Introduction
Although thymoma and thymic carcinoma are relatively
rare types of malignant tumors, they account for most mediastinal
tumors in adults globally (1-3).
Thymomas are a common primary tumor in the anterior mediastinum,
although they are rare (1.5 cases/million). Thymic carcinoma is
rarer than thymomas. In 2016, the National Comprehensive Cancer
Network Guidelines version 2(4)
recommended six combination chemotherapy regimens, excluding
radiotherapy, for patients with unresectable disease (5-7).
According to the guidelines, carboplatin plus paclitaxel is the
recommended regimen for the treatment of patients with thymic
carcinoma, owing to the higher response rate compared with that
noted for other regimens. However, there is a lack of data from
randomized clinical studies to provide a definite indication for
the management of this disease.
Nanoparticle (i.e., 130 nm) albumin-bound paclitaxel
(nab-paclitaxel) utilizes the properties of albumin, namely the
reversible binding of paclitaxel, the subsequent transportation
across the endothelial cells and its concentration in the tumor.
Since it does not contain solvents or ethanol, paclitaxel can be
administered at higher doses than those recommended without
premedication (8). Nab-paclitaxel
is used without dissolving alcohol and so would be available for
treating patients who were allergic to alcohol. In addition, the
safety and efficacy of nab-paclitaxel have been demonstrated in
patients with various types of cancer at a range of doses (100-260
mg/m2) (9-13).
The present retrospective study evaluated the efficacy and safety
of carboplatin plus nab-paclitaxel for the treatment of advanced
thymic carcinoma.
Materials and methods
Patients
The present study was conducted on retrospective
data from patients treated between December 2013 and November 2017.
The last day for survival confirmation was August 30, 2019. During
this period, 12 patients with advanced thymic carcinoma received
treatment with carboplatin plus nab-paclitaxel at the Nippon
Medical School Hospital (Tokyo, Japan). All patients were treated
with carboplatin on day 1 [area under the blood concentration time
curve (AUC), 6] plus nab-paclitaxel (100 mg/m2) on days
1, 8 and 15 in cycles repeated every 3 weeks. The medical records
of the patients were retrospectively reviewed. The inclusion
criteria were as follows: i) Confirmed diagnosis of thymic
carcinoma according to the histopathological criteria proposed by
the World Health Organization (2014 version) (14); ii) stage III (a thoracic surgeon
had rejected these patients as the tumors had infiltrated major
vessels.), IVa or IVb disease according to the Masaoka criteria
(15); and iii) recurrence or
metastases diagnosed through chest or abdominal computed
tomography. There were no exclusion criteria. The protocol of this
study was approved by the Institutional Review Board of the Nippon
Medical School Hospital (approval no. 30-05-933).
Evaluation of response to treatment
and safety
The Response Evaluation Criteria in Solid Tumors
(version 1.1) guidelines (16)
were used to evaluate tumor responses, including complete response
(CR), partial response (PR), stable disease (SD) and progressive
disease (PD). Disease control rate (DCR) was defined as the sum of
CR, PR and SD values.
Progression-free survival (PFS) time was defined as
the period from the first day of administration of carboplatin plus
nab-paclitaxel to the day of documented disease progression or
death. Overall survival time was defined as the period from the
first day of administration of carboplatin plus nab-paclitaxel to
the day of death; patients who remained alive were censored on the
date of the last visit. Follow-up time was defined as the median
time between the first day of treatment and the day of death or
last follow-up visit. Survival curves were plotted using the
Kaplan-Meier method.
Safety was assessed according to the Common
Terminology Criteria for Adverse Events (version 4.0; nih.gov) (17) of the
US National Cancer Institute.
Statistical analysis
Survival curves were plotted using the Kaplan-Meier
method and analyzed by the log-rank test. Analyses were performed
using GraphPad Prism version 8 (GraphPad Software, Inc.). P<0.05
was used to indicate a statistically significant difference.
