Introduction
Prostate cancer is the second most frequently
diagnosed malignancy (after lung cancer) in men worldwide,
accounting for 1,276,106 new cases and causing 358,989 deaths (3.8%
of all deaths caused by cancer in men) in 2018(1). Risk factors can increase the risk of
prostate cancer among men which include age, obesity, smoking, and
inheritance of risk alleles. Notably, prostate cancer incidence and
mortality vary through different ethnic backgrounds. Among the many
risk factors causing prostate cancer, several studies suggest that
virus infections might be important risk factors (2). The role of human papillomavirus (HPV)
in human tumors has been intensively studied for decades; the virus
has been suggested to contribute seriously to the incidence of
ovarian, uterine, and other female reproductive system tumors. HPV
16 and 18 contribute to malignant tumors especially cervical
cancer, and these two types are considered high-risk for 70% of all
cancers caused by HPV in general compared to other types of HPV,
and these two types affect about 20% of the adult population in
Western countries (3-5).
Yet, there is some confusion regarding the presence
of HPV infections in prostate cancer tissues and its role in the
entire tumorigenesis process (6,7), as
research has confirmed the presence of HPV in both cancerous and
healthy tissues. HPV infections have been frequently detected in
prostate cancer tissues but the infection frequencies detected vary
significantly. The most frequently detected high-risk HPVs in
prostate tumors are HPV types 16 and 18 (8-10).
In addition, the retrovirus mouse mammary tumor
virus (MMTV) has been suggested to induce or accelerate
carcinogenesis of healthy breast tissues by proviral integration
(11), but the implications of
MMTV in various human tumors such as prostate cancer remain
unclear.
The correlation between the presence of HPV 18 and
MMTV infections in prostate cancer tissues and clinical tumor
criteria has not been previously analyzed in Moroccan men. The
present study aimed to detect viral infections of high risk HPV 18
and MMTV-like in prostate cancer tissues and to find correlations
between these viral infections and tumor clinical criteria.
Materials and methods
Prostate tissue specimens
A total of 50 fresh prostate biopsies were obtained
from 50 men who were evaluated at the Teaching Hospital of Rabat
City (Morocco) between June 2017 and February 2019. Histological
samples were obtained to confirm a diagnosis of the presence of
prostate adenocarcinoma. Biopsies were obtained according to
standard protocols by physicians directly. Along with the biopsies,
every sample had the relevant attached clinical and pathological
parameters including: age, date of diagnosis, date of biopsy,
Gleason score, prostate-specific antigen (PSA) concentration of
prostate tumors, and the vital status of the patients. The clinical
characteristics of the prostate cancer patients are provided in
Table I.
 | Table IClinical characteristics of the
prostate cancer patients (n=50). |
Table I
Clinical characteristics of the
prostate cancer patients (n=50).
| Characteristics | n (%) |
|---|
| Age at
diagnosis/surgery (years) | |
|
≤60 | 7(14) |
|
>60 | 37(74) |
|
Unknown | 6(12) |
| Preoperative PSA,
ng/ml | |
|
<2.5 | 1(2) |
|
2.5-10 | 10(20) |
|
≥10.0 | 33(66) |
|
Unknown | 6(12) |
| Pathological Gleason
score | |
|
<7 | 11(22) |
|
7 (3+4) | 6(12) |
|
7 (4+3) | 8(16) |
|
>7 | 11(22) |
|
Unknown | 14(28) |
| Pathological
T-stage | |
|
pT1 | 14(28) |
|
pT2x | 17(34) |
|
pT3x | 3(6) |
|
pT4 | 5(10) |
|
Unknown/pTx | 10(20) |
| Radical
prostatectomy | |
|
Yes | 16(32) |
|
No | 21(42) |
|
Unknown | 13(26) |
| Alcohol
consumption | |
|
Yes | 10(20) |
|
No | 32(64) |
|
Unknown | 8(16) |
| Smoking | |
|
Yes | 18(36) |
|
No | 24(48) |
|
Unknown | 8(16) |
Required ethical approval was obtained from the
Committee of Biomedical Research Ethics in Morocco (no. 3/2018/30
April/2018). All patients consented to participate in the study and
their information was handled as anonymous according to the ethical
standards.
