Introduction
It is generally accepted that the management of
colorectal cancer (CRC) has been successfully improved. This is
indicated by the confirmation of declining incidence and mortality
since 1975 and documented in successive annual reports on CRC,
spanning over the last decade in the western world. This success
has been mainly attributed to identifying modifiable lifestyle risk
factors and the wider application of screening and subsequently
endoscopic polypectomy (1-3).
Unfortunately, recent evidence tarnished the current
euphoria. Increasing incidence and mortality in young adults <50
years old have been reported (3,4). An
inconspicuous increase in CRC incidence in younger individuals
appeared in the early 1990s (4,5), but
it was more widely recognized during the last decade (5,6).
This recognition identified CRC as the second most common cancer
after the age of 30 years (7) and
as the fourth leading cause of mortality in adolescents and young
adults (3).
Women seem to be more susceptible to an increased
CRC annual percent change (APC) in younger cohorts (30-49 years
old), which is higher compared with men and consistent across
continents of the western culture (USA, Australia, Europe)
(6,8,9).
Furthermore, an inverse correlation exists in women where the
overall decreasing incidence of CRC is faster in men while the
decreasing overall mortality is slower in women (3).
High rates of proximal colon cancers have been
estimated to be between 30-42% of the total prevalence, being more
common in men compared with women (10,11).
Increasing incidences of proximal cancers in young adults 20-49
years old have also been reported, especially after 2010 (5,12).
The vast majority (80%) of polyps found during
average-risk screening colonoscopy are subcentimetric polyps
(<10 mm) (13,14). At the same time, they constitute a
potential cause of pitfalls for the endoscopist since they may
harbor significant lesions, such as advanced adenomas (≤12.8%)
(15), while the methods for their
resection are imperfect (16,17).
Screening has been considered to contribute to the
decline in CRC incidence and mortality. By contrast, the lack of
adequate screening may have threatening repercussions in younger
individuals regarding the CRC risk (4,18).
To achieve this goal, worldwide guidelines recommend the initiation
of screening at 50 years old since two-thirds of CRC cases are
detected in individuals ≥60 years old and 90% in those >50 years
old (8,19). Recent recommendations suggest that
screening should start earlier at 45 years old (18).
The present study therefore aimed to address the
issues aforementioned by analyzing epidemiological data from an
average risk asymptomatic screening cohort of a wide age range that
was submitted to colonoscopy in an open-access manner in the
endoscopy unit of a tertiary hospital. Since there is no national
organized screening program in Greece, the current study will
provide the opportunity to collect and interpret real-life data
from a population sample in a densely populated urban area of
westernized lifestyle to demonstrate actual needs for health
policymaking.
Materials and methods
The present study prospectively included 716
individuals submitted to screening colonoscopy during a period of 2
years (2017-2018). They have been classified as average risk since
those with a personal or family history of polyps and colorectal
cancer and those with prior positive fecal hemoglobin tests, blood
per rectum or inflammatory bowel disease and anemia were excluded.
They were all asymptomatic except for the occasional sporadic
abdominal discomfort, which was considered non-significant. Their
examination was performed on an open-access endoscopy screening
program after self-referral and personal informed consent. The
procedure was completed under conscious sedation after bowel
cleansing using a polyethylene glycol preparation. The bowel
cleansing was graded on a three-class scale (good, adequate, poor)
similar to the Boston Preparation Scale. In case polyps were
discovered during colonoscopy, they were excised by cold biopsy
forceps or by cold or hot snaring at the endoscopist's discretion.
The size of the polyps was estimated compared with open biopsy
forceps with an opening diameter of 7 mm. The extend of the right
colon was considered up to the splenic flexure and that of the left
colon from the splenic flexure and peripherally up to the anal
verge. Information was collected from the endoscopic and pathologic
reports. During endoscopic sessions, both consultants and trainees
were involved. Per patient analysis was performed according to the
maximum polyp size when more than one polyp of different sizes was
found. Advanced adenomas (AA) were defined as polyps with size ≥10
mm or with high-grade dysplasia (hgd). Since there was a
substantial inter-observer variation for recognizing a villous
component, this parameter was not included in the definition of AA
(20). The protocol of the present
study was accepted by the Sismanogleio-Amalia Fleming Hospital's
scientific review committee (approval number 399/18-4-2019) and
conformed to the amendments of the Helsinki Declaration.
Statistical analysis
Continuous variables were compared between groups
using the t-test and for more than two levels of explanatory
variables using one-way ANOVA. Ordinal data were compared using the
χ2 and post hoc comparisons were performed by the
Bonferroni method. Receiver operating characteristic curves (ROC)
were produced to define the appropriate age providing optimum
sensitivity for adenoma detection. The number needed to screen
(NNS) to detect one adenoma was estimated across different age and
gender groups. The a-level was defined as significant at 0.05. All
P-values were 2-sided. The statistical package used for analytical
statistics was the SPSS 27 (IBM Corp.).
Results
General overview
There were 716 participants with a median age of 63
years (range 30-87 years), and 51.3% were males. The cecum was
reached in 95.7% of the cases. The preparation was considered as
good or adequate in 92% of the endoscopies. A consultant performed
the colonoscopy in 69% of the sessions and the rest by a trainee
under supervision with an overall adenoma detection rate of 28.4%.
The prevalence of lesions including polyps, adenomas, advanced
adenomas, high-grade dysplasia, and cancer are shown in Table I. Polyps were found in 52.4%
(n=375) of the entire cohort. The median number of polyps/person
was 2. Marked proportions of the lesions above were found in this
average risk screening cohort. This has important implications
concerning the epidemiologic burden and the risk of future cancer
development in the population.
 | Table ICharacteristics of participants. |
Table I
Characteristics of participants.
| Total no. | 716 |
|---|
| Age, median,
(range) | 63 (30-87) |
| Sex, n (%) | |
|
Male | 367 (51.3) |
|
Female | 349 (48.7) |
| Prevalence of
lesions, n (%) | |
|
Any
polyp | 375 (52.4) |
|
Adenoma | 205 (28.6) |
|
Advanced
Adenoma | 69 (9.6) |
|
High grade
dysplasia | 31 (4.3) |
|
Cancer | 10 (1.4) |
|
SSP/A | 54 (7.5) |
| Location | |
|
Right colon
lesions, n (%) | 156 (21.7) |
| No polyps/pt,
median | 2 |
| No adenoma/pt,
median | 1 |
Associations of polyp size and
location with important lesions
Most subjects with polyps (58.1%) had a maximum
polyp size of 6-9 mm. Only 14.7% of them had polyps ≥10 mm. There
is a linear association between increasing polyp size and age as
well as the prevalence of adenomas, hgd, cancer, serrated
pathology, and the location at the right colon. Nonetheless, a
significant proportion of the diminutive and small polyps harbor
important lesions (48% adenomas, 5.6% hgd, 12.9% serrated, and 0.9%
coexist with cancer lesions) while 15% of the screened participants
present with isolated right colon lesions of this size (Table II).