Results
Patient characteristics
A total of 12 patients were included in the present
study (Tables I and SI). Among those patients, 2 underwent a
tumor resection. Squamous cell carcinoma was the most common
histological type (75.0%). All patients had a performance status of
0-1, and were treated with carboplatin on day 1 (area under the
blood concentration time curve, 5-6) plus nab-paclitaxel (100
mg/m2) on days 1, 8 and 15 in cycles repeated every 3-4
weeks.
 | Table IClinicopathological characteristics
(n=12). |
Table I
Clinicopathological characteristics
(n=12).
| Characteristic | Value |
|---|
| Sex, n (%) | |
|
Male | 7 (58.3) |
|
Female | 5 (41.7) |
| Age, years | |
|
Median
(range) | 64 (41-73) |
| Histology | |
|
Squamous
cell carcinoma | 9 (75.0) |
|
Undifferentiated
carcinoma | 2 (16.7) |
|
Neuroendocrine
carcinoma | 1 (8.3) |
| Clinical
stagea | |
|
III | 2 (16.7) |
|
Iva | 3 (25.0) |
|
IVb | 5 (41.7) |
|
Postoperative
recurrence | 2 (16.7) |
| Prior therapy | |
|
No | 8 (66.7) |
|
Chemotherapy | 4 (33.3) |
| Performance
status | |
|
0 | 6 (50.0) |
|
1 | 6 (50.0) |
Four patients had received prior chemotherapy: Three
patients had received paclitaxel (200 mg/m2) plus
carboplatin (AUC, 6) 12 months ago, and one patient had received
paclitaxel (200 mg/m2) plus carboplatin (AUC, 6) 7
months ago, irinotecan (100 mg/m2) plus cisplatin (30
mg/m2) (weekly) 6 months ago and docetaxel (60
mg/m2) 6 months ago.
Response and survival analysis
The median number of treatment cycles was 4 (range,
2-6). The relative dose intensity was 66.7%. Reasons for the
reduction of the dose included alcoholic liver injury and
nephropathy. Notably, 3 patients received maintenance treatment
with nab-paclitaxel.
Treatment response data are shown in Table II. CR, PR and DCR were achieved in
1 patient (8.3%), 7 patients (58.3%) and 11 patients (91.7%),
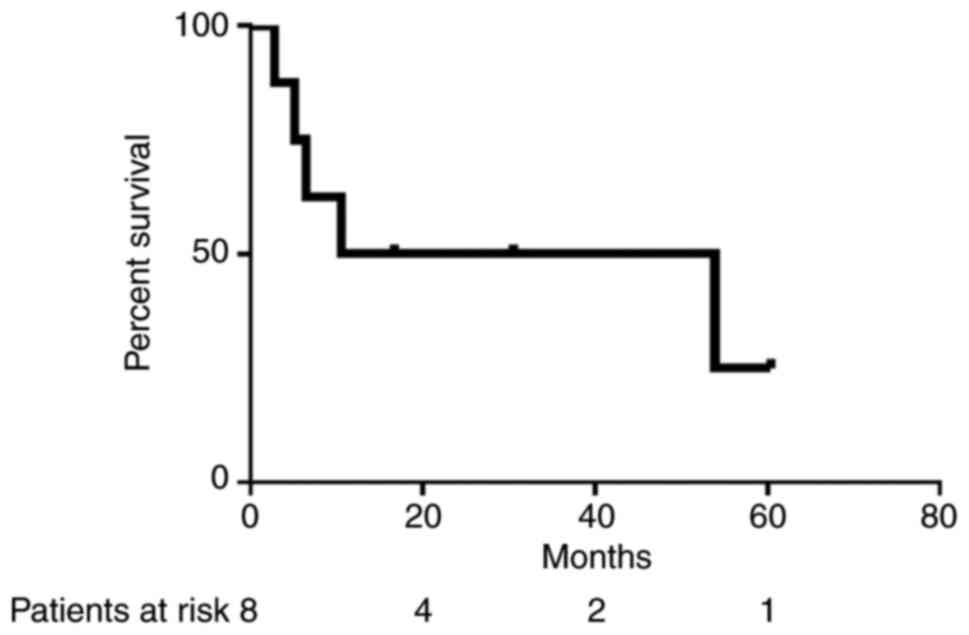
respectively. At the median follow-up time of 27.6 months (range,
6.2-75.1 months), the median PFS time of 12 patients was 16.7
months [95% confidence interval (CI), 13.2-37.7] and the median
first-line PFS time of 8 patients was 13.6 months (95% CI,
4.3-42.3) (Fig. 1). The median
first-line overall survival time was 14.3 months (95% CI,
4.7-54.6). Three patients remained disease-free for >3
years.