DNA extraction and genotyping
DNA samples were obtained from prostate cancer
tissues; ≤25 mg of prostate tissue was placed into a sterile
microcentrifuge tube, and DNA was extracted from the tissue samples
using pure link Invitrogen Genomic DNA Mini Kit (Thermo Fisher
Scientific, Inc.), according to the manufacturers' protocol. DNA
extraction was performed at the place of biopsy collection
(Research and Biosafety Laboratory, Mohammed V Construction
Teaching Hospital, Morocco). Processing and genotyping took place
at the Oncology and Virology Laboratory at the Faculty of Sciences
and Techniques at Mohammedia, Morocco. DNA was quantified using the
Nanodrop spectrophotometer (Thermo Fisher Scientific, Inc.).
Samples with a DNA concentration of 20-50 ng/µl or above were
selected to perform polymerase chain reaction (PCR). To evaluate
the quality and integrity of the extracted DNA, a 268-bp fragment
of the housekeeping β-globin gene was amplified using the GH20/PCO4
primer set as previously described (12). Oligonucleotides for β-globin
amplification are provided in Table
SI.
HPV detection (Fig. S1)
The Specific HPV L1 gene was targeted by PCR using
specific primers described elsewhere (13). The PCR reaction consisted of 25 µl
total volume PCR reaction containing genomic DNA (8 ng), 2X
Taq PCR Master Mix kit (Qiagen USA), 2 µmol forward and
reverse primers. PCR amplification was performed using a Perkin
Elmer 2400 Thermal Cycler® (PerkinElmer, Inc.).
The amplification procedure for HPV-L1 gene
amplification was as follows: initial denaturation at 94˚C for 3
min, followed by 35 cycles of denaturation at 94˚C for 1 min,
annealing at 43˚C for 1 min, and extension at 72˚C for 1 min and
final extension at 72˚C for 10 min. HPV16 and 18 provided by the
biology and medical research unit (CNESTEN) Rabat, Morocco were
used as positive PCR control and Ultra-pure water as negative PCR
control. Primers set data used in HPV-L1 gene amplification are
provided in Table SII.
MMTV-like detection
The 356-bp env sequence of MMTV-like virus as
described previously (14,15) was targeted by PCR using specific
primers: forward, GTAACACAGGCAGATGTAGG and reverse,
GTATGAAGCAGGATGGGTAGA. The conditions for PCR were as follow:
initial denaturation at 94˚C for 3 min, followed by 35 cycles of
denaturation at 94˚C for 1 min, annealing at 54˚C for 1 min, and
extension at 72˚C for 1 min and a final extension at 72˚C for 10
min. Primer sequences and amplicon size expected of MMTV
amplification are provided in Table
SIII.
Sequence identification and molecular
evolution analysis
PCR product sizes were confirmed by gel
electrophoresis for 1.5 h at 70 V on 2% agarose. PCR products were
purified using ExoSAP-IT™ Express PCR Product Cleanup (Thermo
Fisher Scientific, Inc.). Last purification was performed using
BigDye XTerminator® Purification Kit (Thermo Fisher
Scientific, Inc.). Bidirectional Sanger sequencing was performed
using BigDye Terminator v1.1 Cycle Sequencing Kit (Thermo Fisher
Scientific, Inc.).
Forward and reverse PCR primers used in PCR were
used in Sanger sequencing performed by Genetic Analyzer 3130
(Applied Biosystems), All purification and sequencing were
performed at the Innovation Centre Facility, Sidi Mohammed Bin
Abdullah University of Fez (Fez, Morocco). Sequence identity
recognition was performed by NCBI blast tools: https://blast.ncbi.nlm.nih.gov/moleblast/moleblast.cgi.
