cells (obtained from DS
Pharma Biomedical Co., Ltd.) were selected as the pancreatic cancer
cell line. Regarding the liver tissues, non-tumor and tumor
sections were collected from the surgical specimens of patients
with NAFLD and HCC, as aforementioned. Total RNA was extracted from
cells and liver tissues using the TRIzol® reagent
(Thermo Fisher Scientific, Inc.). RNA purity was confirmed by
spectrophotometry, and A260/A280 ratios ranged from 1.9 to 2.1.
First-strand cDNA was synthesized from 500 ng of total RNA using a
PrimeScript RT Master Mix (Takara Bio Inc.) according to the
manufacturer's protocol. Real-time PCR was performed using TB Green
Premix Ex Taq II (Takara Bio Inc.) and the ABI Prism 7700 sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Data were collected and analyzed using the Step One Plus
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Relative gene expression values were calculated using the
comparative ΔΔCq method (15) and
the Cq values were normalized to those of β-actin. The PCR
conditions were as follows: Initial holding period at 95˚C for 30
sec, followed by 40 cycles of a two-step program consisting of
denaturation at 95˚C for 5 sec and annealing, and polymerization at
60˚C for 34 sec (16). The primer
sequences used in this study are provided in Table I.
 | Table IOligonucleotide sequence of primers
for quantitative reverse transcription-quantitative PCR. |
Table I
Oligonucleotide sequence of primers
for quantitative reverse transcription-quantitative PCR.
| Genes | Forward primer
(5'→3') | Reverse primer
(5'→3') |
|---|
| SPTLC3 |
GCTCGGTTTTGTGTTTCAGCGG |
TGCCGGGAATATTTCAGTTGCAAG |
| ACTB |
TGGCACCCAGCACAATGAA |
CTAAGTCATAGTCCGCCTAGAAGCA |
ELISA
SPTLC3 levels were assessed in the serum from
patients with NAFLD and HVs using the ELISA kit For Serine
Palmitoyltransferase, Long Chain Base Subunit 3 (SPTLC3) from
Cloud-Clone Corp. (cat. no. SEH136Hu) according to the
manufacturer's protocol.
Statistical analysis
Results are presented as the mean or median. At
least two repeated experiments were conducted. Statistical analyses
were performed using IBM SPSS version 23 (IBM Corp.). For the test
method, Fisher's exact test, Mann-Whitney U test, Wilcoxon's
signed-rank test, Spearman's rank correlation coefficient and
Tukey's HSD test were used. All possible models of binomial
logistic regression analysis were used for the multivariate
analysis. Items that were significant in the univariate analysis
were used as reference for the selection of possible variables.
Data were considered statistically significant when P<0.05.
Results
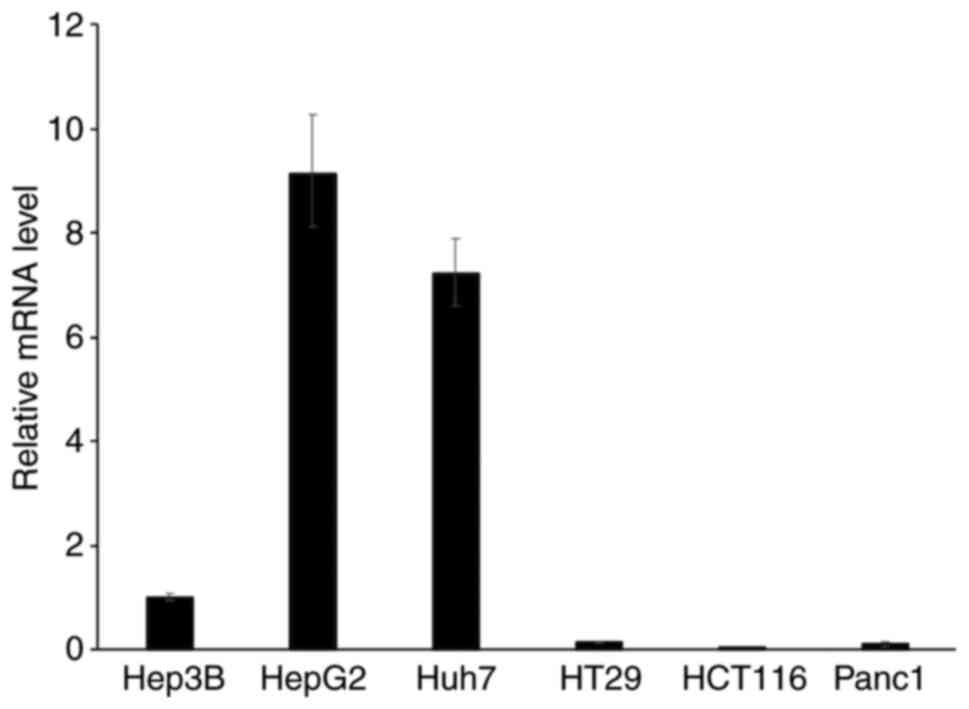
Human liver cancer cell lines express
high levels of SPTLC3
The expression of SPTLC3 was investigated in
cancer cell lines and found to be higher in HepG2, Huh7 and Hep3B
cells compared that in cell lines derived from the colon, rectum
and pancreas (Fig. 1).