 | Table IIResponse to treatment (n=12). |
Table II
Response to treatment (n=12).
| Response | n (%) |
|---|
| Complete
response | 1 (8.3) |
| Partial response | 7 (58.3) |
| Stable disease | 3 (25.0) |
| Progressive
disease | 1 (8.3) |
| Disease-control
rate | 11 (91.7) |
Evaluation of safety
Safety was assessed in all patients. Grade ≥3
hematological adverse events were observed in 7 patients (anemia,
n=3; decreased platelet count, n=2; neutropenia, n=2; and
hyponatremia, n=1). A grade ≥3 non-hematological adverse event
(liver disfunction) was observed in 1 patient. For the 3 patients
with prolonged grade 3 anemia, the dosage was reduced to 80% of the
initial dose. Neuropathy, febrile neutropenia and treatment-related
mortality did not occur in this study (Table III).
 | Table IIIAdverse events. |
Table III
Adverse events.
| Adverse event | All grades, n | % | Grade ≥3, n | % |
|---|
| Hematological | | | | |
|
Leukopenia | 2 | 16.7 | 2 | 16.7 |
|
Neutropenia | 2 | 16.7 | 2 | 16.7 |
|
Anemia | 5 | 41.7 | 3 | 25.0 |
|
Decreased
platelet count | 3 | 25.0 | 2 | 16.7 |
|
Febrile
neutropenia | 0 | 0.0 | 0 | 0.0 |
|
Hyponatremia | 1 | 8.3 | 1 | 8.3 |
|
Non-hematological | | | | |
|
Infection | 0 | 0.0 | 0 | 0.0 |
|
Fever | 0 | 0.0 | 0 | 0.0 |
|
Hepatic
injury | 1 | 8.3 | 1 | 8.3 |
|
Pneumonitis | 1 | 8.3 | 0 | 0.0 |
|
Diarrhea | 1 | 8.3 | 0 | 0.0 |
|
Neuropathy | 0 | 0.0 | 0 | 0.0 |
|
Febrile
neutropenia | 0 | 0.0 | 0 | 0.0 |
|
Treatment-related
mortality | 0 | 0.0 | 0 | 0.0 |
Discussion
To the best of our knowledge, the present study is
the largest investigation conducted thus far to assess the clinical
benefits of carboplatin plus nab-paclitaxel in patients with
advanced thymic carcinoma. As thymoma and thymic carcinoma are
relatively rare types of malignant tumors, this combination may be
an option as a chemotherapy regimen for the treatment of advanced
thymic carcinoma.
Lemma et al (18) advocated the use of combination
chemotherapy consisting of carboplatin plus paclitaxel, in addition
to standard therapeutic regimens, for the treatment of advanced
thymic carcinoma. Of the 23 patients with thymic carcinoma included
in the aforementioned study, 5 patients accomplished a PR, and 12
patients achieved SD (risk ratio, 21.7%; DCR, 73.9%). Notably, the
PFS time was 5 months. Table IV
shows five case reports of patients with thymic carcinoma who
received chemotherapy with carboplatin plus nab-paclitaxel
(19-22).
