Introduction
Triple-negative breast cancer (TNBC), characterised
by the lack of oestrogen and progesterone receptors and human
epidermal growth factor receptor 2 (HER2) expression, occurs in
approximately 12-17% of breast cancer patients (1,2). It
shows an aggressive clinical behaviour and a high rate of local and
distant relapse after treatment compared to other subtypes of
breast cancer (2-4).
Therefore, there is an urgent need to develop new treatments and
biomarkers for TNBC. While normal cells mainly produce energy by
aerobic phosphorylation through the tricarboxylic acid cycle,
cancer cells produce energy via anaerobic glycolysis and other
metabolic pathways. Oncogenic metabolic pathways differ depending
on the tumour type; therefore, developing therapies against tumour
metabolism is not straightforward (5). Lipid metabolism is a crucial pathway
in tumour progression, and cancer cells typically accumulate lipids
(6,7).
Adipophilin (ADP) is a lipid-associated protein that
coats the surface of intracytoplasmic lipid droplets (8,9) ADP
expression in tumour cells is correlated with a poor prognosis in
some types of carcinomas, including lung adenocarcinoma (10) and pancreatic ductal adenocarcinoma
(11). Recently, we demonstrated
via multivariate analysis that ADP expression is an independent
indicator of a poor prognosis for patients with TNBC, while widely
used prognostic factors, such as the Ki-67 labeling index (LI) and
the Nottingham Prognostic Index, and tumor size were not
independent (12). Fatty acid
synthase (FASN) is a critical lipogenic enzyme overexpressed in
various human cancers, including salivary gland tumours (13,14).
FASN expression has been reported to be associated with a poor
prognosis in several types of tumours (15,16);
thus, ADP expression in carcinoma cells might be related and occur
via overexpression of FASN. In addition, FASN expression has been
reported to correlate with the frequency of lymph node metastasis
but is uncorrelated with prognosis in TNBC (17). Although an inverse correlation
between FASN and ADP expression has been reported in salivary duct
carcinomas (18), the relationship
between FASN and ADP in TNBC remains unclear. The present study
aimed to evaluate the prognostic role of FASN expression and assess
the correlation between FASN and ADP expression in TNBC
patients.
Materials and methods
Patient selection
We selected 165 consecutive patients with TNBC who
underwent surgical resection at the Department of Surgery of the
Kansai Medical University Hospital between January 2006 and
December 2018. Patients who were diagnosed with invasive breast
carcinoma of no special type according to the recent World Health
Organization Classification of Breast Tumors (19) were selected. The exclusion criteria
of the present study were as follows: patients who were
administered neoadjuvant chemotherapy and who had a particular type
of invasive carcinoma, such as apocrine carcinoma. The study cohort
comprised 61 TNBC patients.
The patient cohort in the present study overlaps
with that of our previous studies (12,20,21).
Our previous study analysed the prognostic significance of ADP
expression in tissue microarrays using operative specimens from
patients with TNBC (12). The
present study included information regarding the ADP expression
status of operative specimens from the previous study (12). Moreover, we previously examined the
relationship between clinicopathological features and
PD-L1-positive cancer-associated fibroblasts (20) or CD155, an immune-checkpoint
protein (21), in patients with
TNBC using tissue microarrays from operative specimens. The
contents of the present study do not overlap with those of these
two studies (20,21).
This retrospective single-institution study was
conducted following the principles of the Declaration of Helsinki,
and the study protocol was approved by the Institutional Review
Board of the Kansai Medical University Hospital (Approval
#2019234). All the data were anonymised. The institutional review
board waived the requirement for informed consent because of the
retrospective design of the study using medical records and
archival samples, with no risk to the participants. Moreover, the
present study does not include minors. Information regarding this
study, such as the inclusion criteria and opportunity to opt out,
was provided through the institutional website (https://www.kmu.ac.jp/hirakata/hospital/2671t800000136cd-att/a1582783269511.pdf).
Histopathological analysis
Surgically resected specimens were fixed with
formalin, sectioned, and stained with hematoxylin and eosin. More
than two experienced pathologists independently evaluated
histopathological features. We used the TNM Classification of
Malignant Tumours, Eighth edition. The histopathological grading
was based on the Nottingham histological grade (22). The Ki-67 labelling index (LI) was
considered high when ≥40% of neoplastic cells were labelled
(23).