In addition, the phylogeny analysis of the genotypes of the viruses
was performed using MSA viewer built in NCBI-Blast tools, method:
fast minimum evolution, max sequence differences=0.75.
Statistical analysis
We compared the frequency of HPV18 and
MMTV-like infections among the subset of Moroccan men with
tumor characteristic categories. Comparisons between infected and
non-infected men against tumor characteristics of the prostate
cancer (Gleason score, PSA concentrations, tumor stage, and age at
diagnosis) were based on 2x2 or more Chi-squared analysis or
Fisher's exact test to test for statistical significance. All
statistical tests were two-sided, and all P-values of 0.05 or less
were considered statistically significant. All analyses were
conducted using the Minitab 17 (Coventry, UK).
Results
Positive viral infections in prostate cancer tissues
were determined (Fig. 1). Of the
50 patients diagnosed with prostate cancer, 8 (16%) patients were
confirmed to be infected by the high-risk human papillomavirus
(HPV) 18 (Fig. 1B and C), and 2 (4%) patients were infected by
MMTV-like virus (Fig. 1D).
No patient was infected with the two viruses at the same time.
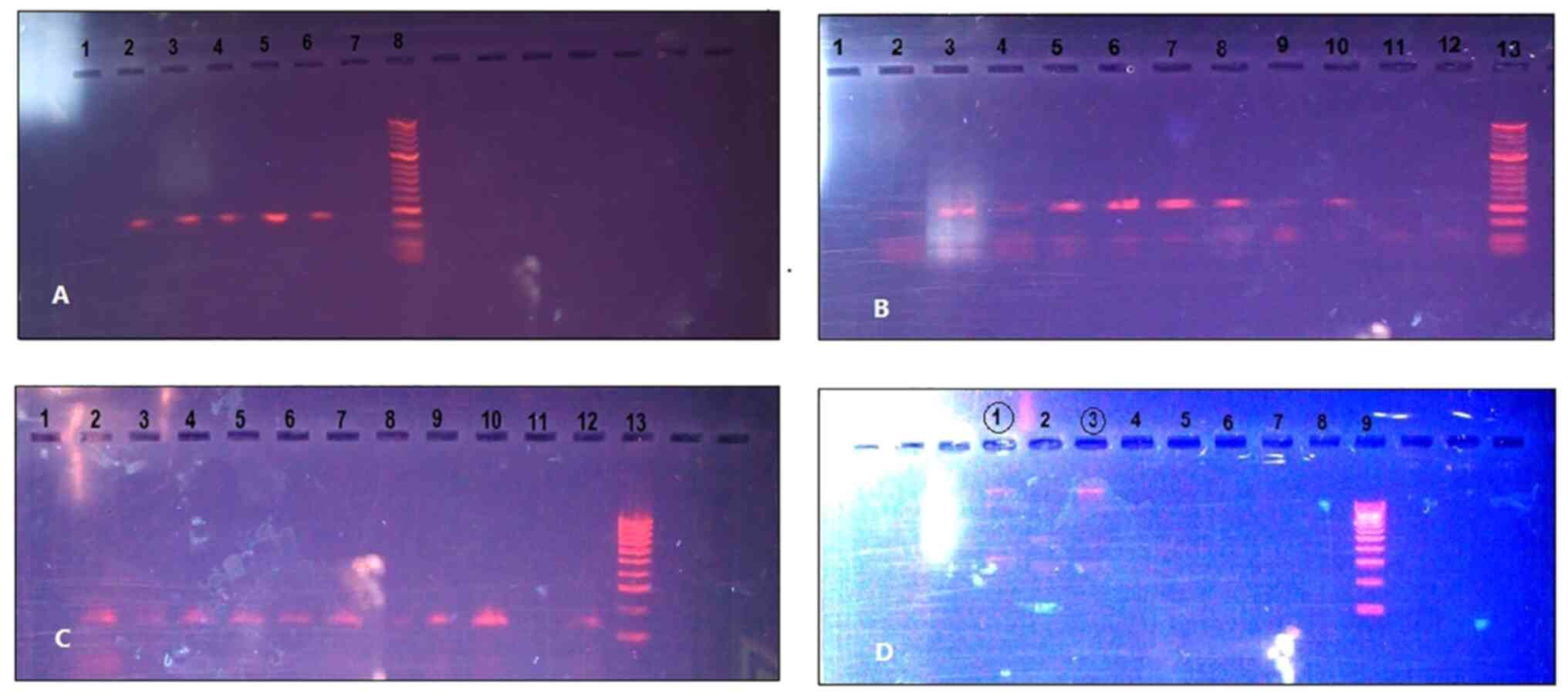 | Figure 1Positive viral infections in prostate
cancer tissues. (A) 268 base pair (bp) β-globin fragments on gel
(lanes 2-6, β-globin fragments; lane 7, negative control; lane 8,
50-bp DNA ladder). (B) HPV L1 450-bp fragment by MY09/MY11 primers
on gel (lane 1, empty; lanes, 3, 4 and 10, positive samples; lanes
2, 9 and 11, negative samples; lane 12, negative control; lane 13,
50-bp DNA ladder). (C) HPV L1 142-bp fragment by GP5+/GP6+ primers
on gel (Lane 1, negative control; lanes 2-10 and 12, HPV fragments;
lane 13, 100-bp DNA ladder. (D) MMTV-like (MMTV1 and 2
primers) on gel (lanes 1 and 3, suspected positive samples; lanes
4-8, negative; lane 9, 100-bp DNA ladder). HPV, human
papillomavirus; MMTV, mouse mammary tumor virus. |