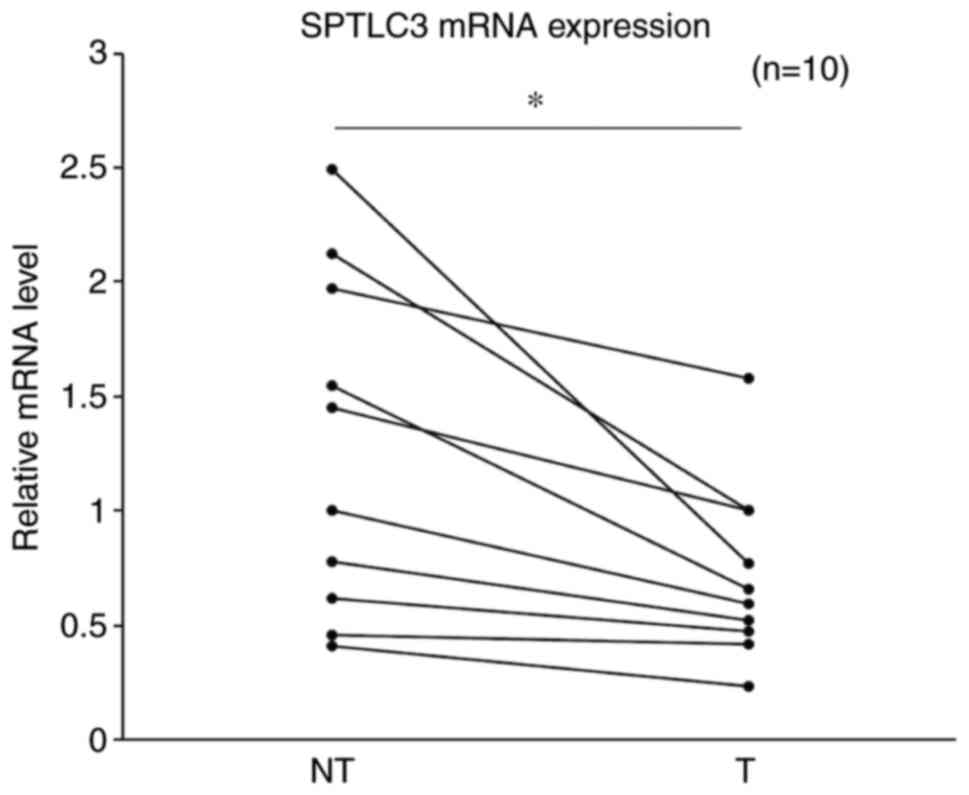
SPTLC3 is highly expressed in
non-tumor liver tissues in patients with NAFLD
Subsequently, the difference in expression level of
SPTLC3 was compared between non-tumor and tumor regions of
the liver in NAFLD patients with HCC. The expression level was
significantly higher in the non-tumor sections compared with that
in the tumor sections of the liver (P=0.034; Fig. 2).
Comparison of clinical characteristics
between the NAFL/NASH and HCC groups
The association between the clinical characteristics
and SPTLC3 expression in patients with NAFL/NASH was investigated.
The results of the biochemical tests performed in the NAFL/NASH
group without HCC and the group with HCC are shown in Table II. Compared with the NAFL/NASH
group, the HCC group had lower platelet count, ALT, albumin,
triglyceride and LDL-Chol levels. The patients were also older and
exhibited higher HbA1c and hyaluronic acid levels. The levels of
AFP and DCP, which are liver tumor markers, were significantly
elevated in the HCC group (P<0.001). Notably, SPTLC3 levels were
significantly higher in the HCC group (P=0.001; Table II).
 | Table IIClinical characteristics of patients
with NAFLD without vs. with HCC. |
Table II
Clinical characteristics of patients
with NAFLD without vs. with HCC.