Tissue microarray
Hematoxylin and eosin-stained slides were used to
select the most morphologically representative carcinoma regions;
three tissue cores of 2 mm in diameter were punched out from the
paraffin-embedded blocks for each patient. Tissue cores were
arrayed in the recipient paraffin blocks. These specimens were also
used in our previous study (12,20,21).
Immunohistochemistry
Immunohistochemical analyses were performed using an
autostainer (Discovery Ultra System; Roche Diagnostics, Basel,
Switzerland) according to the manufacturer's instructions [OptiView
DAB Universal Kit (cat. no. 518-111427; Roche)]. Primary mouse
monoclonal antibody against FASN (clone 23: BD Biosciences; diluted
1:200) was used. Secondary antibody was pre-diluted [OptiView DAB
Universal Kit (cat. no. 518-111427; Roche)]. At least two
researchers independently evaluated immunohistochemical
staining.
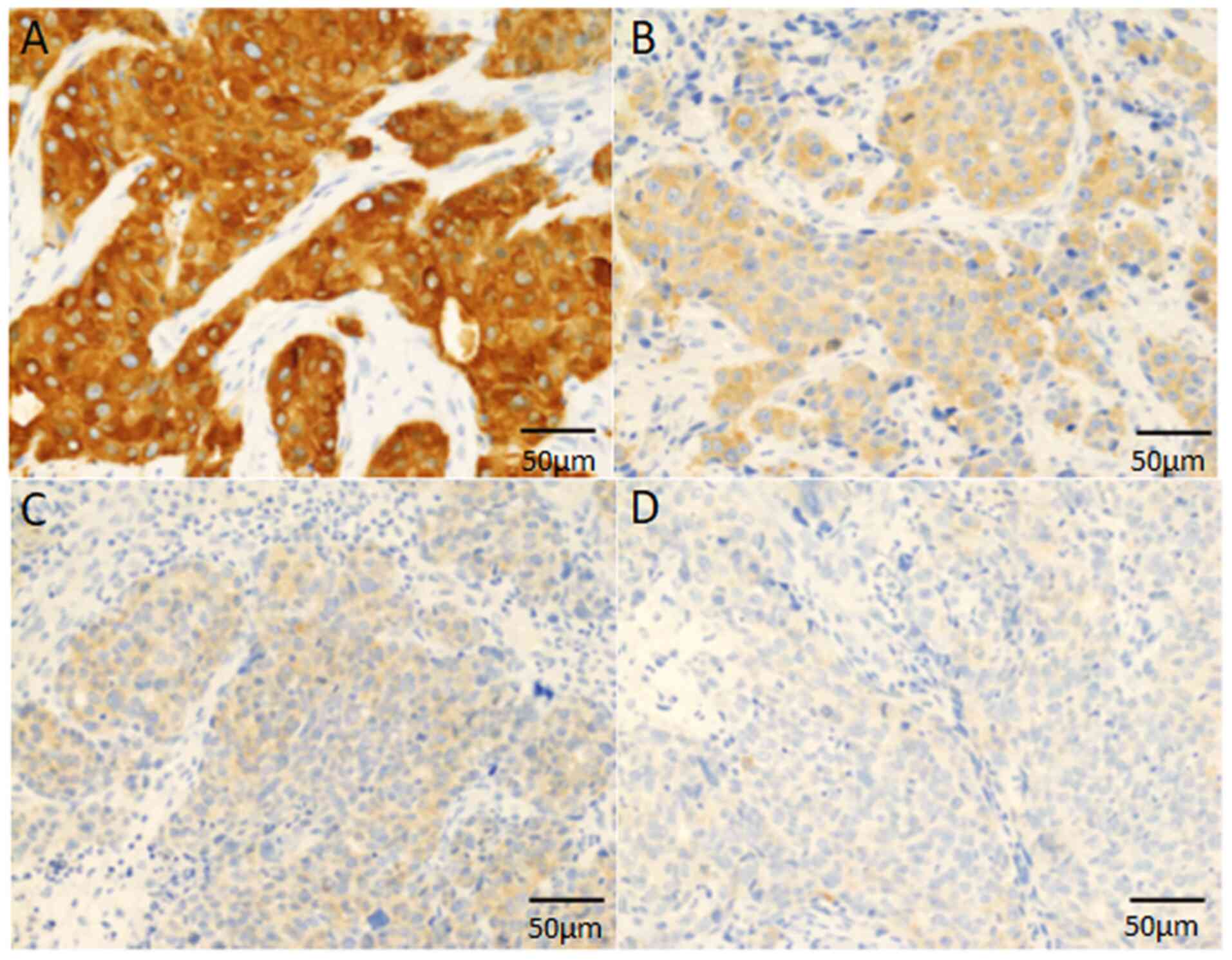
FASN was analysed using a combined scoring system
based on the proportion of positive tumour cells (0-100%) and the
predominant staining intensity in the tumour (18,24).