 | Table IIFrequency of lesions by patient
according to maximum polyp size. |
Table II
Frequency of lesions by patient
according to maximum polyp size.
|
Characteristics | | | | | P-value |
|---|
| Maximum polyp size,
mm | ≤5 | 6-9 | 10-20 | 21-40 | |
| Patients, n | 101 | 218 | 46 | 10 | |
| Age, mean | 61 | 64 | 65.3 | 71 | 0.001 |
| Male (n) | 59.4% (60) | 58.7% (128) | 63% (29) | 90% (9) | 0.25 |
| Lesions (n) | | | | | |
|
Adenomas | 25.7% (26) | 58.3% (127) | 91.3% (42) | 100% (10) | 0.0001 |
|
HGD | 0% | 8.3% (18) | 19.6% (9) | 40% (4) | 0.0001 |
|
Cancer | 1% (1) | 0.9% (2) | 4.3% (2) | 10% (1) | 0.049 |
|
SSP/A | 7.9% (8) | 15% (33) | 28.3% (13) | 0% | 0.009 |
| Isolated Rcolon
(n) | 12.5% (11) | 23.6% (37) | 39.3% (11) | 50% (2) | 0.007 |
| No polyps,
mean | 1.9 | 2.5 | 3.2 | 3.2 | 0.003 |
| No adenomas,
mean | 1.4 | 1.7 | 1.8 | 2.6 | 0.02 |
AA appeared in 9.6% of the attendees, mostly in men
(67% of AA), in the intermediate age group (50-69 years old; 56.2%
of AA), with a significant proportion of them located at the right
colon (39%) and 25% of those were found in 6-9 mm polyps. The mean
size of AA was 15 mm, and the median number per patient was 1. In
Table III, the distribution of
characteristics between the isolated right and left colon lesions
was shown. Isolated right colon lesions were found in older
individuals with increasing frequency, with no significant gender
difference but with a male predominance, harboring important
pathology (~one-third of serious lesions reside in that part of the
bowel). As maximum polyp size increases >9 mm, almost half
(43.3%) of the participants accommodate lesions in the right
colon.
 | Table IIIDistribution of characteristics in
isolated left and right colon lesions. |
Table III
Distribution of characteristics in
isolated left and right colon lesions.
|
Characteristics | Right colon | Left colon | P-value |
|---|
| Participants,
n | 61 | 217 | |
| Age, years,
mean | 65.3 | 62.7 | 0.05 |
| Age category % | | | 0.35 |
|
30-49 | 16.7 | 83.3 | |
|
50-69 | 20 | 80 | |
|
70-87 | 27.5 | 72.5 | |
| Sex % | | | 0.08 |
|
Male | 25.8 | 74.2 | |
|
Female | 16.5 | 83.5 | |
| Lesions % | | | |
|
Adenomas | 29.9 | 70.1 | 0.002 |
|
AA | 39 | 61 | 0.007 |
|
HGD | 27.8 | 72.2 | 0.55 |
|
SSP/A | 34.4 | 65.6 | 0.1 |
| No polyp, mean | 1.8 | 1.9 | 0.9 |
| AA size, mm,
mean | 14.2 | 15.3 | 0.6 |
| Max polyp size
% | | | 0.009 |
|
1-9 mm | 19.5 | 80.5 | |
|
10-40
mm | 43.3 | 56.7 | |
The epidemiologic profile according to
different age groups
A comparison between age groups is presented in
Table IV. It seems that between
the early (30-49 years old) and intermediate (50-69 years old) age
groups, there is no quantitative or qualitative difference in
important pathologies (mean number and size of lesions, proportion
of AA, hgd, cancer and isolated right colon lesions). Apart from
the prevalence of polyps and adenomas that follow a linear
association with increasing age and the disparities in the presence
of SSP/A (sessile serrated polyp/adenoma) that in any case
represent an alternative cancer pathway, it seems that younger
individuals (30-49 years old) have acquired a risk profile that
needs to be taken into account by future screening policy
recommendations.
 | Table IVFrequency of lesions by
patient-Comparison between early-intermediate-late age groups. |
Table IV
Frequency of lesions by
patient-Comparison between early-intermediate-late age groups.
| Age (mean) | n (%) | Sex, male | Any polyp | Adenoma | AA | HGD | Cancer | SSP/A | Rcolon | No polyp mean | No adenoma
mean | HGD size, mm | AA size, mm | 1-10 mm |
|---|
| 30-49(43) | 67 (9.4) | 41.8% | 34.3% | 10.4% | 4.5% | 4.5% | 1.5% | 0 | 16.7% | 2 | 1 | 10 | 9.5 | 91.3% |
| 50-69 (60.4) | 463 (64.7) | 48.8% | 52.7% | 26.3% | 8.2% | 4.1% | 0.4% | 10.2% | 20% | 2.5 | 1.8 | 11.4 | 15.3 | 89.6% |
| 70-87(74) | 186(26) | 60.8% | 58.1% | 40.9% | 15.1% | 4.8% | 3.8% | 3.8% | 27.5% | 2.4 | 1.7 | 15.3 | 15.4 | 79.6% |
| P-value | | 0.006 | 0.004 | 0.0001 | 0.012 | 0.84 | 0.004 | 0.001 | 0.36 | 0.57 | 0.17 | 0.5 | 0.4 | 0.16 |
Gender influences across different age
groups
In Table V, the
findings showed that women 30-49 years old have a higher prevalence
of polyps of larger size and cancer cases compared with men,
although this was not statistically significant. There was no
significant difference in frequency characteristics between women
of 30-49 years old and women of 50-69 years old (data not shown)
except for the prevalence of SSP/A. Females of a younger age had
their polyps, AA and hgd located in the left colon (absence of
isolated right colon lesions). By contrast, men show a marked
increase in the prevalence and size of polyps and prevalence of
adenomas and AA at the next age group of 50-69 years old, with
frequencies higher compared with the respective age group of
females. Men 30-49 years old show polyps distributed throughout the
left and right colon and important lesions, such as AA and hgd in
the right colon (absence of isolated left colon lesions). There was
a linear association for right colon lesions with increasing age in
women. Nonetheless, males maintain the majority of proximal lesions
in all age groups. These findings indicate a trend towards an
additional disease burden in young women, which is located in the
easily accessible left colon.
 | Table VGender differences in different age
groups. |
Table V
Gender differences in different age
groups.