|
Characteristics | NAFL/NASH
(n=66) | HCC (n=33) | P-value |
|---|
| Sex
(male/female) | 24/42 | 13/20 | 0.827 |
| Age (years) | 51 | 70 | <0.001 |
| Platelet count
(1x104/µl) | 23.9 | 12.9 | <0.001 |
| Aspartate
aminotransferase (U/l) | 71 | 39 | <0.001 |
| Alanine
aminotransferase (U/l) | 112.5 | 26 | <0.001 |
| γ-Glutamyl
transferase (U/l) | 65.5 | 58 | 0.456 |
| Total bilirubin
(mg/dl) | 0.7 | 0.7 | 0.278 |
| Albumin (g/dl) | 4.5 | 3.8 | <0.001 |
| Prothrombin index
(%) | 105 | 99 | 0.116 |
| LDL cholesterol
(mg/dl) | 120 | 96 | 0.003 |
| Triglyceride
(mg/dl) | 128 | 114 | 0.037 |
| Glucose
(mg/dl) | 99 | 107 | 0.183 |
| HbA1c (%) | 6 | 6.5 | 0.043 |
| Hyaluronic acid
level (ng/ml) | 35.3 | 181.1 | <0.001 |
| α-fetoprotein
(ng/ml) | 2.7 | 10 | <0.001 |
| DCP (mAU/ml) | 23 | 42 | <0.001 |
| SPTLC3 (ng/ml) | 0.935 | 1.343 | 0.001 |
| FIB-4 index | 1.3775 | 3.8333 | <0.001 |
| APRI | 0.9999 | 0.9649 | 0.812 |
| HCC stage
(I/II/III/IV) | - | 7/12/14/0 | - |
| Tumor vascularity
(hyper/hypo) | - | 30/3 | - |
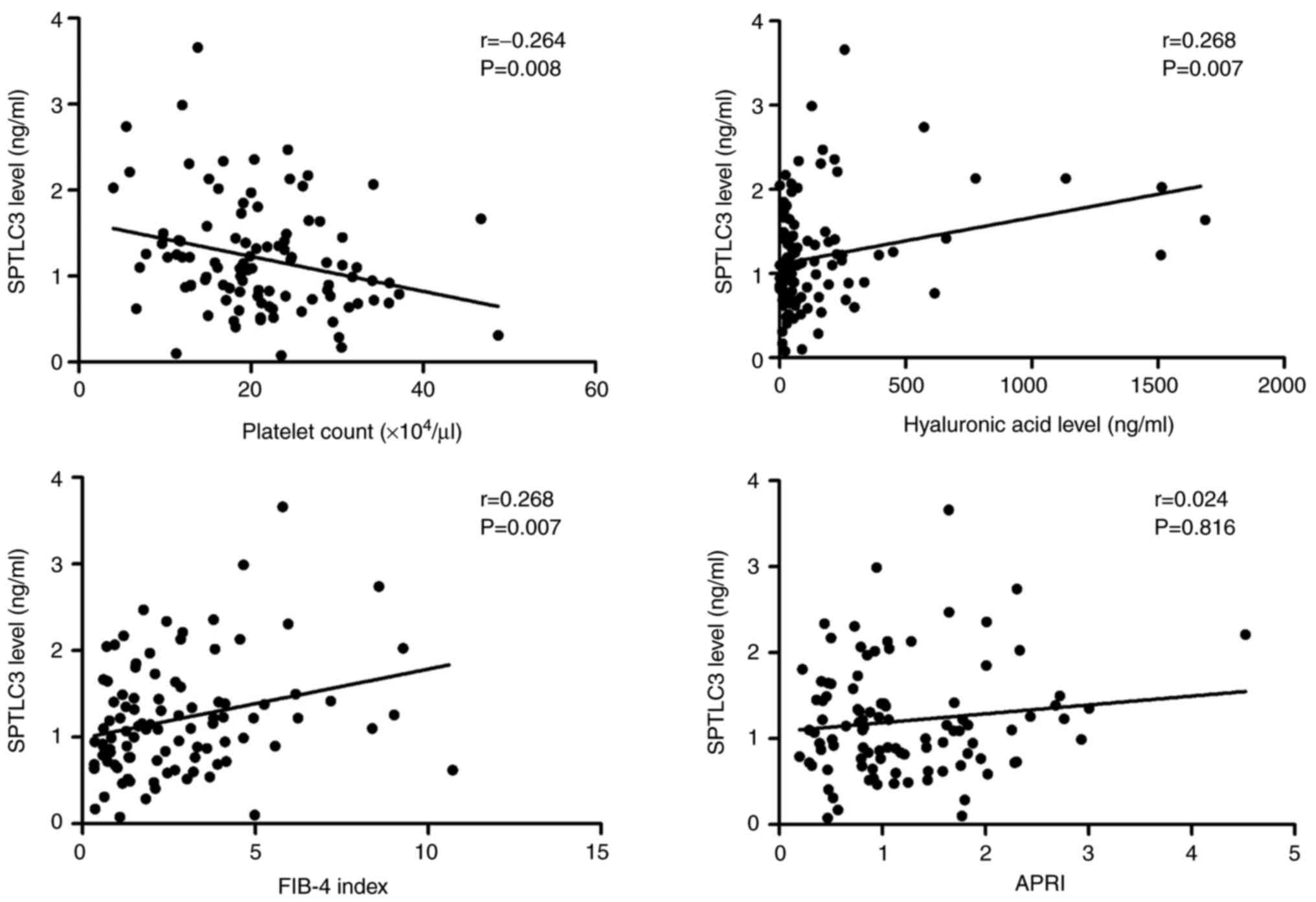
SPTLC3 is associated with advanced
liver fibrosis
The association of platelet count and hyaluronic
acid levels (which are serum biomarkers of liver fibrosis) with
SPTLC3 expression were subsequently investigated. Serum SPTLC3
exhibited a significant negative correlation with platelet count
(P=0.008) and a significant positive correlation with hyaluronic
acid levels (P=0.007; Fig. 3).
