Introduction
Oxaliplatin is a cytostatic antineoplastic drug that
belongs to a novel class of platinum compounds, in which the
platinum ion forms a complex with 1,2-diaminocyclohexane and an
oxalate group (1). Previous
studies on the mechanism of action of oxaliplatin, although not
fully elucidated, have reported that the hydrated derivatives
formed due to the biotransformation of oxaliplatin interact with
DNA. These molecules form intra- and inter-stranded bridges with
DNA and, therefore, interrupt DNA synthesis, which results in the
cytotoxic and antitumor activity of oxaliplatin (1,2).
Cisplatin, carboplatin and oxaliplatin are composed of
double-charged platinum ions surrounded by four ligands. The amine
ligands are situated on the left, which form strong interactions
with the platinum ion, whereas the chloride ligands or carboxylate
compounds are situated on the right, which form leaving groups
allowing the platinum ion to form bonds with DNA bases (3,4).
Oxaliplatin was considered to be a non-vesicant
until the 2000s when tissue necrosis from extravasation was
described (5). However, in certain
reviews oxaliplatin is classified as an irritant (6). At present oxaliplatin is considered
to have both irritant and vesicant properties (6,7).
In cancer therapy, extravasation refers to the
inadvertent infiltration of chemotherapy into the subcutaneous or
subdermal tissues surrounding the intravenous or intra-arterial
administration site (6). When
extravasation occurs the degree of damage is dependent on the
agent, the drug concentration, dose and volume, its pH (7.35-7.40)
and osmolarity distance from normality (281-282 mOsm/l), as well as
the location of the extravasation event and the duration of cell
exposure to the drug (8).
Patient-dependent risk factors of chemotherapy extravasation
include small and/or fragile veins, lymphedema, obesity, impaired
level of consciousness and numerous previous venipunctures.
Iatrogenic factors, which can contribute to extravasation, include
lack of proper nursing staff training, selection of the wrong
cannula size, suboptimal location, accidental puncturing of the
vein or movement of the cannula due to patient movement or insecure
fixing (9,10). The extravasation severity and the
potential sequelae highlight the fact that it is important to
distinguish extravasation from other local reactions to
chemotherapy. It is also crucial that the associated risk of the
cannulation procedure is reduced and that medical staff are aware
of the signs and symptoms of extravasation and are familiar with
its management.
Case report
A 64-year-old female patient presented with a
4-month history of anorectal pain, tenesmus, asthenia and weakness.
The patient's medical history included arterial hypertension and
diabetes mellitus. A colonoscopy revealed an ulcerated mass within
10 cm of the anal verge and the histological report determined this
to be a colorectal adenocarcinoma. Molecular analysis of the mass
determined the tumor to have microsatellite stability and a KRAS
mutation. A computed tomography (CT) scan of the chest, abdomen and
pelvis revealed a voluminous rectal mass with vaginal fistulation
and vesical infiltration, as well as numerous mesenteric and
retroperitoneal adenopathies. The patient was diagnosed with a
rectal adenocarcinoma with unresectable metastatic adenopathies. A
folinic acid, fluorouracil and oxaliplatin (FOLFOX-4) chemotherapy
regimen was selected as the most suitable approach. Treatment was
initiated using a subcutaneous pump for ambulatory administration,
with venous access to the right subclavian vein via a port-a-cath
device. The port-a-cath was inserted in 2017, using ultrasound to
guide the catheter along the subclavian vein. Following its
insertion, an X-ray was performed to check the central line
position was correct and to scan for any initial complications.