The FASN staining intensity was scored as follows: 0, negative; 1,
weak; 2, moderate; 3, strong (Fig.
1). The FASN score (0-300) was calculated by multiplying the
percentage by the staining intensity. FASN was classified into two
groups based on the FASN score: low (<120) and high (≥120),
according to a previous report (18).
Statistical analysis
All analyses were performed using SPSS Statistics
27.0 (IBM, Inc.). Correlations between two groups were determined
using the chi-squared test or Fisher's exact test for categorical
variables and the Mann-Whitney U test for continuous variables. The
rates of relapse-free survival (RFS) and overall survival (OS) were
evaluated using Kaplan-Meier analysis. Log-rank tests were used to
compare the groups. The statistical significance was set at
P<0.05.
Results
Patient characteristics
Table I summarises
the clinicopathological features of the present cohort. The cohort
of this study is fundamentally identical to that previously
reported regarding ADP expression in TNBC (12). This study included 61 women with
TNBC. The median age at the time of initial diagnosis was 58 years
(range, 31-93 years). All patients were diagnosed with TNBC based
on biopsy results. All samples were invasive carcinomas of no
special type. No discrepancy was found in the pathological
diagnosis and molecular subtype between the preoperative biopsy and
operative specimens. The median observation period was 61 months
(range: 11-173 months). Eleven (18.0%) patients experienced relapse
(all had distant metastasis, and none experienced local
recurrence), and nine (14.3%) patients died of the disease.
 | Table IClinical characteristics of patients
with triple-negative breast cancer. |
Table I
Clinical characteristics of patients
with triple-negative breast cancer.
| Factors | Value |
|---|
| Total, n | 61 |
| Median age, years
(range) | 68 (31-93) |
| Menopausal status,
n (%) | |
|
Premenopausal | 9 (14.8) |
|
Postmenopausal | 51 (83.6) |
|
Unknown | 1 (1.6) |
| Median BMI
(range) | 23.3
(16.2-32.2) |
| Median tumor size,
mm (range) | 20 (2-55) |
| Pathological stage,
n (%) | |
|
I | 25 (41.0) |
|
IIA | 23 (37.7) |
|
IIB | 5 (8.2) |
|
IIIA | 4 (6.6) |
|
IIIB | 3 (4.9) |
|
IIIC | 1 (1.6) |
| Lymph node status,
n (%) | |
|
Positive | 14 (23.0) |
|
Negative | 33 (54.1) |
|
Not
tested | 14 (23.0) |
| Lymphatic invasion,
n (%) | |
|
Positive | 53 (86.9) |
|
Negative | 8 (13.1) |
| Venous invasion, n
(%) | |
|
Positive | 37 (60.7) |
|
Negative | 24 (39.3) |
| Nottingham
histological grade, n (%) | |
|
1 | 2 (3.3) |
|
2 | 27 (44.3) |
|
3 | 32 (52.5) |
| Ki-67 labeling
index, n (%) | |
|
High | 37 (60.7) |
|
Low | 21 (34.4) |
|
Not
tested | 3 (4.9) |
| Adjuvant
chemotherapy, n (%) | |
|
Performed | 35 (57.4) |
|
Not
performed | 23 (37.7) |
|
Undetermined | 3 (4.9) |
Correlation between
clinicopathological factors and FASN expression
Table II shows the
correlation between FASN expression and the clinicopathological
factors in the study cohort. Forty patients (65.6%) were
FASN-positive and 21 (34.4%) were FASN-negative. Typically, FASN
expression was observed in the cytoplasm of neoplastic cells
(Fig. 1).
 | Table IIAssociation between
clinicopathological factors and FASN expression. |
Table II
Association between
clinicopathological factors and FASN expression.