These case reports showed that the administration of carboplatin
plus nab-paclitaxel resulted in favorable antitumor effects against
thymic carcinoma. Funaishi et al (23) reported a case with a PFS time of
10.3 months. Ley et al (24) and Maurer et al (25) suggested the clinical benefit of
nab-paclitaxel in recurrent/metastatic gynecological and head and
neck carcinomas, which are resistant to paclitaxel and docetaxel.
These results are consistent with the present findings, indicating
that carboplatin plus nab-paclitaxel may be an option for the
treatment of advanced thymic carcinoma. Recently, the efficacy and
safety of lenvatinib in patients with advanced or metastatic thymic
carcinoma was confirmed in a single-arm, phase 2 trial conducted in
eight institutions in Japan (five cancer centers, two medical
university hospitals and one public hospital) (26). In this phase 2 trial, carboplatin
and paclitaxel were used as first-line treatment in 71% of cases.
The use of lenvatinib after treatment with carboplatin plus
nab-paclitaxel was also an effective alternative.
 | Table IVStudies of carboplatin plus
nab-paclitaxel as salvage chemotherapy in patients with thymic
carcinoma. |
Table IV
Studies of carboplatin plus
nab-paclitaxel as salvage chemotherapy in patients with thymic
carcinoma.
| First author,
year | Patient sex | Age, years | Histology | Response | PFS, months | (Refs.) |
|---|
| Makimoto et
al, 2014 | Male | 40 | Sq | PR | - | (19) |
| Igawa et al,
2015 | Male | 59 | LCNEC | PR | <6 | (20) |
| Zhan et al,
2015 | Female | 63 | Sq | PR | <36 | (21) |
| Shima et al,
2016 | Male | 22 |
Lymphoepithelioma-like | PR | - | (22) |
| Funaishi et
al, 2017 | Male | 78 | Sq | PR | 10.3 | (23) |
Gong et al (27) showed that nab-paclitaxel treatment
had a high response rate in non-small cell lung cancer (NSCLC) when
used as second-line chemotherapy. No significant difference was
found between clinical features and the short-term effect of
nab-paclitaxel, such as taxanes, or other second-line chemotherapy.
It was also determined that nab-paclitaxel may be an appropriate
second-line treatment for patients with thymic cancer who had
previously received chemotherapy. Additionally, maintenance
monotherapy with nab-paclitaxel may be an option to prolong the PFS
time of patients with thymic carcinoma. These results are
consistent with those obtained after maintenance monotherapy with
nab-paclitaxel for NSCLC (28).
In conclusion, the results presented within the
present study suggest that carboplatin plus nab-paclitaxel is a
promising salvage chemotherapy regimen for the treatment of
advanced thymic carcinoma. Thymic cancer is a very rare type of
cancer and the present study contained a limited number of patients
as the clinical study was conducted in a single facility.
Prospective studies are therefore warranted to further evaluate the
efficacy of carboplatin plus nab-paclitaxel chemotherapy for the
treatment of thymic carcinoma.
Supplementary Material
Patient characteristics.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AT, RN, MS, KK and AG were responsible for the
conception and design of the study. Provision of study materials or
patients, data collection and analysis, and manuscript writing were
completed by AT, RN, NT, KH, ST, AF, MO, TS, ST, SN, AM, YM, KK, MS
and AG. AT and RN confirm the authenticity of all the raw data. All
authors have read and approved the manuscript.
Ethics approval and consent to
participate
The protocol of this study was approved by the
Institutional Review Board of the Nippon Medical School Hospital
(Tokyo, Japan; approval no. 30-05-933).
Patient consent for publication
Not applicable.