| | 30-49 (birth cohort
1980s) | 50-69 (birth cohort
1960s) | 70-87 (birth cohort
1940s) |
|---|
| | 67 | 463 | 186 |
|---|
| Age category
Participants, n | Male | Female | P-value | Male | Female | P-value | Male | Female | P-value |
|---|
| M/F % (n) | 41,8(28) | 58.2(39) | | 48.8(226) | 51.2(237) | | 60.8(113) | 39.2(73) | |
| Any polyp % | 28.6 | 38.5 | 0.44 | 64.6 | 41.4 | 0.0001 | 63.7 | 49.3 | 0.06 |
| No polyps,
mean | 2 | 2.13 | 0.8 | 2.7 | 2.3 | 0.2 | 2.6 | 2 | 0.07 |
| Polyp location | | | 0.04 | | | 0.07 | | | 0.2 |
|
L % | 57.1 | 100 | | 74,2 | 83.5 | | 66.7 | 82.8 | |
|
R % | 42.9 | 0 | | 25.8 | 16.5 | | 33.3 | 17.2 | |
| Adenoma % | 14.3 | 7.7 | 0.44 | 33.2 | 19.8 | 0.001 | 44.2 | 35.6 | 0.28 |
| No adenoma,
mean | 1 | 1 | NS | 1.8 | 1.6 | 0.3 | 1.8 | 1.5 | 0.3 |
| HGD % | 7.1 | 2.6 | 0.56 | 5.3 | 3 | 0.24 | 3.5 | 6.8 | 0.3 |
| HGD size, mm,