As determined from Table II, the age of diagnosis varied
among those infected by HPV 18 (58-75 years). The PSA
concentrations also varied significantly (6.56-26.16 ng/ml). While
the Gleason score for 50% of these patients was 6; the Gleason
parameter was unknown for 1 patient. Three patients were not
analyzed for Gleason score. One patient was treated by radical
prostatectomy, three patients were treated by hormone therapy, and
1 patient was treated by both. Regarding tumor stage at the time of
biopsy, 4 men presented with stage 1 tumors, 3 presented with
histological stage 3 tumors, and 1 patient had third stage
tumor.
 | Table IIClinical characteristics of the 10
men infected by HPV or MMTV viruses. |
Table II
Clinical characteristics of the 10
men infected by HPV or MMTV viruses.
| ID | HPV 18+ve
(Yes/No) | MMTV+ve
(Yes/No) | Age at diagnosis
(years) | PSA (ng/ml) | Gleason
scorea | Treatment | Pathological
T-stage |
|---|
| PR-04 | N | Y | 57 | 8.09 | 6 | RP | T2a |
| PR-01 | Y | N | 72 | 18.92 | 6 | RP+HT | T1c |
| PR-03 | Y | N | 67 | 24.25 | 0 | - | T3 |
| PR-08 | N | Y | 61 | 7.27 | 0 | - | T1c |
| PR-05 | Y | N | 67 | 17 | 6 | HT | T2b |
| PR-06 | Y | N | 67 | 11.8 | 6 | HT | T1c |
| PR-07 | Y | N | 75 | 9.99 | - | - | T2b |
| PR-18 | Y | N | 70 | 19.31 | 6 | HT | T1c |
| PR-10 | Y | N | 58 | 6.56 | 0 | HT | T2b |
| PR-13 | Y | N | 63 | 26.16 | - | RP | T1c |
Those infected by the MMTV-like virus were
aged 57 and 61 years. Both patients had low PSA concentrations
(8.09 and 7.27 ng/ml). One patient presented with a Gleason score
of 6 and was treated by radical prostatectomy and presented with
early stage 2 after histological evaluation of the tumor. The other
patient was not analyzed for Gleason score and presented with tumor
stage one at the time of biopsy, and was not treated at the place
of therapy.
The clinical characteristics of the 10 men infected
by HPV 18 or MMTV-like viruses are documented in Table II.