Following three cycles of chemotherapy, the patient
exhibited no signs of significant toxicity. However, during the
fourth cycle of FOLFOX-4 treatment, the patient reported a sudden
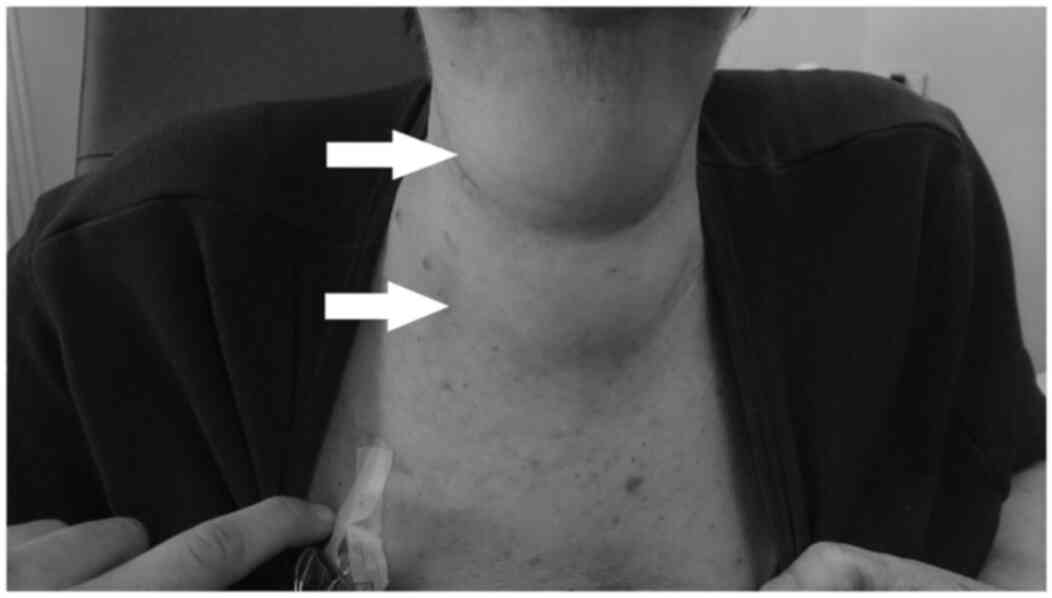
throbbing pain in the chest wall and anterior cervical region, with
the appearance of a soft edema that coincided with these painful
areas. As extravasation of oxaliplatin was suspected, the infusion
was interrupted, the content of the port-a-cath device was
aspirated and local dry heat was applied. A bolus of intravenous
dexamethasone (12 mg) was administered and the patient was referred
to the emergency department for further monitoring and
treatment.
An urgent blood test revealed the elevation of
C-reactive protein; however, this was the only abnormality detected
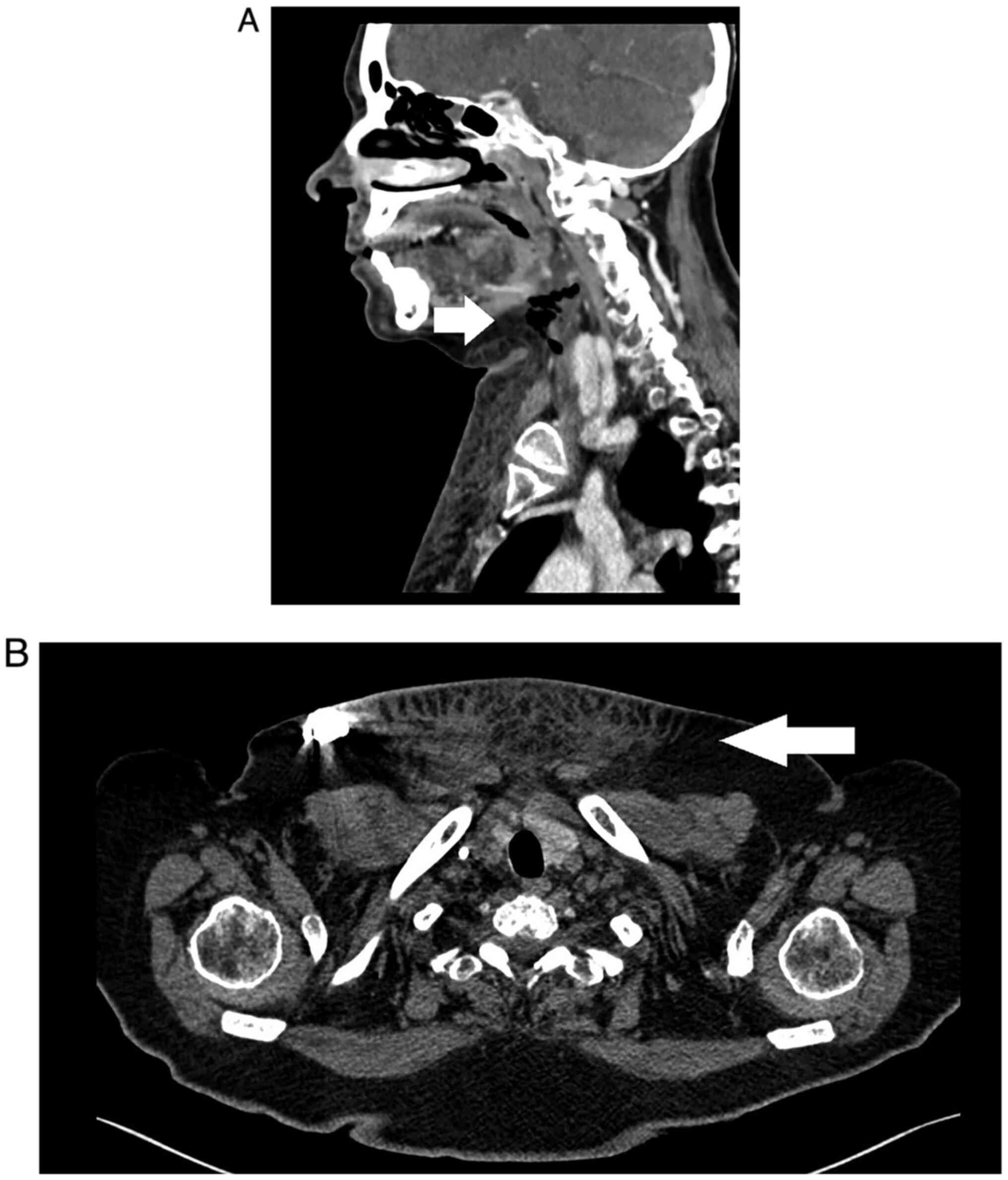
in the patient's blood. A CT scan identified an anterior cervical
collection and jugular-subclavian venous confluence at the distal
end, with presence of bubbles close to the venous access site of
the subcutaneous port-a-cath, which extended cranially dissecting
laterocervical planes and formed a hydro-aerial collection located
in the submaxillary region of 8x15x25 mm. Furthermore, subcutaneous
inflammation was identified in the upper third and anterior thorax,
ascending through laterocervical planes to the homolateral
submaxillary region (Figs. 1 and
2).
The patient was admitted to the hospital for
monitoring and continued steroid (dexamethasone 4 mg iv/8 h) and
analgesic treatment. The patient was not treated with antibiotics,
as she remained afebrile and exhibited no signs of infection. Edema
and pain had improved at 12 h following admission and erythema and
local induration were present in the thoracic and cervical area