mean | 9 | 12 | NS | 9.8 | 13.8 | 0.16 | 25.7 | 8.8 | 0.2 |
| HGD location | | | NS | | | 0.6 | | | NS |
|
L % | | 100 | | 50 | 62.5 | | 66.7 | 60 | |
|
R % | 100 | | | 50 | 37.5 | | 33.3 | 40 | |
| AA % | 7.1 | 2.6 | NS | 10.6 | 5.9 | 0.08 | 17.7 | 11 | 0.3 |
| AA size, mm,
mean | 9 | 10 | NS | 15 | 16 | 0.6 | 16.3 | 13.2 | 0.45 |
| AA location | | | 0.33 | | | 0.09 | | | 0.2 |
|
L % | | 100 | | 48.1 | 78.6 | | 42.1 | 75 | |
|
R % | 100 | | | 51.9 | 21.4 | | 57.9 | 25 | |
| Cancer % | 0 | 2.6 | NS | 0 | 0.8 | 0.49 | 4.4 | 2.7 | 0.7 |
| Serrated
polyp/adenoma % | 0 | 0 | | 11.5 | 8.9 | 0.36 | 5.3 | 1.4 | 0.25 |
| Maximum polyp size,
mm | | | 0.27 | | | 0.5 | | | 0.14 |
|
≤5 | 62.5% | 26.7% | | 28.1% | 31.6% | | 19.4% | 16.7% | |
|
6-10 | 37.5% | 60% | | 58.9% | 55.1% | | 54,2% | 75% | |
|
11-20 | 0 | 13.3% | | 11% | 13.3% | | 18.1% | 5.6% | |
|