Competing interests
RN received honoraria from AstraZeneca and Chugai
Pharmaceutical. ST received honoraria for lectures, presentations
and speakers bureaus from Taiho Pharmaceutical. YM received payment
or honoraria for lectures and presentations from Boehringer
Ingelheim Phamaceuticals and Taiho Pharmaceutical. MS received
payment or honoraria for lectures and presentations from Boehringer
Ingelheim Phamaceuticals, Taiho Pharmaceutical and Eli Lilly Japan
K.K. AG received payment or honoraria for lectures and
presentations from Boehringer Ingelheim Phamaceuticals. KK received
payment or honoraria for lectures and presentations from Chugai
Pharmaceutical, Taiho Pharmaceutical, MSD, Nippon Boehringer
Ingelheim, Bristol-Myers Squibb, Kyowa-Hakko Kirin, AstraZeneca and
Ono Pharmaceutical.
References
|
1
|
de Jong WK, Blaauwgeers JL, Schaapveld M,
Timens W, Klinkenberg TJ and Groen HJ: Thymic epithelial tumours: A
population-based study of the incidence, diagnostic procedures and
therapy. Eur J Cancer. 44:123–130. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mariusdottir E, Nikulasson S, Bjornsson J
and Gudbjartsson T: Thymic epithelial tumours in iceland: Incidence
and histopathology, a population-based study. APMIS. 118:927–933.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gadalla SM, Rajan A, Pfeiffer R,
Kristinsson SY, Bjorkholm M, Landgren O and Giaccone G: A
population-based assessment of mortality and morbidity patterns
among patients with thymoma. Int J Cancer. 128:2688–2694.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
National Comprehensive Cancer Network
(NCCN): Thymomas and Thymic Carcinomas. Version 2.2016. NCCN,
Plymouth Meeting, PA, 2016. https://thymicuk.org/wp-content/uploads/2019/10/NCCN-Thymoma-and-Thymic-cancer-guidelines.pdf.
Accessed March 16, 2016.
|
|
5
|
Engels EA: Epidemiology of thymoma and
associated malignancies. J Thorac Oncol. 5 (10 Suppl 4):S260–S265.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Strollo DC, Rosado de Christenson ML and
Jett JR: Primary mediastinal tumors. Part 1: Tumors of the anterior
mediastinum. Chest. 112:511–522. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Engels EA and Pfeiffer RM: Malignant
thymoma in the United States: Demographic patterns in incidence and
associations with subsequent malignancies. Int J Cancer.
105:546–551. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gradishar WJ: Albumin-bound paclitaxel: A
next-generation taxane. Expert Opin Pharmacother. 7:1041–1053.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Socinski MA, Bondarenko I, Karaseva NA,
Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P,
Zhang H, et al: Weekly nab-paclitaxel in combination with
carboplatin versus solvent-based paclitaxel plus carboplatin as
first-line therapy in patients with advanced non-small-cell lung
cancer: Final results of a phase III trial. J Clin Oncol.
30:2055–2062. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hirsh V, Okamoto I, Hon JK, Page RD,
Orsini J, Sakai H, Zhang H, Renschler MF and Socinski MA:
Patient-reported neuropathy and taxane-associated symptoms in a
phase 3 trial of nab-paclitaxel plus carboplatin versus
solvent-based paclitaxel plus carboplatin for advanced
non-small-cell lung cancer. J Thorac Oncol. 9:83–90.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koizumi W, Morita S and Sakata Y: A
randomized phase III trial of weekly or 3-weekly doses of
nab-paclitaxel versus weekly doses of cremophor-based paclitaxel in
patients with previously treated advanced gastric cancer (ABSOLUTE
trial). Jpn J Clin Oncol. 45:303–306. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shitara K, Takashima A, Fujitani K, Koeda
K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Makari Y, Amagai K,
et al: Nab-paclitaxel versus solvent-based paclitaxel in patients
with previously treated advanced gastric cancer (ABSOLUTE): An
open-label, randomised, non-inferiority, phase 3 trial. Lancet
Gastroenterol Hepatol. 2:277–287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicolson AG: Introduction to The 2015 World health organization
classification of tumors of the lung, Pleura, thymus, and heart. J
Thorac Oncol. 10:1240–1242. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Masaoka A, Monden Y, Nakahara K and
Tanioka T: Follow-up study of thymomas with special reference to
their clinical stages. Cancer. 48:2485–2492. 1981.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). J Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
U.S. Department of Health and Human
Services, National Institutes of Health and National Cancer
Institute: Common Terminology Criteria for Adverse Events (CTCAE).
Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf.
Accessed May 28, 2009.
|
|
18
|
Lemma GL, Lee JW, Aisner SC, Langer CJ,
Tester WJ, Johnson DH and Loehrer PJ Sr: Phase II study of
carboplatin and paclitaxel in advanced thymoma and thymic
carcinoma. J Clin Oncol. 29:2060–2065. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Makimoto G, Fujiwara K, Watanabe H,
Kameyama N, Matsushita M, Rai K, Sato K, Yonei T, Sato T and
Shibayama T: nab-paclitaxel in combination with carboplatin for a
previously treated thymic carcinoma. Case Rep Oncol. 7:14–17.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Igawa S, Yanagisawa N, Niwa H, Ishihara M,
Hiyoshi Y, Otani S, Katono K, Sasaki J, Satoh Y and Masuda N:
Successful chemotherapy with carboplatin and nab-paclitaxel for
thymic large cell neuroendocrine carcinoma: A case report. Oncol
Lett. 10:3519–3522. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhan P, Xie H and Yu LK: Response to
nab-paclitaxel and nedaplatin in a heavily-metastatic thymic
carcinoma: A case report. Oncol Lett. 9:1715–1718. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shima H, Ozasa H, Tsuji T, Ajimizu H,
Nomizo T, Yagi Y, Sakamori Y, Nagai H, Minamiguchi S, Kim YH and
Mishima M: Response to chemotherapy with carboplatin plus
albumin-bound paclitaxel in a patient with lymphoepithelioma-like
thymic carcinoma: A case report. Mol Clin Oncol. 4:715–718.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Funaishi K, Yamasaki M, Saito N, Daido W,
Ishiyama S, Deguchi N, Taniwaki M and Ohashi N: First-line
treatment with carboplatin plus nab-paclitaxel and maintenance
monotherapy with nab-paclitaxel for a thymic carcinoma: A case
report. Case Rep Oncol. 10:571–576. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ley J, Wildes TM, Daly K, Oppelt P and
Adkins D: Clinical benefit of nanoparticle albumin-bound-paclitaxel
in recurrent/metastatic head and neck squamous cell carcinoma
resistant to cremophor-based paclitaxel or docetaxel. Med Oncol.
34(28)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maurer K, Michener C, Mahdi H and Rose PG:
Universal tolerance of nab-paclitaxel for gynecologic malignancies
in patients with prior taxane hypersensitivity reactions. J Gynecol
Oncol. 28(e38)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sato J, Satouchi M, Itoh S, Okuma Y, Niho
S, Mizugaki H, Murakami H, Fujisaka Y, Kozuki T, Nakamura K, et al:
Lenvatinib in patients with advanced or metastatic thymic carcinoma
(REMORA): A multicentre, phase 2 trial. Lancet Oncol. 21:843–850.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gong W, Sun P, Mu Z, Liu J, Yu C and Liu
A: Efficacy and safety of nab-paclitaxel as second-line
chemotherapy for locally advanced and metastatic non-small cell
lung cancer. Anticancer Res. 37:4687–4691. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakao A, Uchino J, Igata F, On R, Ikeda T,
Yatsugi H, Hirano R, Sasaki T, Tanimura K, Imabayashi T, et al:
Nab-paclitaxel maintenance therapy following carboplatin +
nab-paclitaxel combination therapy in chemotherapy naive patients
with advanced non-small cell lung cancer: Multicenter, open-label,
single-arm phase II trial. Invest New Drugs. 36:903–910.
2018.PubMed/NCBI View Article : Google Scholar
|