To explore the relevance between tumor parameters
and infections by HPV 18 or MMTV-like virus, statistical
significance tests were performed (Table III). Five patients infected by
HPV 18 or MMTV-like had a Gleason score of 6. In contrast, 5
non-infected patients had the same Gleason score. There was a
significant difference between Gleason score and infection by one
of the two viruses analyzed (P=0.0008). Regarding PSA, 4 infected
patients had a low PSA concentration less than 10 ng/ml, and 4 had
a moderate rate of PSA concentration and 2 patients presented with
a high PSA concentration (>20 ng/ml). In contrast, 7 prostate
cancer patients among the 40 men not infected by any virus
presented with a low PSA concentration, and 27 out of 50 had a PSA
concentration more than 10 ng/ml. No significant differences
between viral infections and PSA concentration was noted
(P=0.2179).
 | Table IIIAssociation between prostate tumor
criteria and HPV 18, MMTV-like infected vs. non-infected
patients. |
Table III
Association between prostate tumor
criteria and HPV 18, MMTV-like infected vs. non-infected
patients.
| Tumor
characteristics | Infected by HPV or
MMTV-likea
(n=10) | Not infected
(n=40) |
P-valueb |
|---|
| Pathological
Gleason score | | | 0.0008 |
|
=6 | 5 | 5 | |
|
>6 | 0 | 25 | |
|
Unknown | 5 | 10 | |
| PSA | | | 0.2179 |
|
<10 | 4 | 7 | |
|
10-20 | 4 | 10 | |
|
>20 | 2 | 17 | |
|
Unknown | 0 | 6 | |
| Pathological T
stage | | | 0.4702 |
|
T1 | 5 | 9 | |
|
T2 | 4 | 13 | |
|
>T3 | 1 | 7 | |
|
Unknown | 0 | 11 | |
| Age at
diagnosis/surgery | | | 0.8101 |
|
≤60
years | 2 | 5 | |
|
>60
years | 8 | 28 | |
|
Unknown | 0 | 7 | |
| Radical
prostatectomy | | | 0.9644 |
|
Yes | 3 | 13 | |
|
No | 4 | 18 | |
|
Unkown | 3 | 9 | |
Nine out of 10 infected by viruses were at an early
histological stage of the tumor (T1 or T2), at the same time 22 out
of 40 of noninfected men treated for prostate cancer also presented