| Factors | FASN-high
(n=40) | FASN-low
(n=21) | P-value |
|---|
| Age, years (median
± SD) | 64±15 | 66±15 | 0.773 |
| Body mass index,
kg/m2 (median ± SD) | 23.5±3.5 | 23.3±4.1 | 0.820 |
| Menopausal status,
n | | | |
|
Premenopausal | 6 | 3 | >0.999 |
|
Postmenopausal | 34 | 17 | |
|
Unknown | 0 | 1 | |
| Tumor size, n | | | |
|
≤20 mm | 16 | 13 | 0.104 |
|
>20
mm | 24 | 8 | |
| Pathological stage,
n | | | |
|
I+II | 34 | 19 | 0.703 |
|
III | 6 | 2 | |
| Lymph node status,
n | | | |
|
Positive | 11 | 3 | 0.321 |
|
Negative | 20 | 13 | |
|
Not
tested | 9 | 5 | |
| Lymphatic invasion,
n | | | |
|
Positive | 33 | 20 | 0.243 |
|
Negative | 7 | 1 | |
| Venous invasion,
n | | | |
|
Positive | 25 | 12 | 0.684 |
|
Negative | 15 | 9 | |
| Nottingham
histological grade, n | | | |
|
1+2 | 19 | 10 | 0.993 |
|
3 | 21 | 11 | |
| Ki-67 labeling
index, n | | | |
|
High | 19 | 18 | 0.011 |
|
Low | 18 | 3 | |
|
Not
tested | 3 | 0 | |
| Adjuvant
chemotherapy, n | | | |
|
Performed | 24 | 11 | 0.546 |
|
Not
performed | 14 | 9 | |
|
Undetermined | 2 | 1 | |
FASN expression did not correlate with any clinical
factors, including age, menopausal status, body mass index, or
adjuvant chemotherapy. A lower Ki-67 LI was significantly
correlated with FASN expression (P=0.011), but not with
other factors, such as tumour diameter, pathological stage,
histological grade, lymphatic and venous invasion, or lymph node
status.
Correlation between FASN expression
and prognosis
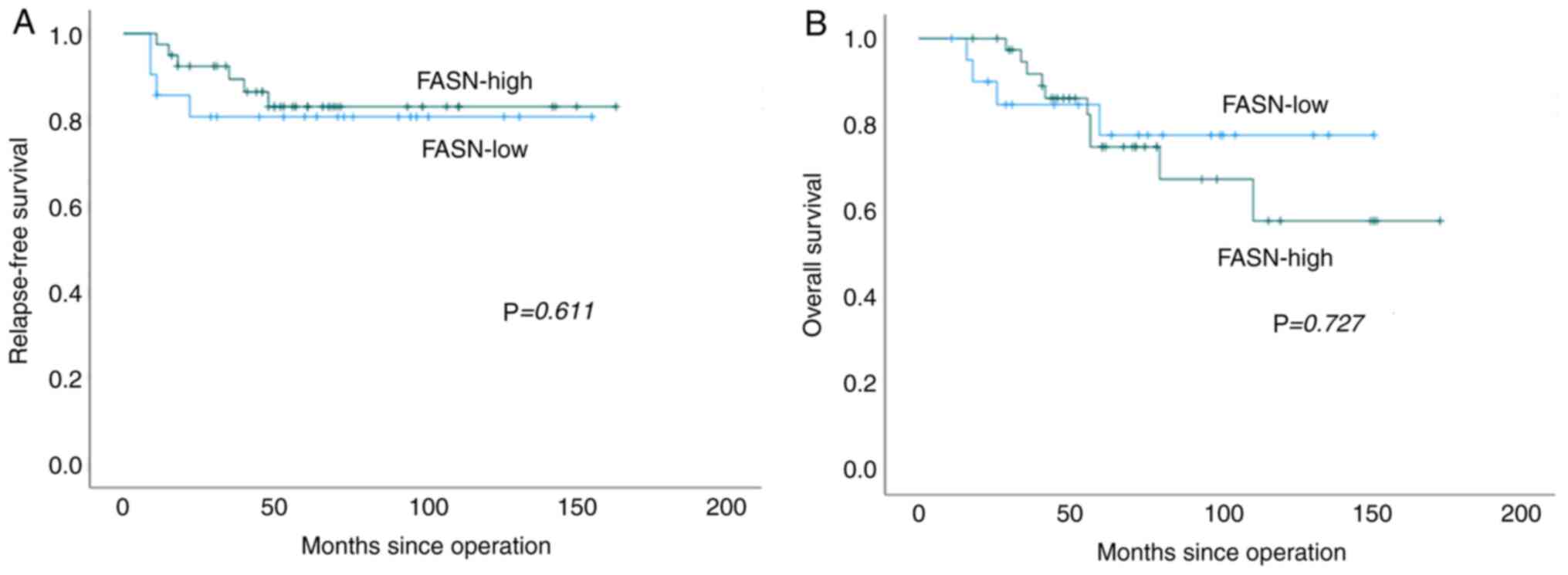
The median RFS of FASN-high and -low patients was 53
and 64 months, respectively, and the median OS of FASN-high and
-low patients was 59 and 64 months, respectively. FASN expression
was not correlated with RFS or OS (Fig. 2, P=0.611 and P=0.727,
respectively).