21-40 | 0 | 0 | | 2.1% | 0 | | 8.3% | 2.8% | |
| NNS-adenoma | 18 | 22 | | 6 | 10 | | 4 | 7 | |
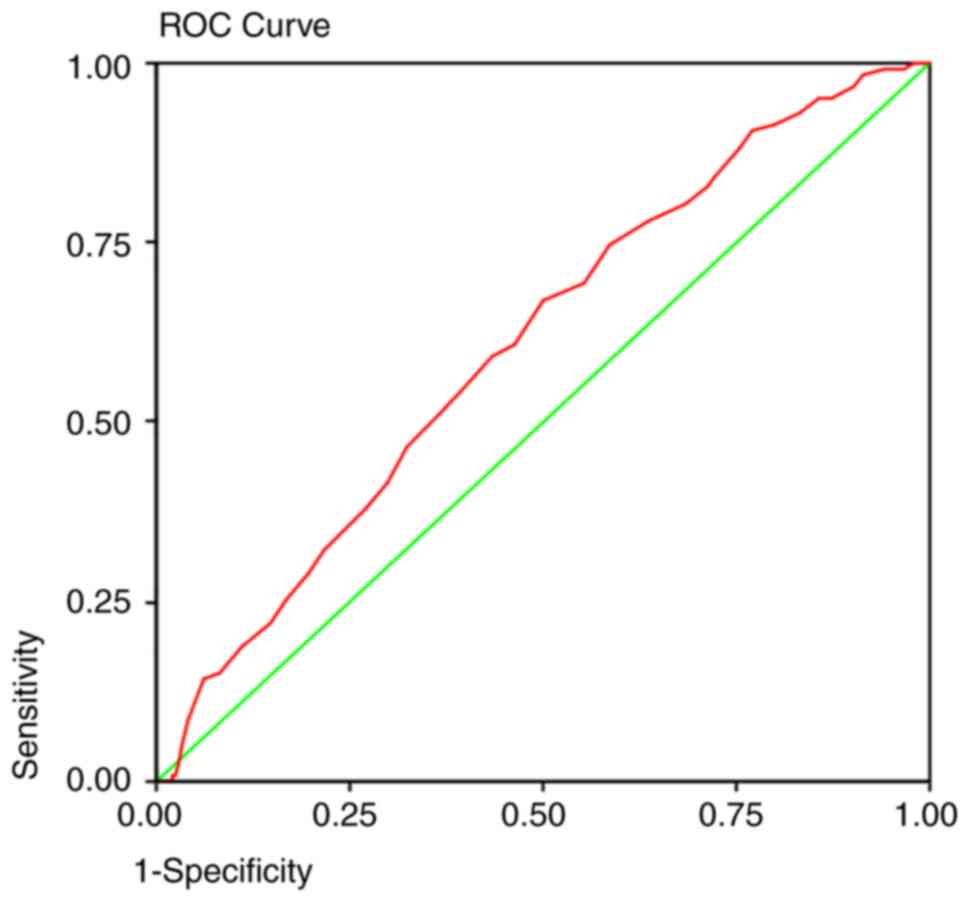
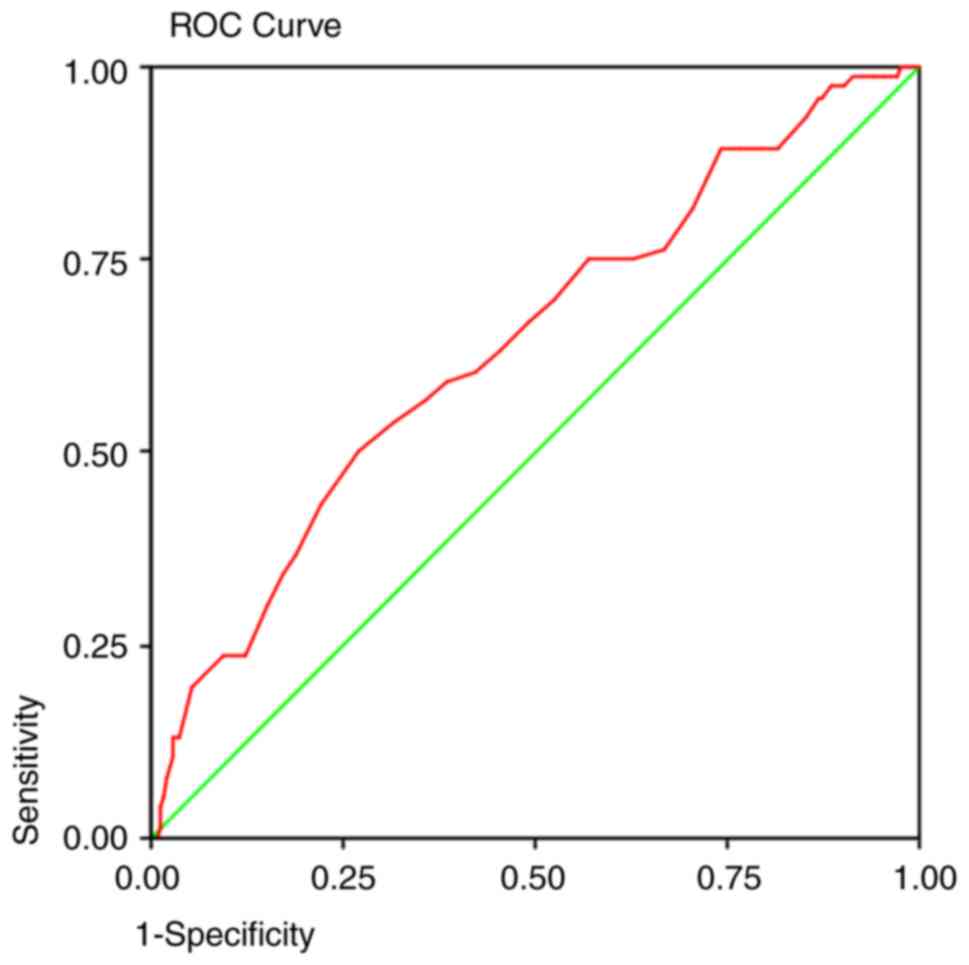
Implications based on ROC curves
analysis
ROC curves (Table
VI) were generated to predict the detection of adenomas at each
age group by colonoscopic screening. In every screening program,
one of the most important determinants is the sensitivity of the
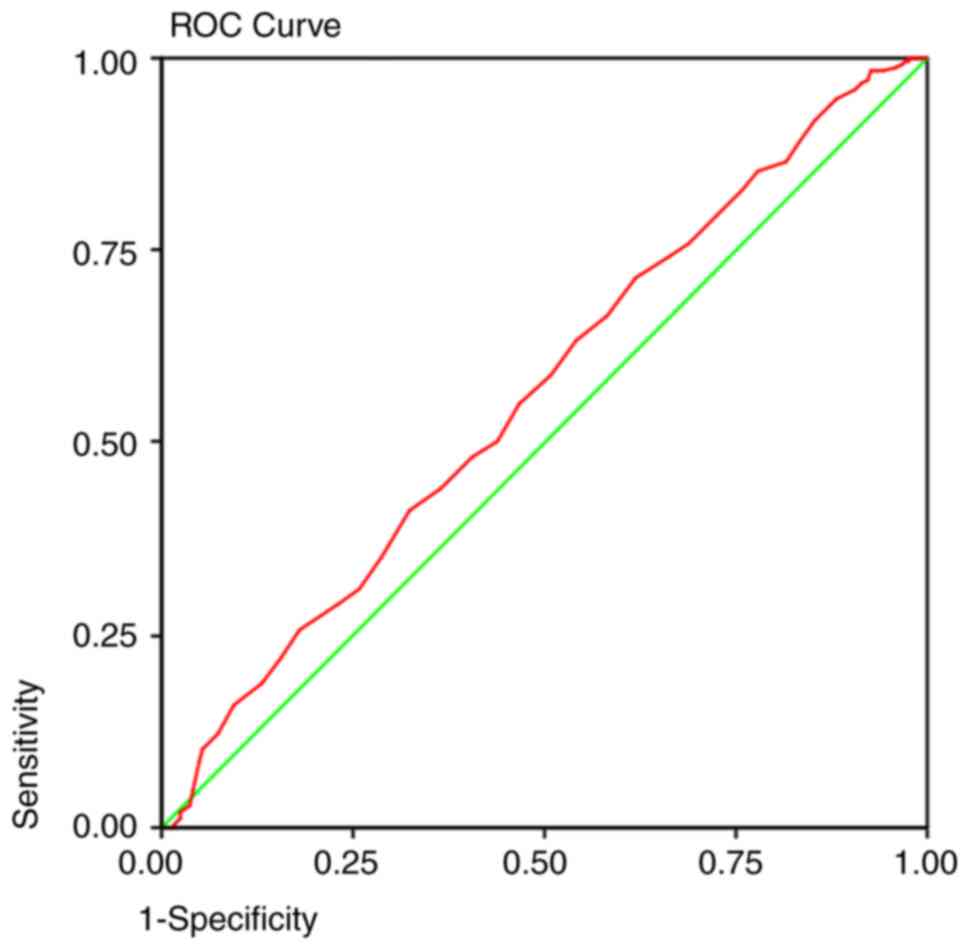
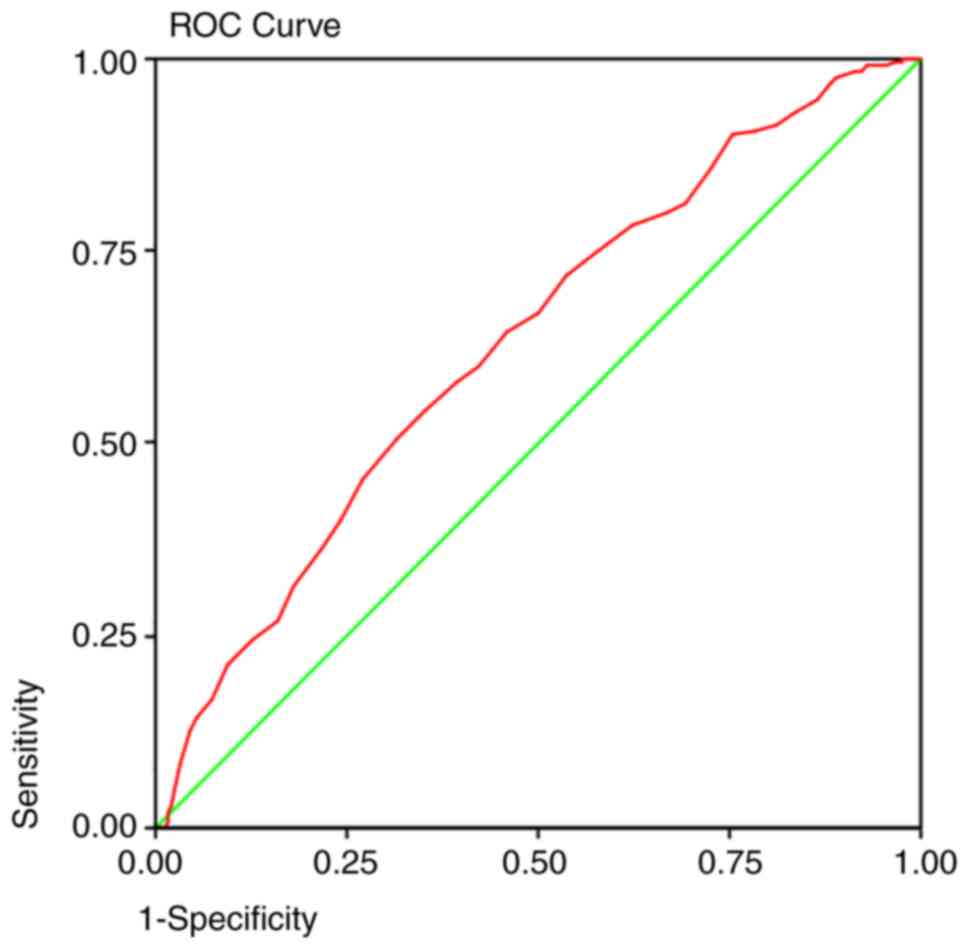
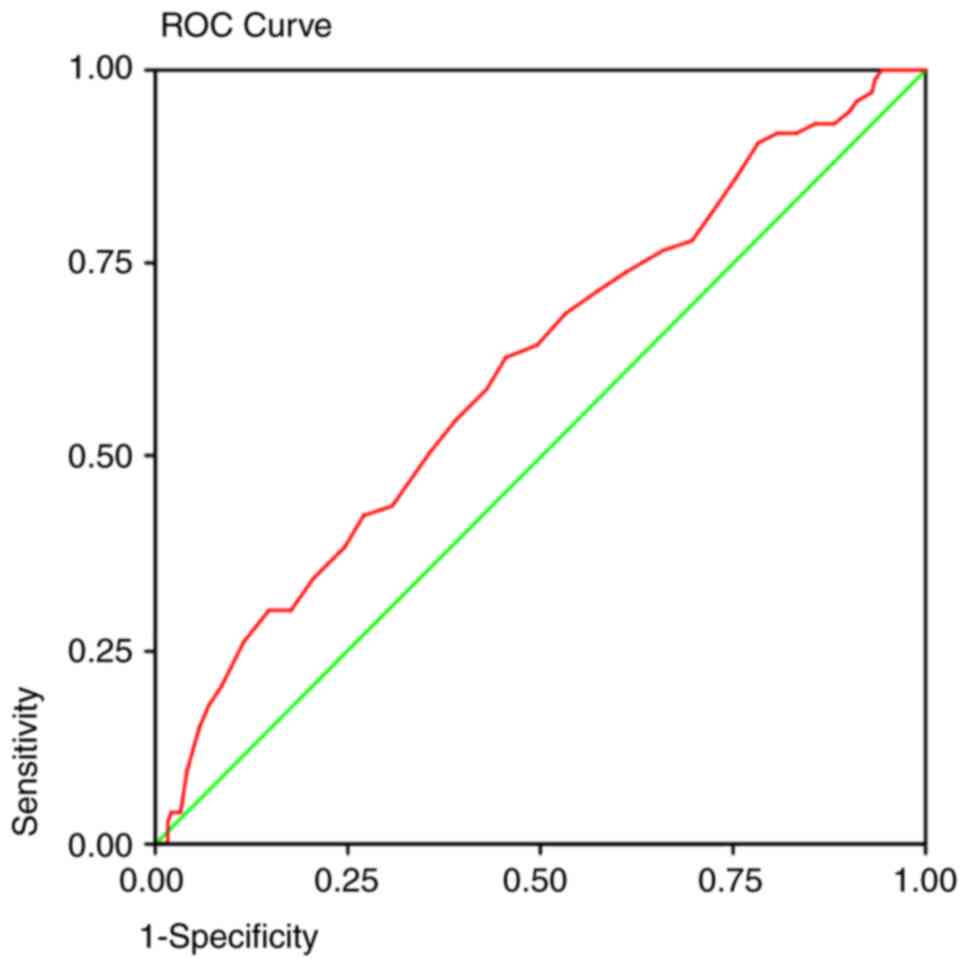
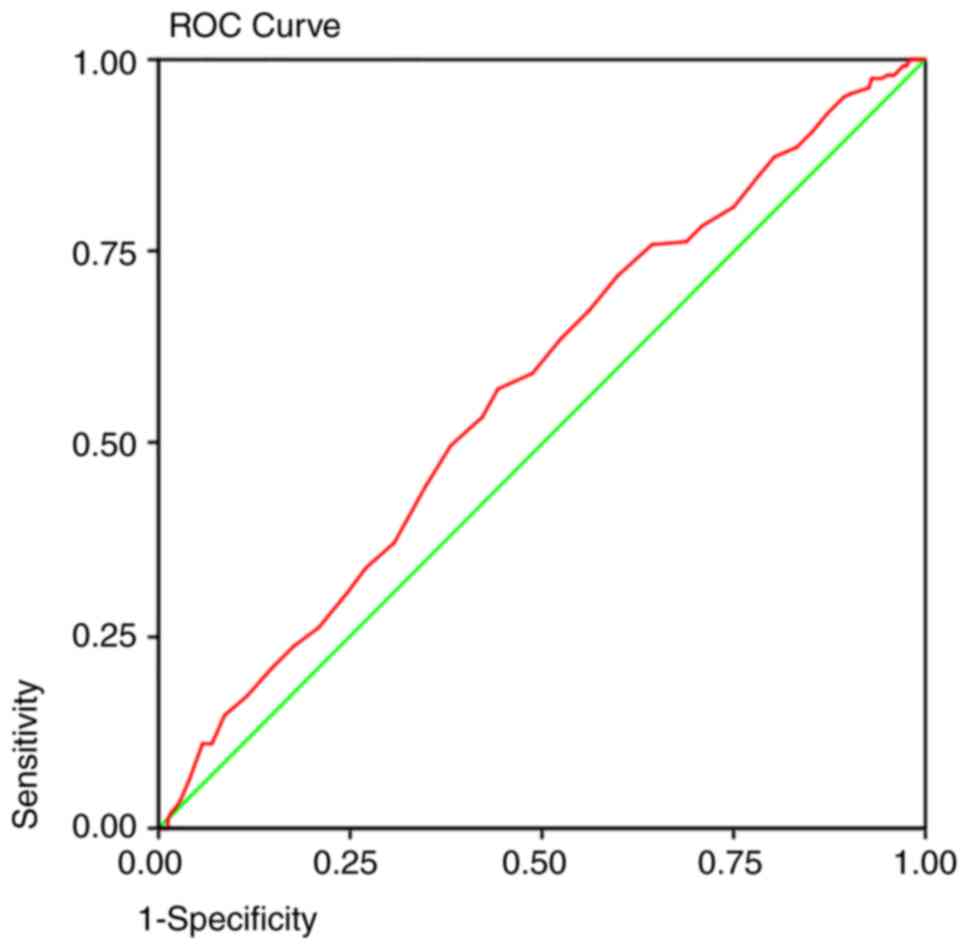
detection method. The area under the curve predicted that at 50
years old, a colonoscopy will detect 92% of the polyps (Fig. 1), 95% of the adenomas (Fig. 2), and 93% of the AA (Fig. 3) and proximal lesions (Fig. 4). When ROC curves were applied for
males and females separately (Figs.