with early tumor stage T1 or T2. No significant differences between
viral infections and tumor stage were noted (P=0.4702). A total of
80% of the prostate cancer patients infected by viruses had an age
range of more than 60 years. In addition, the majority of those not
infected had the same range of age. No significant differences
between viral infections and age at diagnosis/surgery was noted
(P=0.8101). Three prostate cancer patients (30%) infected by HPV 18
or MMTV-like viruses were subjected to radical prostatectomy
while 13 non-infected patients (33% of the study group) were
subjected to radical prostatectomy. Eighteen patients were treated
with hormone therapy or other types of medication. A phylogenetic
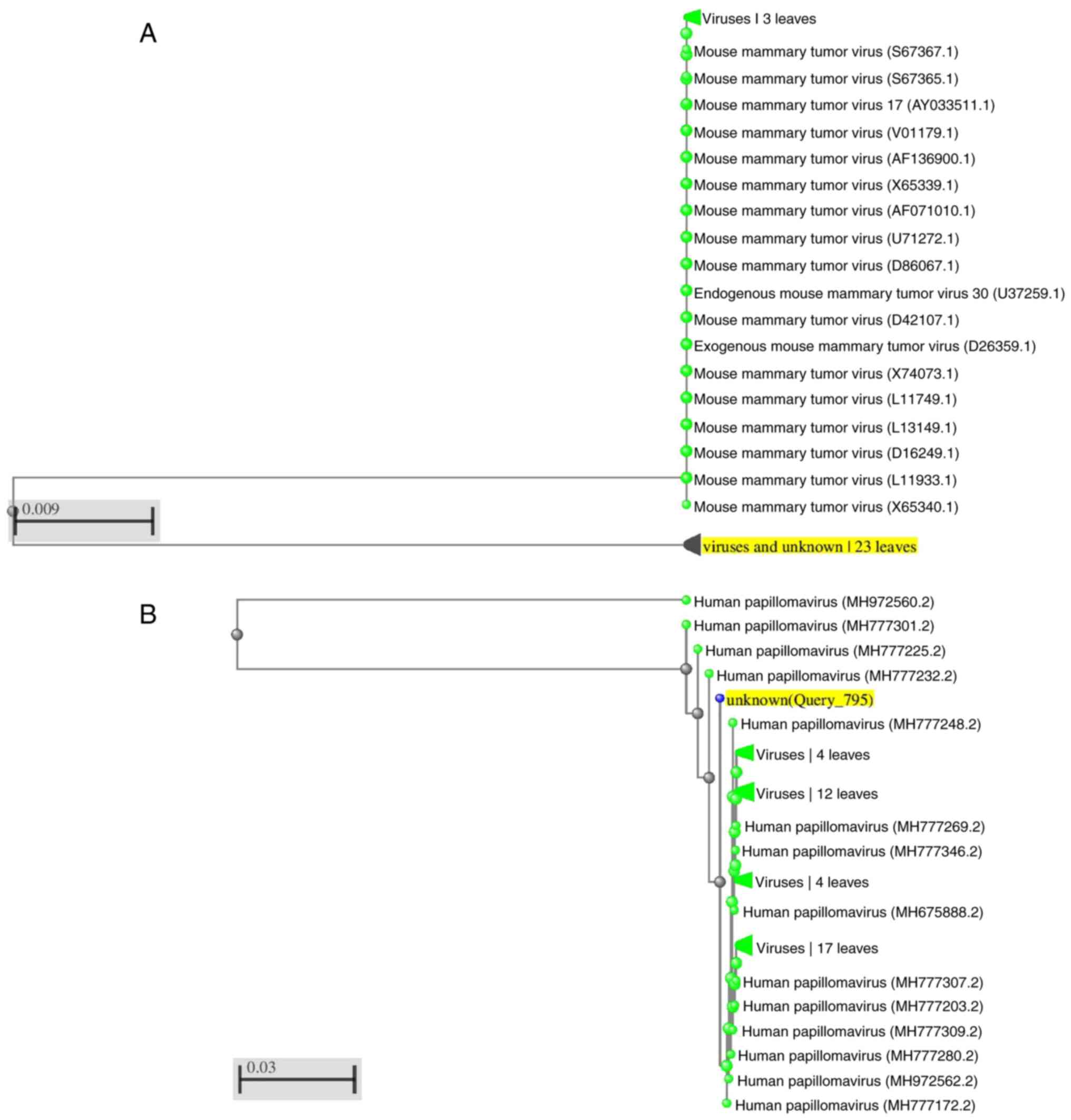
pairwise tree for MMTV-like was constructed using the
sequence data already registered in NCBI databases in comparisons
with our study sequence (Fig. 2A
and B).
The molecular evolution homology with other HPV 18
genotypes and MMTV-like is reported in Fig. 2A and B. Certain homologies of our sequence are
demonstrated with genotypes found in the NCBI database. Our
sequence meets the reference sequence at 4067-4078 base pair (bp),
the taxonomic classification of papillomaviruses in origin based on
nucleotide similarity of the L1 gene.
Discussion
Human papillomavirus (HPV) infections in healthy and
cancerous prostate tissues have been previously reported, but vary
in regards to infection frequencies among prostate cancer patients,
with no absolute confirmation concerning a susceptibility role of
HPV infections in prostate tumor development (16,17).
The present study findings concerning HPV 18, well-described as a
high-risk genotype, reported 8 HPV 18 infections (out of the 50
prostate cancer patients) in the prostate cancer biopsies. Two
mouse mammary tumor virus (MMTV) infections in the same subset of
subjects were identified; MMTV infections in prostate cancer
tissues have been rarely reported to date (18).