Conversely, no significant correlations were found with AFP or DCP
levels, which are biomarkers of HCC. Furthermore, FIB-4 index and
APRI, which are a combination of laboratory parameters indicating
liver fibrosis, were assessed. SPTLC3 expression level exhibited a
significant positive correlation with FIB4-index (P=0.007; Fig. 3).
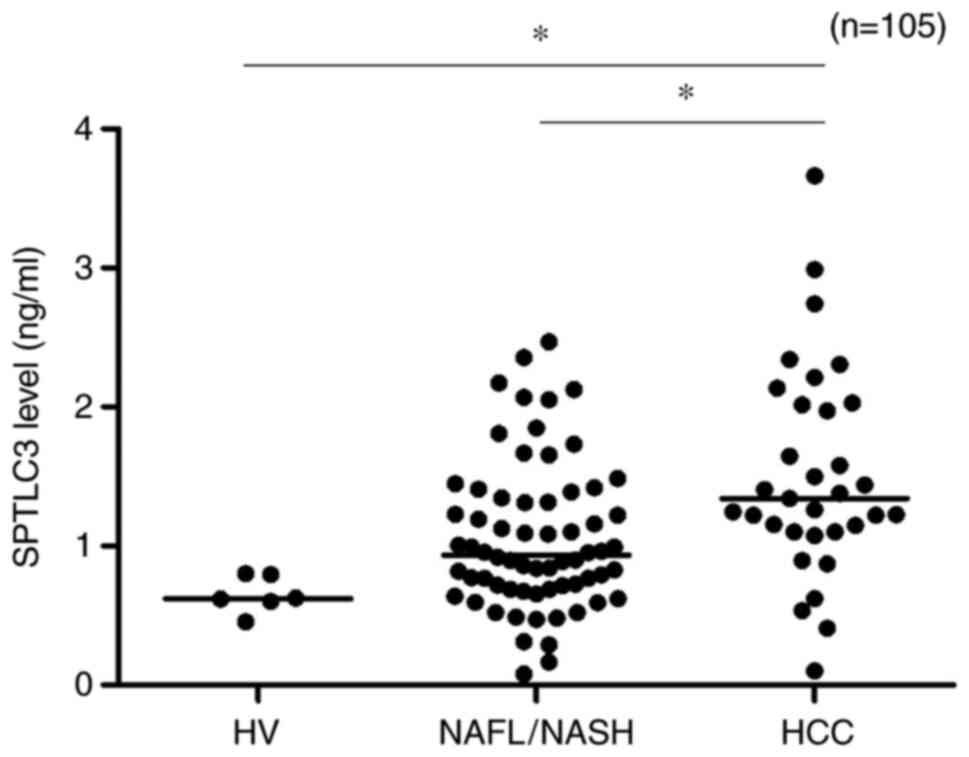
SPTLC3 levels are significantly higher
in patients with HCC
Next, it was verified whether serum SPTLC3 levels
increase during the process of carcinogenesis. SPTLC3 serum levels
of the HV, NAFL/NASH and HCC groups were compared (Fig. 4). SPTLC3 levels were significantly
higher in the HCC group compared with those in the HV and NAFL/NASH
groups (P<0.01).
SPTLC3 is associated with HCC
Subsequently, multivariate statistical analysis was
performed to determine whether serum SPTLC3 level was associated
with carcinogenesis. Multivariate analysis of HCC-related factors
was conducted using 11 factors that were significantly higher
(P<0.05) in the HCC group following the univariate analysis;
these revealed that platelet COUNT, ALT, albumin and SPTLC3 levels
were independent factors associated with HCC (Table III).
 | Table IIIFactors associated with HCC in
patients with NAFLD. |
Table III
Factors associated with HCC in
patients with NAFLD.