Correlation between ADP and FASN
expression
As previously reported, ADP expression was positive
in 14 patients (23%) and negative in 47 patients (77%) (12). The correlations between ADP and
FASN expression are shown in Table
III. A significant negative correlation was observed between
ADP and FASN expression (P=0.041).
 | Table IIIAssociation between adipophilin and
fatty acid synthase expression. |
Table III
Association between adipophilin and
fatty acid synthase expression.
| | Fatty acid
synthase | |
|---|
| Adipophilin | High, n | Low, n | P-value |
|---|
| Positive | 6 | 8 | |
| Negative | 34 | 13 | 0.041 |
Discussion
The present study demonstrated that FASN-high was
significantly negatively correlated with ADP expression and a lower
Ki-67 LI. FASN expression was not correlated with RFS and OS in
patients with TNBC.
Fatty acids are essential components of all cells as
they constitute the lipid membrane and are important substrates for
energy metabolism. FASN synthesises long-chain fatty acids using
acetyl-CoA as a primer, malonyl-CoA as a two-carbon donor, and the
predominant product of this enzyme is a 16-carbon fatty acid,
palmitate (13). Under normal
conditions, FASN converts excess carbohydrates into fatty acids,
leading to esterification to store triacylglycerols. In
non-neoplastic tissues, FASN expression is observed in the high
lipid metabolic tissues, including adipocytes, hepatocytes,
sebaceous glands, and hormone-sensitive tissues, such as the
endometrium, prostate, and adrenal cortex, and its expression is
low in other non-neoplastic cells (24). It is well known that lactic acid
synthesis via anaerobic glycolysis is highly upregulated in cancer
cells (Warburg effect), and excess pyruvate is synthesised for
de novo fatty acid synthesis via acetyl-CoA to maintain cell
membrane production in proliferative cancer cells (13). Therefore, upregulation of FASN has
been reported in some types of carcinomas (25,26),
including non-small cell lung cancer (27), oral squamous cell carcinoma
(28), colon cancer (15), bladder cancer (16), salivary gland tumour (18) and malignant melanoma (29).
In breast cancer, FASN expression has been addressed
in some studies. FASN expression was significantly higher in the
HER2 subtype and lower in the luminal subtype and TNBC (30). In one report, high FASN was
significantly correlated with lymph node metastasis but not with
pathological stage, tumour cell proliferative activity (Ki-67), and
disease-free and OS in patients with TNBC (17). In another report, FASN expression
was significantly correlated with pathological stage and lymph node
metastasis in patients with TNBC (31). In the present cohort, FASN-high was
significantly correlated with a lower Ki-67 LI and was not
correlated with patient prognosis.
Interestingly, ADP expression was significantly
negatively correlated with FASN expression. A previous study
demonstrated that ADP expression was significantly correlated with
a higher Ki-67 LI (12);
therefore, lower FASN expression was significantly correlated with
ADP expression and a higher Ki-67 LI. The correlation between ADP
and FASN has only been evaluated in salivary duct carcinoma, a
highly aggressive type of salivary gland carcinoma (18), and the present study is the first
to address this correlation in TNBC. In salivary duct carcinoma,
ADP expression was also a significantly poor prognostic marker of
progression-free and OS by multivariate analysis and was negatively
correlated with FASN expression, which is consistent with the
results of our present and previous studies in patients with TNBC
(12). These results suggest that
de novo fatty acid synthesis by FASN is not the main pathway
of lipogenesis and a source of energy for cancer cells in
ADP-positive highly proliferative TNBC and salivary duct
carcinoma.