5 and 6 respectively), the
area under the curve for the detection of adenomas demonstrated 95
and 93% sensitivity, respectively, at the same age limit (50 years
old).
 | Table VIArea Under The Curve and Cut-off
points of screening colonoscopy for identifying disease positive
patients. |
Table VI
Area Under The Curve and Cut-off
points of screening colonoscopy for identifying disease positive
patients.
| | Area | Asymptomatic
significance | 95% CI | Cut-off value | Sensitivity | Specificity |
|---|
| Polyps | 0.564 | 0.003 | 0.522-0.606 | 50 years | 0.92 | 0.85 |
| Adenomas | 0.631 | 0.000 | 0.586-0.675 | 50 years | 0.95 | 0.86 |
| AA | 0.613 | 0.002 | 0.545-0.682 | 50 years | 0.93 | 0.88 |
| Proximal
lesions | 0.573 | 0.005 | 0.523-0.622 | 50 years | 0.93 | 0.87 |
| Adenomas-male | 0.607 | 0.001 | 0.548-0.666 | 50 years | 0.95 | 0.87 |
|
Adenomas-female | 0.641 | 0.036 | 0.569-0.712 | 50 years | 0.93 | 0.85 |
| Adenomas | | | | 48 years | 0.98 | 0.90 |
| Adenomas-male | | | | 48 years | 0.98 | 0.92 |
|
Adenomas-female | | | | 46 years | 0.98 | 0.91 |
Discussion
Since 2003, when the European Council recommended
implementing CRC screening, a number of European countries have
initiated screening programs starting at the age of 50 years
(21). In Greece, although there
has been an attempt for an organized screening program according to
the National Oncological Plan during 2008-2012, until now,
screening has been applied only in certain hospitals on an
individual basis (22). The
present study revealed an overall prevalence of adenomas, AA, and
cancer of 28.6, 9.6 and 1.4%, respectively, among asymptomatic
average risk first-time participants presented by self-reference in
an open access colonoscopy screening program. Similar studies of
asymptomatic average-risk screening programs have shown rates of
adenomas, AA and cancer of 12.3-29, 2.5-7.1 and 0.7%, respectively
(13,23,24).
The results of the present study indicated an increased prevalence
rate, although the higher mean age of the participants compared
with the other studies may account for the difference.
The present study demonstrated a substantial rate of
adenomas in the 30-49 year old group (10.4%) and also a high
prevalence of AA (4.5%) and cancer (1.5%). Previous publications
from the USA, the Middle, and the Far East, including asymptomatic
average risk cohorts spanning from 1994-2014, have demonstrated a
similar prevalence of adenomas (8.3-17.3%) but lower AA (1-2.5%)
and cancer (0.2%) at 40-49 years old (25-29).
Consequently, it has been proposed that this age group with risk
factors such as obesity should start screening at 45 years old
(25).
Subcentimetric polyps constituted 85% of the cohort,
which is consistent with other studies previously mentioned
(13,14). The present study noted that 8.3% of
the 6-9 mm polyps could host advanced lesions, such as hgd, or
coexist with cancer in 0.9% of the cases. In the literature so far,
the pooled rate of AA in small polyps (6-9 mm) is ~4.9% (30) in asymptomatic average-risk groups.
Other studies from Europe and America, including asymptomatic but
not exclusively average-risk individuals, report the presence of AA
in 1.6-12.8% of the small polyps (15,31,32).
This is to remind the endoscopists that participate in screening
programs not to dismiss subcentimetric polyps easily and to be
cautious about the radical excision of such lesions.
These data regarding young adults and small polyps
necessitate reconsideration of current recommendations. The natural
history of precancerous lesions suggests that 37% of the adenomas
and 91% of AA will eventually progress to cancer with an annual
rate of 2.5-5.5% for AA, while ~6% of subcentimetric adenomas will
become AA in 2-3 years (33-35).
This indicates that advanced lesions at the early age group will
become cancer almost by certainty after 20-30 years, meaning in the
6th or 7th decade of life.
There are several but consistent data gathered from
different continents (America, Australia and Europe) (6,9,11)
noting that although absolute CRC incidence rates are lower in
women compared with men (8,11),
the annual percentage changes for women 30-49 years old are higher
between 1994 and 2016 ranging between APC 4.73-6.8% (6,10)
and they appeared to have been tripled (APC: 12) since
2009(5). In Europe, this
percentage change was associated with a cohort effect, with the
turning point being in the early 1990s (5,6).
Another study, including first-time asymptomatic average-risk
individuals, showed that women <50 years old had more advanced
neoplasia compared with men (6.2 vs. 0.6% respectively) (23). The present study noted an increase
in prevalence rates of polyps and a larger maximum polyp size in
women 30-49 years old relative to men. The majority of the serious
lesions were located in the left colon the opposite to that in men.
If this tendency is validated in other studies, it would be
reasonable to lower the screening age for younger women <50
years old with a flexible sigmoidoscopy as the initial examination
followed by colonoscopy at a later age according to current
guidelines.
The above findings differed in older age groups,
where men >50 years had statistically more serious lesions
compared with women while maintaining an increased prevalence of
right colon lesions. These findings were consistent with other
studies presenting increased incidence of proximal malignant
neoplasia in men 20-39 years old, especially since 2010 (5,12),
with higher left-sided lesions in women (10).
Furthermore, in the present study, proximal lesions
were found in a substantial proportion of participants (21.9%) and
39% of AA were located solely in the right colon. Right colon
lesions were detected in older age compared with distal lesions
with a predilection in men and larger maximum polyp size. This
notification is crucial for developing a screening program and the
determination of screening methods as sigmoidoscopy and fecal tests
are inadequate or less sensitive, respectively, for the detection
of proximal lesions (36). The
location has significant implications for screening since there is
a higher missing rate for proximal lesions (37) and these lesions are out of the
reach of sigmoidoscopy. In one meta-analysis, once only
sigmoidoscopy at the age of 55 years decreased the incidence and
mortality of CRC by 18 and 28%, respectively (38). This could be an option for younger
age women in whom the majority of lesions lie in the left colon and
since the NNS for the finding of one adenoma is higher compared
with men (22 vs. 18), it is difficult to justify a screening
colonoscopy at ages <50 years.