| | | Multivariate
analysis |
|---|
| Variables | Univariate analysis
P-valuea | Odds ratio | 95% CI |
P-valueb |
|---|
| Sex | <0.001 | 1.008 | 0.890-1.141 | 0.905 |
| Platelet count | <0.001 | 0.629 | 0.415-0.953 | 0.029 |
| Alanine
aminotransferase | <0.001 | 0.987 | 0.974-1.000 | 0.045 |
| Albumin | <0.001 | 0.008 | 0.000-0.793 | 0.040 |
| Triglyceride | 0.037 | 0.990 | 0.968-1.012 | 0.358 |
| LDL
cholesterol | 0.003 | 1.046 | 0.987-1.108 | 0.133 |
| Hyaluronic acid
level | <0.001 | 0.993 | 0.985-1.000 | 0.051 |
| HbA1c | 0.043 | 1.986 | 0.961-4.163 | 0.064 |
| α-fetoprotein | <0.001 | 1.620 | 0.990-2.652 | 0.055 |
| DCP | <0.001 | 1.001 | 0.993-1.010 | 0.754 |
| SPTLC3 | 0.001 | 12.935 | 1.002-167.016 | 0.050 |
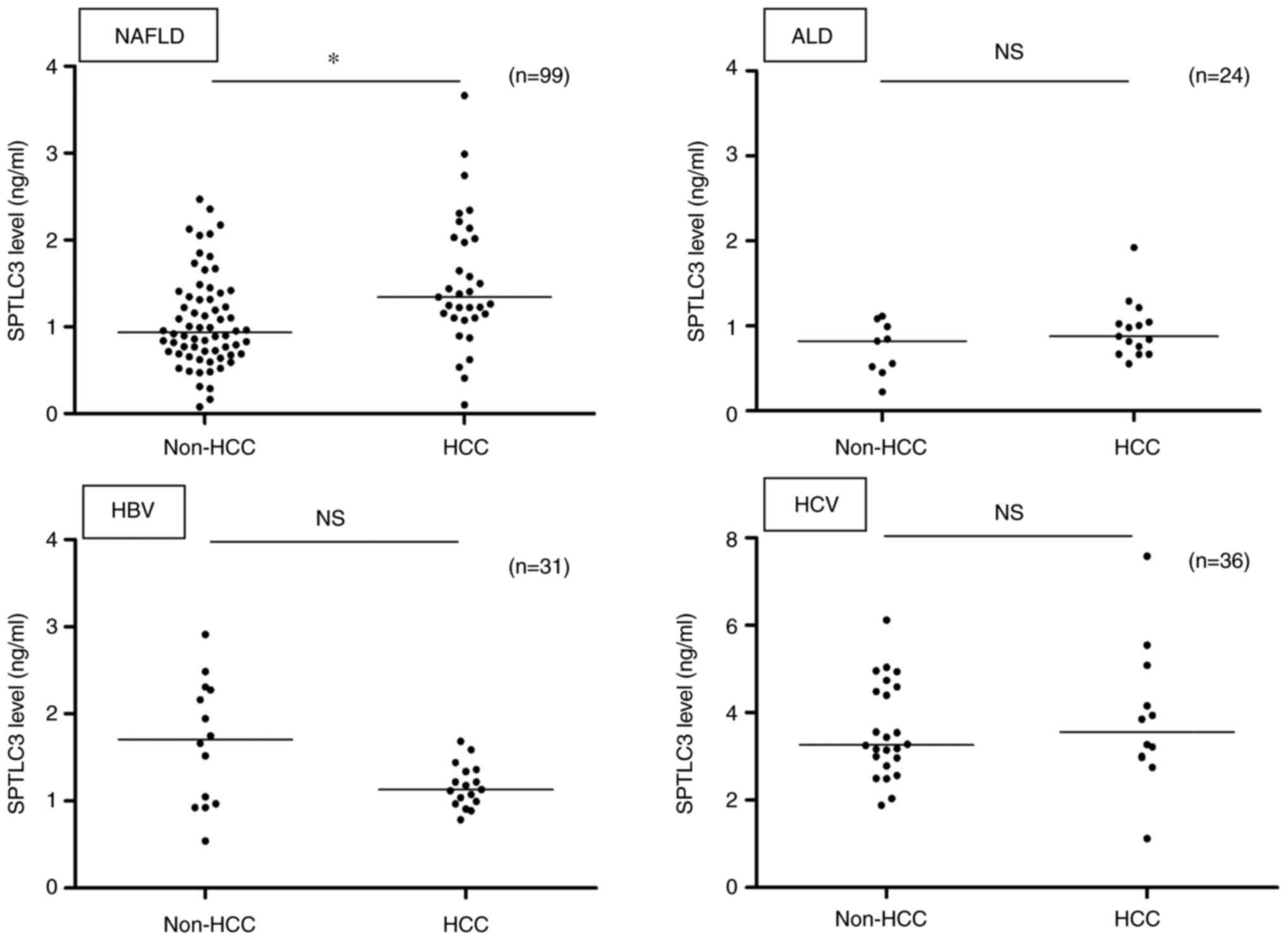
SPTLC3 is significantly elevated in
liver cancer specifically during NAFLD
SPTLC3 serum levels in patients with liver disease
of different etiologies were compared. Among patients with HBV, HCV
and alcoholic liver injury, the serum SPTLC3 levels of the non-HCC
and HCC groups were compared, but no significant increase was
observed in the HCC group (P>0.05; Fig. 5). Thus, it was confirmed that
SPTLC3 levels increased significantly only in the NAFLD patients
with HCC compared with those without HCC.