ADP expression reflects the intracellular lipid
accumulation in cancer cells (10-12,18).
ADP expression was significantly associated with higher
proliferative activity in cancer cells in breast cancer, including
TNBC (12,32) and salivary duct carcinoma (18). Thus, ADP expression might be
associated with higher proliferative activity, leading to a poor
prognosis. Although the detailed mechanism of ADP expression in
cancer cells remains unclear, ADP expression in cancer cells might
reflect upregulation of lipid metabolism correlating with a higher
proliferative capacity and production of cell membranes of cancer
cells in a hypoxic tumour microenvironment (12). As described earlier, FASN is well
known to be a central enzyme complex in de novo fatty acid
synthesis, and both ADP and FASN have been known to be activated
under hypoxic conditions (13,33,34).
ADP expression was significantly negatively correlated with FASN
expression in TNBC and salivary duct carcinoma (18). Therefore, lipid accumulation in
TNBC and salivary duct carcinoma was not correlated with
upregulation of de novo fatty acid synthesis. Lipid
acumination can be derived from lipid uptake and neutral lipid
synthesis (35). Thus, the
mechanism of ADP expression in TNBC other than the FASN pathway
must be clarified to address the new therapeutic strategy in
ADP-positive TNBC patients with a poorer prognosis.
Although the prognostic significance of FASN
expression in TNBC remains controversial, FASN is considered a
potential therapeutic target (36). It has been shown that blocking FASN
has anticancer effect via the apoptotic pathway in vitro and
in vivo (37-39).
Moreover, the effectiveness of simultaneous blocking of FASN and
epidermal growth factor receptors has also been reported in
preclinical models of chemoresistant TNBC (40). The usefulness of orlistat, an
anti-obesity drug, in epidermal growth factor receptor mutated
non-small cell lung cancer has also been reported (28). Accordingly, FASN can be a potential
therapeutic target for patients with TNBC, and the detailed
mechanism of FASN expression and correlation of ADP expression in
TNBC using both experimental animal model and human cultured cells
must be clarified.
There are some limitations to the present study.
First, this was a retrospective single-institution study with a
small sample size, which could have led to selection bias. Second,
tissue microarray cores of 2 mm diameter were used to determine
FASN and ADP expression. Hence, there could have been a
heterogeneous expression in the cancer tissues, despite our
selection of regions that were morphologically most representative
of cancer. Third, since chemotherapy may affect FASN expression,
this study excluded patients who had undergone neoadjuvant
chemotherapy. Therefore, additional studies with larger patient
populations are needed to clarify these issues.
In conclusion, FASN expression was significantly
negatively correlated with ADP expression in TNBC. ADP expression
reflects lipid acumination in cancer cells; however, its mechanism
other than de novo lipogenesis synthesised by FASN via
acetyl-CoA might be present. Thus, additional studies are needed to
analyse the mechanism of ADP expression, a significantly poor
prognostic marker, leading to a new therapeutic strategy for
patients with ADP-positive TNBC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported in part by Japan Agency
for Medical research and Development (grant no. JP21lm0203006), the
Osaka Community Foundation 2020, and research grants D1 and D2 from
Kansai Medical University.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
KY and MI conceived and designed the study. KY and
MI performed immunohistochemical analyses. KY, MI, HY, KT, MS and
TS acquired and analyzed data. KY and MI confirm the authenticity
of all the raw data. KY and MI drafted the manuscript and prepared
tables and figures. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki, and the study protocol was approved by
the Institutional Review Board of the Kansai Medical University
Hospital (protocol no. 2019234; Hirakata, Osaka, Japan). The
institutional review board waived the requirement for informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: Therapeutic options. Lancet Oncol.
8:235–244. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the carolina
breast cancer study. JAMA. 295:2492–2502. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Metzger-Filho O, Tutt A, De Azambuja E,
Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA Jr, Ellis
P, et al: Dissecting the heterogeneity of triple-negative breast
cancer. J Clin Oncol. 30:1879–1887. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moreno-Sánchez R, Rodríguez-Enríquez S,
Marín-Hernández A and Saavedra E: Energy metabolism in tumor cells.