where extravasation had occurred. The port-a-cath device was
removed without complications. The device did not show signs of
damage, holes or any leakage points.
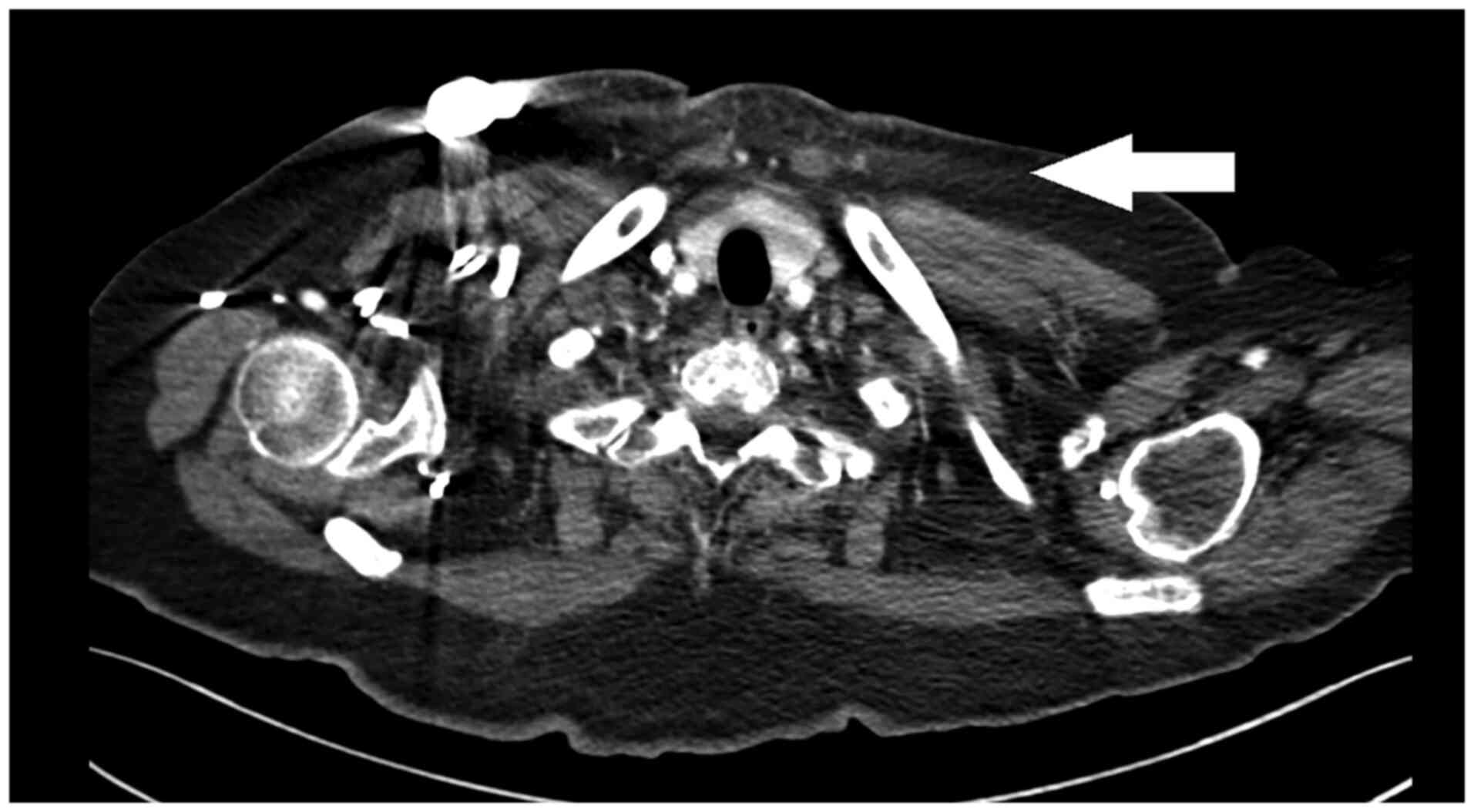
A CT scan was performed 10 days after admission and
demonstrated resolution of the cervical hydro-aerial collection and
a significant improvement in inflammation, which was non-existent
in the cervical area but persisted in the anterior thoracic region
(Fig. 3). The patient did not
present with any sequelae at the cervical level 2 weeks following
discharge from hospital. In the thoracic region, a scar-like
induration was observed in the area of greatest exposure to the
extravasation event; however, this was not painful. Skin integrity
was maintained throughout the process. Due to this extravasation
event, the patient refused to continue chemotherapy treatment and
succumbed to disease progression after 12 months.
Discussion
Extravasation of chemotherapy agents administered
via a central venous access device (CVAD) is a rare complication.
Its prevalence can range from 0.1-6% when chemotherapy is
administered via a peripheral intravenous access point and from
0.26-4.7% when administered via a CVAD (11,12).
In the case of CVAD, when extravasation occurs the drug solution
may accumulate in the mediastinum, pleura or in a subcutaneous area
of the chest or neck. The most frequent symptom of central line
extravasation is acute thoracic pain. Diagnosis of this condition
should be based on clinical presentation and further confirmed by
imaging, such as a thoracic CT scan. Current data on the management
and evolution of chemotherapy extravasation of CVAD are currently
based on previous case reports (7,13).
As demonstrated in the present study, the management of oxaliplatin