Discussion
In the present study, SPTLC3 mRNA was found
to be highly expressed in human liver cancer cell lines. In
addition, SPTLC3 was associated with the development of liver
fibrosis in NASH. Furthermore, serum SPTLC3 levels in patients with
liver cancer and NAFLD were significantly higher compared with
those in non-cancer-bearing patients.
Tabassum et al (17) performed genome-wide association
analyses of 141 lipid species, followed by phenome-wide scans with
25 cardiovascular disease-related phenotypes. They identified two
variants near SPTLC3 and ZNF385D that modulate the
plasma levels of ceramide (CER) d18:1/24:1 and d18:1/24:0,
respectively, and this was associated with the risk of
intracerebral hemorrhage. Therefore, SPTLC3 has been
attracting attention as a lipid-related gene that may predict
cardiovascular disease risk.
SPT is a membrane-bound protein localized in the
endoplasmic reticulum membrane in eukaryotes. It is a complex with
a molecular weight of 480 kDa, composed of three different subunits
(SPTLC1, SPTLC2 and SPTLC3). SPT as a whole is composed of four
dimers, including two subtypes of dimers: SPTLC1 and SPTLC2; and
SPTLC1 and SPTLC3. The N-terminus of SPTLC1 binds to the
endoplasmic reticulum membrane, while the C-terminus binds to the
paired SPTLC2 or SPTLC3 in the cytoplasm and exhibits enzymatic
activity (18). Notably, silencing
SPTLC3 expression in HepG2 or human trophoblast cells using
SPTLC3-specific siRNA significantly attenuates intracellular
SPT activity. This suggests that SPTLC3 represents a key subunit of
SPT activity in liver cancer cells (19).
SPT is a rate-determining enzyme that metabolizes
and synthesizes membrane sphingolipids (20). CER, sphingosine and
sphingosine-1-phosphate, which are metabolites of sphingolipids,
act as intra- and intercellular lipid mediators and are involved in
cell proliferation, differentiation and apoptosis (21,22).
As regards the association between NASH and
sphingolipids, non-diabetic obese patients with a fatty liver have
higher concentrations of CER and sphingomyelin (which is a type of
sphingolipid), in their adipose tissue, compared with patients with
healthy livers (23). It has also
been reported that hepatic CER levels were increased in mice fed a
high-fat diet, and that this elevation was suppressed by the
administration of an insulin sensitizer or SPT inhibitor (24). As mentioned above, sphingolipids
and their metabolite, ceramide, are implicated in the development
of fatty liver. Therefore, it was hypothesized that SPTLC3, which
was examined in the present study, is also associated with the
progression of NASH pathology.
Previous reports have indicated an association
between SPT and carcinogenesis in malignant melanomas. SPT
inhibitors have been shown to attenuate carcinogenesis by
inhibiting the G2/M transition of malignant melanoma
cells (25) and have been reported
to suppress the progression of tumors by inhibiting the de
novo synthesis of sphingolipids in a mouse model of malignant
melanoma (26).
However, there are few reports on the association
between SPT and liver cancer. It has been determined that SPT
inhibition promotes melatonin-induced apoptosis of HepG2 cells
(27); however, to the best of the
authors' knowledge, no studies prior to the present study
investigated the role of SPTLC3 in human liver cancer.
The non-cancerous sections of the liver in patients
with NAFLD expressed significantly higher SPTLC3 mRNA levels
compared with the cancerous sections. By contrast, the serum SPTLC3
levels were significantly higher in patients with liver cancer and
NAFLD compared with those in patients with NAFLD but without liver
cancer. In our previous study, SPTLC3 mRNA levels in
non-cancerous liver tissue in a mouse model of NAFLD carcinogenesis
were found to increase over time as NASH and carcinogenesis
progressed (8). It was
hypothesized that, rather than being expressed by cancerous
hepatocytes, SPTLC3 may be expressed by normal hepatocytes
that have the potential to undergo lipotoxicity and become
cancerous, which may be supported by the increase in serum SPTLC3
levels in patients with liver cancer and NAFLD.
Progression of hepatic fibrosis is one of the
factors determining the prognosis of patients with NASH (7,28),
and it is the most important factor in the pathological progression
of NASH (29). In the present
study, serum SPTLC3 levels were found to be significantly
associated with known liver fibrosis markers, including platelet
count, hyaluronic acid levels and FIB-4 index, and with NASH
pathogenesis (liver fibrosis progression). This suggests that serum
SPTLC3 levels may also be associated with the prognosis of patients
with NASH.
AFP and DCP are well-known tumor markers of existing
liver cancer; however, AFP levels are not increased in patients
with NAFLD-related liver carcinogenesis. In a comparative study of
34 cases of NASH-related liver cancer and 56 cases of HCV-related
liver cancer in Japan, the mean values of AFP were 7.0 and 24.0
ng/ml, respectively (P=0.007) (30). In addition, the level of DCP may
increase due to vitamin K deficiency and, thus, false positives may
be associated with malnutrition, oral administration of warfarin,
suppression of vitamin production by intestinal bacteria upon oral
administration of antibiotics, and impaired absorption of
fat-soluble vitamins owing to obstructive jaundice. Therefore,
patient medical history should always be taken into
consideration.