The ROC curves revealed that with the current
recommendation of screening colonoscopy at 50 years, 5-7% of
adenomas will remain undetected either due to the inability of the
detection method itself or because of a late referral of
individuals. Since the first proposal for initiation of screening
at 45 years for African Americans was suggested in 2005(39), certain Medical Societies, such as
in Saudi Arabia in 2015(19), the
US Multi-society Task Force in 2017(20) and recently the American Cancer
Society (18) have adopted this
recommendation for all adult average-risk population. In the
present case, we would expect to detect 98% of the overall adenomas
in both genders if we decided to lower the screening age at 48
years in men and 46 years in women. This age group is also
compatible with epidemiological data showing that CRC is an
important health risk, being the fourth most common cancer with
74.3% of its cases clustering in between 40 and 49 years old
(40). Cost-effectiveness analysis
modeling using estimated adenoma prevalence in different age
cohorts similar to the present study has concluded positively for
starting screening at 45 years (41). The selection of the acceptable age
for initiation of screening is a matter of public health policy and
available resources. Since the purpose of screening is to detect
precancerous lesions, meaning in practice adenomas, it would be
reasonable to detect as many of them as possible and as early in
life as possible since the cancers that are diagnosed at the age of
60 years stem from adenomas at the age of 40 years. Following this
concept, if it is a public necessity to detect 98% of the adenomas
in the screened population, one would need to start screening at
the age of 48 years and if one would like to detect the same number
in women as in men, then the initiation of screening should be at
46 years for women.
The present study has certain limitations. Some
limitations are inherent to the study type and the sample size.
Although the participants were prospectively entered into the
database, this is an observational cross-sectional study with an
adequate overall sample size but with a restricted earlier age
cohort and consisted of self-referred individuals since there is no
organized national screening program in Greece. This might
introduce type II errors and selection bias because the included
individuals could be considered more health-oriented with
health-seeking behaviors. A future meta-analysis could properly
collect and incorporate more data from various studies regarding
young adults who unfortunately remain behind screening
intentions.
Additionally, in one-third of the procedures,
trainees were actively involved, theoretically increasing the risk
of missing lesions. The present study was aware that proposals
regarding screening procedures at a population level should
consider multidimensional prerequisites concerning the disease
itself, health resources and local cost-effectiveness analysis.
This epidemiological analysis aims to provide answers about the
active disease profile of the Greek population, which is of
fundamental importance in activating the cascade of further
endeavor.
In conclusion, this is the largest study of
colorectal cancer screening of a Greek average risk population
sample so far. It noted emerging trends of increased prevalence in
precancerous and malignant colon lesions, especially important for
younger adults and, more specifically, women. It also focused on
the small polyps that host significant lesions in the screening
cohort and also focused on the proximal location of lesions in the
right colon as an additional parameter of missing lesions and
access strategy. Based on this disturbing data and the
corroborative epidemiologic surveillance profile from other
countries, it was proposed to lower the initiation age of screening
at 48 years for men with colonoscopy and at 46 years for women with
once-only sigmoidoscopy with subsequent surveillance following
current guidelines from the age of 50 years by colonoscopy. These
proposals are based on a Greek population sample and cannot be
extrapolated to other countries.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VP was responsible for conception and design of the
present study. Analysis and interpretation of the data was
performed by VP, GF, PK, NV, SV, GL, ID and EK. VP and EK drafted
the article and VP, ID and EK critically revised the article for
important intellectual content. VP, NV and SV confirm the
authenticity of all raw data. All authors reviewed and approved the
final manuscript.
Ethics approval and consent to
participate
All participants provided written informed consent.
The study has been performed in accordance with the ethical
standards of the 1964 Declaration of Helsinki and its later
amendments. The Institutional Review board of the Sismanogleio
General Hospital approved the study protocol (approval number
399/18-4-2019).
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
Authors' information
Vasileios Panteris: ORCID 0000-0003-1165-8927.
References
|
1
|
Edwards BK, Noone AM, Mariotto AB, Simard
EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA,
et al: Annual Report to the Nation on the status of cancer,
1975-2010, featuring prevalence of comorbidity and impact on
survival among persons with lung, colorectal, breast, or prostate
cancer. Cancer. 120:1290–1314. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jemal A, Ward EM, Johnson CJ, Cronin KA,
Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, et al:
Annual report to the Nation on the status of cancer, 1975-2014,
featuring survival. J Natl Cancer Inst. 109(djx030)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Henley SJ, Ward EM, Scott S, Ma J,
Anderson RN, Firth AU, Thomas CC, Islami F, Weir HK, Lewis DR, et
al: Annual report to the nation on the status of cancer, part I:
National cancer statistics. Cancer. 126:2225–2249. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Fedewa SA, Anderson WF, Miller
KD, Ma J, Rosenberg PS and Jemal A: Colorectal cancer incidence
patterns in the United States, 1974-2013. J Natl Cancer Inst.
109(djw322)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Exarchakou A, Donaldson LJ, Girardi F and
Coleman MP: Colorectal cancer incidence among young adults in
England: Trends by anatomical sub-site and deprivation. PLoS One.
14(e0225547)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vuik FE, Nieuwenburg SA, Bardou M,
Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, Zadnik V, Pellisé
M, Esteban L, Kaminski MF, et al: Increasing incidence of
colorectal cancer in young adults in Europe over the last 25 years.
Gut. 68:1820–1826. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bhandari A, Woodhouse M and Gupta S:
Colorectal cancer is a leading cause of cancer incidence and
mortality among adults younger than 50 years in the USA: A
SEER-based analysis with comparison to other young-onset cancers. J
Investig Med. 65:311–315. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ohri A, Robinson A, Liu B, Bhuket T and
Wong R: Updated assessment of colorectal cancer incidence in the
U.S. by age, sex, and race/ethnicity. Dig Dis Sci. 65:1838–1849.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Troeung L, Sodhi-Berry N, Martini A,
Malacova E, Ee H, O'Leary P, Lansdorp-Vogelaar I and Preen DB:
Increasing incidence of colorectal cancer in adolescents and young
adults aged 15-39 years in Western Australia 1982-2007: Examination
of Colonoscopy History. Front Public Health. 5(179)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Frostberg E and Rahr HB: Clinical
characteristics and a rising incidence of early-onset colorectal
cancer in a nationwide cohort of 521 patients aged 18-40 years.
Cancer Epidemiol. 66(101704)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singh KE, Taylor TH, Pan CG, Stamos MJ and
Zell JA: Colorectal cancer incidence among young adults in
California. J Adolesc Young Adult Oncol. 3:176–184. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Crosbie AB, Roche LM, Johnson LM, Pawlish
KS, Paddock LE and Stroup AM: Trends in colorectal cancer incidence
among younger adults-Disparities by age, sex, race, ethnicity, and
subsite. Cancer Med. 7:4077–4086. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bokemeyer B, Bock H, Hüppe D, Düffelmeyer
M, Rambow A, Tacke W and Koop H: Screening colonoscopy for
colorectal cancer prevention: Results from a German online registry
on 269000 cases. Eur J Gastroenterol Hepatol. 21:650–655.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lowenfels AB, Williams JL, Holub JL,
Maisonneuve P and Lieberman DA: Determinants of polyp size in
patients undergoing screening colonoscopy. BMC Gastroenterol.