extravasation should include discontinuing the infusion and
aspirating as much of the chemotherapeutic agent as possible via
the CVAD. If the extravasated agent is an anthracycline,
dexrazoxane administration may be considered as an antidote.
However, in previously reported cases, conventional therapy was the
preferred approach, whereby surgical procedures, with the objective
of draining the remaining solution, may also be considered
(14,15). Antibiotics, endovenous
corticosteroids, analgesia and other treatments leading to the
control of the symptoms derived from the mediastinitis or pleuritis
secondary to extravasation, should also be administered as
appropriate (7,13).
Common symptoms related to extravasation include
tingling, burning, discomfort/pain, swelling and redness at the
injection site (16,17). Late symptoms may include
blistering, necrosis and ulceration. Signs that indicate
extravasation may occur are the absence of blood return, resistance
on the plunger of the syringe during delivery of a bolus drug, or
an interruption to the free flow of an infusion (7). Supportive and non-specific approaches
have been described in managing cytostatic drug extravasation, as
there are currently no direct antidotes (8). Prolonged peripheral line infusions of
vesicants are associated with an increased risk of extravasation
(8). Therefore, vesicants should
not be administered as prolonged unsupervised infusions via
peripheral veins, as they are more likely to cause complications,
including tissue necrosis. If this occurs, the infusion should be
stopped immediately. When several rounds of treatment are needed
and there are pre-existing extravasation risk factors, it is
recommended that the drug is administered via a central line
(8,9,16).