In the present study, serum SPTLC3 levels were
significantly elevated in patients with NAFLD-related HCC, while
they remained unchanged in patients with liver diseases of
different etiologies (HBV, HCV and alcoholic liver injury). In
these groups, no significant increases were observed compared with
the non-HCC group, suggesting that SPTLC3 may be a specific tumor
marker in patients with NAFLD.
There were two main limitations of the present
study. The first limitation was the selection bias of patients. The
selected patients with NAFLD with or without HCC were individuals
admitted to a medical institution specializing in liver diseases
for the purpose of detailed examination and treatment and were not
randomly selected from the general population. In the future, it
would be beneficial to conduct validation studies in a sample that
is larger and more representative of the general population. The
second was the insufficient evaluation of SPTLC expression. In the
present study, western blotting was not performed to evaluate the
level of SPTLC3 protein in the liver cancer tissues. It is
necessary to evaluate the protein content in the liver tissues in
order to evaluate how SPTLC3 protein expression is reflected in
serum SPTLC3 levels, and this will be further studied in the
future.
In conclusion, SPTLC3 levels were specifically
increased in the serum of patients with NAFLD and HCC, suggesting
that SPTLC3 may be involved in liver carcinogenesis in these
patients. These findings may highlight the clinical significance of
SPTLC3 in the pathogenesis of NAFLD.
Acknowledgements
The authors would like to thank Dr Nobuhiro
Hiraishi, Ms. Hiromi Eguchi and Ms. Yuko Morinaga for their
technical assistance.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors upon reasonable
request.
Authors' contributions
SI, KO and AI designed the study. OT, AT, HS and KT
collected the clinical samples. SI and NH performed reverse
transcription-quantitative PCR. SI, KO and SM drafted and revised
the manuscript. SI, KK, SK, TT, AM and HU performed statistical and
clinical analyses for this study. KO and KT confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Kagoshima University Hospital (approval no. 28-107).
Written informed consent was obtained from all patients. All
procedures were performed according to the World Medical
Association's Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eslam M, Sanyal AJ and George J:
International Consensus Panel. MAFLD: A consensus-driven proposed
nomenclature for metabolic associated fatty liver disease.
Gastroenterology. 58:1999–2014, e1. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of nonalcoholic fatty
liver disease-Meta-analytic assessment of prevalence, incidence,
and outcomes. Hepatology. 64:73–84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Estes C, Anstee QM, Arias-Loste MT, Bantel
H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day
CP, et al: Modeling NAFLD disease burden in China, France, Germany,
Italy, Japan, Spain, United Kingdom, and United States for the
period 2016-2030. J Hepatol. 69:896–904. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Oda K, Uto H, Mawatari S and Ido A:
Clinical features of hepatocellular carcinoma associated with
nonalcoholic fatty liver disease: A review of human studies. Clin J
Gastroenterol. 8:1–9. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tateishi R, Uchino K, Fujiwara N, Takehara
T, Okanoue T, Seike M, Yoshiji H, Yatsuhashi H, Shimizu M, Torimura
T, et al: A nationwide survey on non-B, non-C hepatocellular
carcinoma in Japan: 2011-2015 update. J Gastroenterol. 54:367–376.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dulai PS, Singh S, Patel J, Soni M, Prokop
LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, et al:
Increased risk of mortality by fibrosis stage in nonalcoholic fatty
liver disease: Systematic review and meta-analysis. Hepatology.
65:1557–1565. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Angulo P, Kleiner DE, Dam-Larsen S, Adams
LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC,
Lafferty HD, Stahler A, et al: Liver fibrosis, but no other
histologic features, is associated with long-term outcomes of
patients with nonalcoholic fatty liver disease. Gastroenterology.
149:389–397, e10. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yoshimine Y, Uto H, Kumagai K, Mawatari S,
Arima S, Ibusuki R, Mera K, Nosaki T, Kanmura S, Numata M, et al:
Hepatic expression of the Sptlc3 subunit of serine
palmitoyltransferase is associated with the development of
hepatocellular carcinoma in a mouse model of nonalcoholic
steatohepatitis. Oncol Rep. 33:1657–1666. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the American association for the study of
liver diseases. Hepatology. 67:328–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
European Association for the Study of the
Liver (EASL); European Association for the Study of Diabetes
(EASD); European Association for the Study of Obesity (EASO).