11(101)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kolligs FT, Crispin A, Graser A, Munte A,
Mansmann U and Göke B: Risk factors for advanced neoplasia within
subcentimetric polyps: Implications for diagnostic imaging. Gut.
62:863–870. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Panteris V, Vezakis A and Triantafillidis
JK: Should hot biopsy forceps be abandoned for polypectomy of
diminutive colorectal polyps? World J Gastroenterol. 24:1579–1582.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Panteris V: The problem with cold snare
polypectomy of diminutive colorectal polyps. Scand J Gastroenterol.
55(1389)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wolf AMD, Fontham ETH, Church TR, Flowers
CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC,
Shih YT, et al: Colorectal cancer screening for average-risk
adults: 2018 guideline update from the American Cancer Society. CA
Cancer J Clin. 68:250–281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bénard F, Barkun AN, Martel M and von
Renteln D: Systematic review of colorectal cancer screening
guidelines for average-risk adults: Summarizing the current global
recommendations. World J Gastroenterol. 24:124–138. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rex DK, Boland CR, Dominitz JA, Giardiello
FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D and Robertson
DJ: Colorectal cancer screening: Recommendations for physicians and
patients from the U.S. Multi-society task force on colorectal
cancer. Gastroenterology. 153:307–323. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Navarro M, Nicolas A, Ferrandez A and
Lanas A: Colorectal cancer population screening programs worldwide
in 2016: An update. World J Gastroenterol. 23:3632–3642.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Altobelli E, Lattanzi A, Paduano R,
Varassi G and di Orio F: Colorectal cancer prevention in Europe:
Burden of disease and status of screening programs. Prev Med.
62:132–141. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Boursi B, Halak A, Umansky M, Galzan L,
Guzner-Gur H and Arber N: Colonoscopic screening of an average-risk
population for colorectal neoplasia. Endoscopy. 41:516–521.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang MH, Rampal S, Sung J, Choi YH, Son
HJ, Lee JH, Kim YH, Chang DK, Rhee PL, Rhee JC, et al: The
prevalence of colorectal adenomas in asymptomatic Korean men and
women. Cancer Epidemiol Biomarkers Prev. 23:499–507.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hong SN, Kim JH, Choe WH, Han HS, Sung IK,
Park HS and Shim CS: Prevalence and risk of colorectal neoplasms in
asymptomatic, average-risk screenees 40 to 49 years of age.
Gastrointest Endosc. 72:480–489. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hemmasi G, Sohrabi M, Zamani F, Ajdarkosh
H, Rakhshani N, Khoonsari M, Ameli M and Hatami K: Prevalence of
colorectal adenoma in an average-risk population aged 40-50 versus
50-60 years. Eur J Cancer Prev. 24:386–390. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rundle AG, Lebwohl B, Vogel R, Levine S
and Neugut AI: Colonoscopic screening in average-risk individuals
ages 40 to 49 vs 50 to 59 years. Gastroenterology. 134:1311–1315.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Strul H, Kariv R, Leshno M, Halak A,
Jakubowicz M, Santo M, Umansky M, Shirin H, Degani Y, Revivo M, et
al: The prevalence rate and anatomic location of colorectal adenoma
and cancer detected by colonoscopy in average-risk individuals aged
40-80 years. Am J Gastroenterol. 101:255–262. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Leshno A, Moshkowitz M, David M, Galazan
L, Neugut AI, Arber N and Santo E: Prevalence of colorectal
neoplasms in young, average risk individuals: A turning tide
between East and West. World J Gastroenterol. 22:7365–7372.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hassan C, Pickhardt PJ, Kim DH, Di Giulio
E, Zullo A, Laghi A, Repici A, Iafrate F, Osborn J and Annibale B:
Systematic review: Distribution of advanced neoplasia according to
polyp size at screening colonoscopy. Aliment Pharmacol Ther.
31:210–217. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Butterly LF, Chase MP, Pohl H and Fiarman
GS: Prevalence of clinically important histology in small adenomas.
Clin Gastroenterol Hepatol. 4:343–348. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gupta N, Bansal A, Rao D, Early DS,
Jonnalagadda S, Wani SB, Edmundowicz SA, Sharma P and Rastogi A:
Prevalence of advanced histological features in diminutive and
small colon polyps. Gastrointest Endosc. 75:1022–1030.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brenner H, Hoffmeister M, Stegmaier C,
Brenner G, Altenhofen L and Haug U: Risk of progression of advanced
adenomas to colorectal cancer by age and sex: Estimates based on
840,149 screening colonoscopies. Gut. 56:1585–1589. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vleugels JLA, Hazewinkel Y, Fockens P and
Dekker E: Natural history of diminutive and small colorectal
polyps: A systematic literature review. Gastrointest Endosc.
85:1169–1176.e1. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pickhardt PJ, Kim DH, Pooler BD, Hinshaw
JL, Barlow D, Jensen D, Reichelderfer M and Cash BD: Assessment of
volumetric growth rates of small colorectal polyps with CT
colonography: A longitudinal study of natural history. Lancet
Oncol. 14:711–720. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Meza R, Jeon J, Renehan AG and Luebeck EG:
Colorectal cancer incidence trends in the United States and United
kingdom: Evidence of right- to left-sided biological gradients with
implications for screening. Cancer Res. 70:5419–5429.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Haug U, Knudsen AB, Brenner H and Kuntz
KM: Is fecal occult blood testing more sensitive for left-versus
right-sided colorectal neoplasia? A systematic literature review.
Expert Rev Mol Diagn. 11:605–616. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Massat NJ, Moss SM, Halloran SP and Duffy
SW: Screening and primary prevention of colorectal cancer: A review
of sex-specific and site-specific differences. J Med Screen.
20:125–148. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Agrawal S, Bhupinderjit A, Bhutani MS,
Boardman L, Nguyen C, Romero Y, Srinivasan R and Figueroa-Moseley
C: Committee of Minority Affairs and Cultural Diversity, American
College of Gastroenterology. Colorectal cancer in African
Americans. Am J Gastroenterol. 100:515–523; discussion 514.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fairley TL, Cardinez CJ, Martin J, Alley
L, Friedman C, Edwards B and Jamison P: Colorectal cancer in U.S.
adults younger than 50 years of age, 1998-2001. Cancer. 107 (Suppl
5):S1153–S1161. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Knudsen AB, Zauber AG, Rutter CM, Naber
SK, Doria-Rose VP, Pabiniak C, Johanson C, Fischer SE,
Lansdorp-Vogelaar I and Kuntz KM: Estimation of benefits, burden,
and harms of colorectal cancer screening strategies: Modeling study
for the US preventive services task force. JAMA. 315:2595–2609.
2016.PubMed/NCBI View Article : Google Scholar
|