Extravasation via a central line is most commonly
caused by a dislodged needle in the port (15). It is therefore important that the
proper positioning of the port is assessed using imaging following
the procedure. Previous case studies have also described this
procedure in patients with peripherally inserted central catheters
(11). Oncology nurses must follow
institutional protocols for the assessment of central venous access
if extravasation occurs while using a CVAD (14). Furthermore, when managing
extravasation, it has been reported that hypertonic solutions can
further increase tissue injury and lead to tissue necrosis
(9), whereas cold treatment can
aggravate neuropathy. A warm compress may increase drug removal by
local vasodilation, which may help avoid peripheral neuropathy
(18); however, it may potentially
increase cellular uptake and, therefore, injury. Further research
into this area is needed (4,19).
A study identifying five oxaliplatin extravasation
cases, including a case involving high doses of oxaliplatin (>40
mg), where local cooling was applied and induration and localized
pain were exhibited by several patients, was previously published
(20). However, this treatment
strategy is not recommended, as the application of localized cold
treatment with a cytostatic compound may increase the risk of
peripheral neuropathy. Therefore, the application of local heat is
currently recommended, although, to the best of the authors'
knowledge, there are no studies describing this treatment. It is a
common practice to apply heat, for 30 min, or for 15 min every 6 h
over 2 days (4,5,11,20-22).
Treatment recommendations rarely come from controlled clinical
trials, but are often based on animal models, case studies or small
uncontrolled studies, due to the following: Ethics, the rarity of
the event, the lack of clear definition in the efficacy of
treatments (such as surgical intervention), impairment of mobility
and aesthetic sequelae and poorly studied anecdotal treatment
strategies (including sulphadiazine and corticoids) (12,18).
In summary, there are currently few guidelines on
how to manage oxaliplatin extravasation. In most cases, a warm, dry
compress is applied, in contrast to other platins, which require
wet dressings.
The cervical extravasation of other specific drugs
has also previously been described in detail (17,18),
such as in a patient treated with bevacizumab for colorectal
cancer. In that case, extravasation occurred during chemotherapy
infusion due to a catheter migration of the port outside of the
superior vena cava, causing cervical pain without skin
manifestations. Conservative management was proposed. The patient
fully recovered from all symptoms within 3 weeks. Physicians should
also be aware that, in cases of bevacizumab extravasation, a
non-surgical approach may be effective. To the best of the authors'
knowledge, there has only been a single case report on tissue
damage following paravasal infusion of oxaliplatin. In this
previous case report, a 62-year-old male patient with colon cancer
received adjuvant chemotherapy and presented with extensive tissue
damage following oxaliplatin extravasation in the left antecubital
region. Despite the severity of the extravasation event and a
prolonged stay in hospital, the patient had almost fully recovered
at 8 months, without surgical intervention. The patient had
exhibited a high temperature and presented with clinical signs of
infection; however, directed treatment using several antibiotics
was ineffective. Recovery occurred gradually following
extravasation, including lymph drainage and the administration of
prednisone (12).
In conclusion, cervical extravasation of oxaliplatin
is a unique event that has not previously been reported in the
literature, to the best of the authors' knowledge. The increased
use of central venous catheters to infuse this drug may lead to
similar cases being reported in the future. Although severe tissue
inflammation and necrosis have been reported in cases involving
soft tissue, the present study suggested that cervical oxaliplatin
extravasation can be managed with close observation and symptomatic
treatment alone. Individual cases, however, may require a more
aggressive approach. It is crucial that roper catheter positioning
is confirmed prior to drug administration.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH and JRA contributed to the literature review,
writing the manuscript, analysis of clinical information and case
discussion. MR, AG and JC analyzed patient data and advised on
patient treatment. JH and JRA confirm the authenticity of all the
raw data. All the authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Patient consent for publication was approved by
ethics approval from Vall d'Hebron University Hospital (Spain).