FEBS J. 274:1393–1418. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Porporato PE, Payen VL, Baselet B and
Sonveaux P: Metabolic changes associated with tumor metastasis,
part 2: Mitochondria, lipid and amino acid metabolism. Cell Mol
Life Sci. 73:1349–1363. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Straub BK, Gyoengyoesi B, Koenig M,
Hashani M, Pawella LM, Herpel E, Mueller W, Macher-Goeppinger S,
Heid H and Schirmacher P: Adipophilin/perilipin-2 as a lipid
droplet-specific marker for metabolically active cells and diseases
associated with metabolic dysregulation. Histopathology.
62:617–631. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bickel PE, Tansey JT and Welte MA: PAT
proteins, an ancient family of lipid droplet proteins that regulate
cellular lipid stores. Biochim Biophys Acta. 1791:419–440.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sztalryd C and Kimmel AR: Perilipins:
Lipid droplet coat proteins adapted for tissue-specific energy
storage and utilization, and lipid cytoprotection. Biochimie.
96:96–101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fujimoto M, Yoshizawa A, Sumiyoshi S,
Sonobe M, Menju T, Hirata M, Momose M, Date H and Haga H:
Adipophilin expression in lung adenocarcinoma is associated with
apocrine-like features and poor clinical prognosis: An
immunohistochemical study of 328 cases. Histopathology. 70:232–241.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hashimoto Y, Ishida M, Ryota H, Yamamoto
T, Kosaka H, Hirooka S, Yamaki S, Kotsuka M, Matsui Y, Yanagimoto
H, et al: Adipophilin expression is an indicator of poor prognosis
in patients with pancreatic ductal adenocarcinoma: An
immunohistochemical analysis. Pancreatology. 19:443–448.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Adipophilin expression is an independent
marker for poor prognosis of patients with triple-negative breast
cancer: An immunohistochemical study. PLoS One.
15(e0242563)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Díaz KP, Gondak R, Martins LL, de Almeida
OP, León JE, Mariano FV, Altemani A and Vargas PA: Fatty acid
synthase and Ki-67 immunoexpression can be useful for the
identification of malignant component in carcinoma ex-pleomorphic
adenoma. J Oral Pathol Med. 48:232–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ogino S, Nosho K, Meyerhardt JA, Kirkner
GJ, Chan AT, Kawasaki T, Giovannucci EL, Loda M and Fuchs CS:
Cohort study of fatty acid synthase expression and patient survival
in colon cancer. J Clin Oncol. 26:5713–5720. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abdelrahman AE, Rashed HE, Elkady E,
Elsebai EA, El-Azony A and Matar I: Fatty acid synthase, Her2/neu,
and E2F1 as prognostic markers of progression in non-muscle
invasive bladder cancer. Ann Diagn Pathol. 39:42–52.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Giró-Perafita A, Sarrats A, Pérez-Bueno F,
Oliveras G, Buxó M, Brunet J, Viñas G and Miquel TP: Fatty acid
synthase expression and its association with
clinico-histopathological features in triple-negative breast
cancer. Oncotarget. 8:74391–74405. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hirai H, Tada Y, Nakaguro M, Kawakita D,
Sato Y, Shimura T, Tsukahara K, Kano S, Ozawa H, Okami K, et al:
The clinicopathological significance of the adipophilin and fatty
acid synthase expression in salivary duct carcinoma. Virchows Arch.
477:291–299. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rakha EA, Allison KH, Bu H, Ellis IO,
Foschini MP, Horii R, et al: Invasive breast carcinoma of no
special type. In: WHO Classification of Tumours, 5th edition.
Breast Tumours IARC, Lyon, pp102-109, 2019.
|
|
20
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Prognostic significance of PD-L1-positive
cancer-associated fibroblasts in patients with triple-negative
breast cancer. BMC Cancer. 21(239)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Immunohistochemical analysis of CD155
expression in triple-negative breast cancer patients. PLoS One.
16(e0253176)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu Q, Ma G, Deng Y, Luo W, Zhao Y, Li W
and Zhou Q: Prognostic value of Ki-67 in patients with resected
triple-negative breast cancer: A meta-analysis. Front Oncol.
9(1068)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kusakabe T, Maeda M, Hoshi N, Sugino T,
Watanabe K, Fukuda T and Suzuki T: Fatty acid synthase is expressed
mainly in adult hormone-sensitive cells or cells with high lipid
metabolism and in proliferating fetal cells. J Histochem Cytochem.