EASL-EASD-EASO clinical practice guidelines for the management of
non-alcoholic fatty liver disease. J Hepatol. 64:1388–1402.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono
M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, et al:
Validation of the FIB4 index in a Japanese nonalcoholic fatty liver
disease population. BMC Gastroenterol. 12(2)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee J, Vali Y, Boursier J, Spijker R,
Anstee QM, Bossuyt PM and Zafarmand MH: Prognostic accuracy of
FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A
systematic review. Liver Int. 41:261–270. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kruger FC, Daniels CR, Kidd M, Swart G,
Brundyn K, Van Rensburg C and Kotze M: APRI: A simple bedside
marker for advanced fibrosis that can avoid liver biopsy in
patients with NAFLD/NASH. S Afr Med J. 101:477–480. 2011.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nishikoba N, Kumagai K, Kanmura S,
Nakamura Y, Ono M, Eguchi H, Kamibayashiyama T, Oda K, Mawatari S,
Tanoue S, et al: HGF-MET signaling shifts M1 macrophages toward an
M2-like phenotype through PI3K-mediated induction of Arginase-1
Expression. Front Immunol. 11(2135)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tabassum R, Rämö JT, Ripatti P, Koskela
JT, Kurki M, Karjalainen J, Palta P, Hassan S, Nunez-Fontarnau J,
Kiiskinen TTJ, et al: Genetic architecture of human plasma lipidome
and its link to cardiovascular disease. Nat Commun.
10(4329)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hornemann T, Wei Y and von Eckardstein A:
Is the mammalian serine palmitoyltransferase a high-molecular-mass
complex? Biochem J. 405:157–164. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hornemann T, Richard S, Rütti MF, Wei Y
and von Eckardstein A: Cloning and initial characterization of a
new subunit for mammalian serine-palmitoyltransferase. J Biol Chem.
281:37275–37281. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Menaldino DS, Bushnev A, Sun A, Liotta DC,
Symolon H, Desai K, Dillehay DL, Peng Q, Wang E, Allegood J, et al:
Sphingoid bases and de novo ceramide synthesis: Enzymes involved,
pharmacology and mechanisms of action. Pharmacol Res. 47:373–381.
2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zheng W, Kollmeyer J, Symolon H, Momin A,
Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, et al:
Ceramides and other bioactive sphingolipid backbones in health and
disease: Lipidomic analysis, metabolism and roles in membrane
structure, dynamics, signaling and autophagy. Biochim Biophys Acta.
1758:1864–1884. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gomez-Larrauri A, Presa N,
Dominguez-Herrera A, Ouro A, Trueba M and Gomez-Muñoz A: Role of
bioactive sphingolipids in physiology and pathology. Essays
Biochem. 64:579–589. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kolak M, Westerbacka J, Velagapudi VR,
Wågsäter D, Yetukuri L, Makkonen J, Rissanen A, Häkkinen AM,
Lindell M, Bergholm R, et al: Adipose tissue inflammation and
increased ceramide content characterize subjects with high liver
fat content independent of obesity. Diabetes. 56:1960–1968.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cinar R, Godlewski G, Liu J, Tam J,
Jourdan T, Mukhopadhyay B, Harvey-White J and Kunos G: Hepatic
cannabinoid-1 receptors mediate diet-induced insulin resistance by
increasing de novo synthesis of long-chain ceramides. Hepatology.
59:143–153. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lee YS, Choi KM, Choi MH, Ji SY, Lee S,
Sin DM, Oh KW, Lee YM, Hong JT, Yun YP, et al: Serine
palmitoyltransferase inhibitor myriocin induces growth inhibition
of B16F10 melanoma cells through G(2)/M phase arrest. Cell Prolif.
44:320–329. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee YS, Choi KM, Lee S, Sin DM, Lim Y, Lee
YM, Hong JT, Yun YP and Yoo HS: Myriocin, a serine
palmitoyltransferase inhibitor, suppresses tumor growth in a murine
melanoma model by inhibiting de novo sphingolipid synthesis. Cancer
Biol Ther. 13:92–100. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ordoñez R, Fernández A, Prieto-Domínguez
N, Martínez L, García-Ruiz C, Fernández-Checa JC, Mauriz JL and
González-Gallego J: Ceramide metabolism regulates autophagy and
apoptotic cell death induced by melatonin in liver cancer cells. J
Pineal Res. 59:178–189. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hagström H, Nasr P, Ekstedt M, Hammar U,
Stål P, Hultcrantz R and Kechagias S: Fibrosis stage but not NASH
predicts mortality and time to development of severe liver disease
in biopsy-proven NAFLD. J Hepatol. 67:1265–1273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Loomba R and Chalasani N: The hierarchical
model of NAFLD: Prognostic significance of histologic features in
NASH. Gastroenterology. 149:278–281. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tokushige K, Hashimoto E, Yatsuji S,
Tobari M, Taniai M, Torii N and Shiratori K: Prospective study of
hepatocellular carcinoma in nonalcoholic steatohepatitis in
comparison with hepatocellular carcinoma caused by chronic
hepatitis C. J Gastroenterol. 45:960–967. 2010.PubMed/NCBI View Article : Google Scholar
|