Patient consent for publication
Written informed consent was obtained from the
family delegate of the patient for publication of the clinical data
and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yuan X, Zhang W, He Y, Yuan J, Song D,
Chen H, Qin W, Qian X, Yu H and Guo Z: Proteomic analysis of
cisplatin- and oxaliplatin-induced phosphorylation in proteins
bound to Pt-DNA adducts. Metallomics. 12:1834–1840. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Spanish Agency for Medicines and Health
Products (AEMPS): Technical datasheet of oxaliplatin. AEMPS,
Madrid, 2021. https://cima.aemps.es/cima/publico.html. Accessed
January 22, 2021.
|
|
3
|
Goodsell DS: The molecular perspective:
Cisplatin. Oncologist. 11:316–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bahadori F and Demiray M: Management of
extravasation of oxaliplatin by mimicking its biotransformation.
Clin Transl Oncol. 20:1353–1357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Baur M, Kienzer HR, Rath T and Dittrich C:
Extravasation of oxaliplatin (Eloxatin((R)))-clinical course.
Onkologi. 23:468–471. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pérez Fidalgo JA, García Fabregat L,
Cervantes A, Margulies A, Vidall C and Roila F: ESMO Guidelines
Working Group. Management of chemotherapy extravasation: ESMO-EONS
clinical practice guidelines. Ann Oncol. 23 (Suppl
7):vii167–vii173. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pericay C, López A, Soler JR, Bonfill T,
Dotor E and Saigí E: Extravasation of oxaliplatin: An infrequent
and irritant toxicity. Clin Transl Oncol. 11:114–116.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wickham R, Engelking C, Sauerland C and
Corbi D: Vesicant extravasation part II: Evidence-based management
and continuing controversies. Oncol Nurs Forum. 33:1143–1150.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kreidieh FY, Moukadem HA and El Saghir NS:
Overview, prevention and management of chemotherapy extravasation.
World J Clin Oncol. 7:87–97. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Narducci F, Jean-Laurent M, Boulanger L,
El Bédoui S, Mallet Y, Houpeau JL, Hamdani A, Penel N and Fournier
C: Totally implantable venous access port systems and risk factors
for complications: A one-year prospective study in a cancer centre.
Eur J Surg Oncol. 37:913–918. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Masters B, Hickish T and Cidon EU: A
midline for oxaliplatin infusion: The myth of safety devices. BMJ
Case Rep. 2014(bcr2014204360)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Boesen C and Nielsen SE: Reversible tissue
damage after paravasal infusion of oxaliplatin. Ugeskr Laeger.
177(V07140390)2015.PubMed/NCBI(In Danish).
|
|
13
|
Leon-Ferre RA, Abu Hejleh TB and
Halfdanarson TR: Extravasation of oxaliplatin into the mediastinum:
A case report and review of the literature. Clin Adv Hematol Oncol.
10:546–548. 2012.PubMed/NCBI
|
|
14
|
Haslik W, Hacker S, Felberbauer FX,
Thallinger C, Bartsch R, Kornauth C, Deutschmann C and Mader RM:
Port-a-Cath extravasation of vesicant cytotoxics: Surgical options
for a rare complication of cancer chemotherapy. Eur J Surg Oncol.
41:378–385. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Azaïs H, Bresson L, Bassil A, Katdare N,
Merlot B, Houpeau JL, El Bedoui S, Meurant JP, Tresch E and
Narducci F: Chemotherapy drug extravasation in totally implantable
venous access port systems: How effective is early surgical lavage?
J Vasc Access. 16:31–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Makrilia N, Syrigou E, Kaklamanos I,
Manolopoulos L and Saif MW: Hypersensitivity reactions associated
with platinum antineoplastic agents: A systematic review. Met Based
Drugs. 2010(207084)2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sorich J, Taubes B, Wagner A and Hochster
H: Oxaliplatin: Practical guidelines for administration. Clin J
Oncol Nurs. 8:251–256. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Markman M: Toxicities of the platinum
antineoplastic agents. Expert Opin Drug Saf. 2:597–607.
2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
de Lemos ML and Walisser S: Management of
extravasation of oxaliplatin. J Oncol Pharm Pract. 11:159–162.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kretzschmar A, Pink D, Thuss-Patience P,
Dörken B, Reichert P and Eckert R: Extravasations of oxaliplatin. J
Clin Oncol. 21:4068–4069. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Foo KF, Michael M, Toner G and Zalcberg J:
A case report of oxaliplatin extravasation. Ann Oncol. 14:961–962.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Conde-Estévez D and Mateu-de Antonio J:
Update in the management of extravasations of cytocytostatic agent.
Farm Hosp. 36:34–42. 2012.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|