48:613–622. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khan W, Augustine D, Rao RS, Patil S, Awan
KH, Sowmya SV, Haragannavar VC and Prasad K: Lipid metabolism in
cancer: A systematic review. J Carcinog. 20(4)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang J, Song Y, Shi Q and Fu L: Research
progress on FASN and MGLL in the regulation of abnormal lipid
metabolism and the relationship between tumor invasion and
metastasis. Front Med. 15:649–656. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ali A, Levantini E, Teo JT, Goggi J,
Clohessy JG, Wu CS, Chen L, Yang H, Krishnan I, Kocher O, et al:
Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated
non-small cell lung cancer. EMBO Mol Med. 10(e8313)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aquino IG, Bastos DC, Cuadra-Zelaya FJM,
Teixeira IF, Salo T, Coletta RD and Graner E: Anticancer properties
of the fatty acid synthase inhibitor TVB-3166 on oral squamous cell
carcinoma cell lines. Arch Oral Biol. 113(104707)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
de Andrade BA, León JE, Carlos R,
Delgado-Azañero W, Mosqueda-Taylor A, Graner E and de Almeida OP:
Expression of fatty acid synthase (FASN) in oral nevi and melanoma.
Oral Dis. 17:808–812. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jung YY, Kim HM and Koo JS: Expression of
lipid metabolism-related proteins in metastatic breast cancer. PLoS
One. 10(e0137204)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jiang W, Xing XL, Zhang C, Yi L, Xu W, Ou
J and Zhu N: MET and FASN as prognostic biomarkers of triple
negative breast cancer: A systematic evidence landscape of clinical
study. Front Oncol. 11(604801)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kuniyoshi S, Miki Y, Sasaki A, Iwabuchi E,
Ono K, Onodera Y, Hirakawa H, Ishida T, Yoshimi N and Sasano H: The
significance of lipid accumulation in breast carcinoma cells
through perilipin 2 and its clinicopathological significance.
Pathol Int. 69:463–471. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saarikoski ST, Rivera SP and Hankinson O:
Mitogen-inducible gene 6 (MIG-6), adipophilin and tuftelin are
inducible by hypoxia. FEBS Lett. 530:186–190. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ni T, He Z, Dai Y, Yao J, Guo Q and Wei L:
Oroxylin A suppresses the development and growth of colorectal
cancer through reprogram of HIF1α-modulated fatty acid metabolism.
Cell Death Dis. 8(e2865)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chang R, Chou MC, Hung LY, Wang ME, Hsu MC
and Chiu CH: Study of valproic acid-enhanced hepatocyte steatosis.
BioMed Res Int. 2016(9576503)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Menendez JA and Lupu R: Fatty acid
synthase (FASN) as a therapeutic target in breast cancer. Expert
Opin Ther Targets. 21:1001–1016. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Puig T, Turrado C, Benhamú B, Aguilar H,
Relat J, Ortega-Gutiérrez S, Casals G, Marrero PF, Urruticoechea A,
Haro D, et al: Novel inhibitors of fatty acid synthase with
anticancer activity. Clin Cancer Res. 15:7608–7615. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Blancafort A, Giró-Perafita A, Oliveras G,
Palomeras S, Turrado C, Campuzano Ò, Carrion-Salip D, Massaguer i
Vall-llovera A, Brugada R, Palafox Sánchez M, et al: Dual fatty
acid synthase and HER2 signaling blockade shows marked antitumor
activity against breast cancer models resistant to anti-HER2 drugs.
PLoS One. 10(e0131241)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Qu H, Shan K, Tang C, Cui G, Fu G, Qi Y,
Cui J, Li J, Wang R, Feng N, et al: A novel small-molecule fatty
acid synthase inhibitor with antitumor activity by cell cycle
arrest and cell division inhibition. Eur J Med Chem.
219(113407)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Giró-Perafita A, Palomeras S, Lum DH,
Blancafort A, Viñas G, Oliveras G, Pérez-Bueno F, Sarrats A, Welm
AL and Puig T: Preclinical evaluation of fatty acid synthase and
EGFR inhibition in triple-negative breast cancer. Clin Cancer Res.
22:4687–4697. 2016.PubMed/NCBI View Article : Google Scholar
|