The association between tumor parameters and viral
infections was also discussed, revealing a significant association
between viral infections and Gleason score (P=0.0008). The majority
of infected men (5 patients) had a Gleason score of 6. From the
pathological angle, scores of 6 or less describe cancer cells that
look similar to normal cells and indicate that the cancer is likely
to grow slowly. Furthermore, the tumor pathological stage for 90%
of the infected patients was at stage one or two (T1 50%, T2 40%)
which indicate that the prostate tumors were in the early stages of
development. This indicates that viral infections were presented at
an early stage as the tumors were being initiated. This led us to
conclude that there is a possible role of high-risk HPV genotypes
or MMTV in the early development of prostate tumors. Yet, this
result should be considered with restraint since other tumor
criteria data especially the PSA concentration, tumor histological
stage, age at diagnosis or radical prostatectomy treatment, were
not significantly different between men infected by HPV 18 or
MMTV-like vs. the non-infected men (P=0.2179, 0.4702,
0.8101, and 0.9644, respectively). In addition; we considered the
tumor stage as a clinical parameter, while radical prostatectomy
was considered as a pathological indicators; thus, the result of
HPV and MMTV infections in those men treated with radical
prostatectomy should be given special attention than viral
infections in the clinical stages.
High homology of our sequence is demonstrated with
genotypes previously described by Tirosh et al (18). The research mentioned has described
the complete genome of HPV 18. Our sequence meets the reference
sequence at 4067-4078 base pair (bp), the taxonomic classification
of papillomaviruses in origin was based on nucleotide similarity of
the L1 gene sequence. Analyzing the molecular evolution tree, the
homology of MMTV-like sequence was concluded to be similar
to this described by Tsiagbe et al (19), providing the complete coding region
of the superantigen gene (SAG) in mouse models, meeting perfectly
at 784-800 of the reference sequence.
The comparisons were inclusive to the targeted
sequence superantigen gene (SAG). The homology of the superantigen
was notable with MMTV 3' LTR-derived superantigen gene, and
endogenous MMTV (RCS-MTV) superantigen gene from SJL/J mouse strain
(20,21), in addition to similarity with the
sequence of the MMTV proviral DNA (from Mus musculus mammary
gland) for gag-protease-pol polyprotein and env protein (19), and MMTV long terminal repeat region
(22,23). Concerning HPV sequences, the
similar homology was with HPV isolated from humans in the USA
(Fig. 2A and B) (24).
The origin of viral infections in the prostate has
been a point of argument for years. Taken in mind that the
associations between viral infections and prostate tumors has not
been perfectly established yet, the circulation of such viruses
between males and females should be considered, and further studies
concerning the assumed circulation are important. The present study
results revealed that HPV 18 or MMTV-like infections were
found in the prostate gland early in tumorgenesis. Therefore, other
studies with a wide sample size aimed at explaining the
relationship between viral infections, on the one hand, and Gleason
score and other tumor parameters, on the other hand, could be
statistically meaningful to further confirm our conclusion.
Supplementary Material
HPV detection on gel. HPV16 and 18
control test. Lane 1, 1:100 bp DNA ladder; lane 2, HPV16; lane 3,
HPV18, lane 4, 50 bp DNA ladder.
Oligonucleotides for β-globin
amplification.
Primer set data used in HPV-L1 gene
amplification.
Primer sequences and amplicon size
expected of MMTV amplification.
Acknowledgements
The authors would like to thank the biopsy donors
and their families, and many thanks to the members of the Virology,
Oncology and Medical Biotechnology team at FSTM-Hassan II
University of Casablanca, for all their efforts to support us
during all work stages on this research.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BA and MME conceived and designed the experiments.
BA, AL, MMz and MME optimized the experimental approach. BA, ML and
YE performed the experiments. BA, ML, YE, IA and SIK analyzed all
of the sequencing data and performed the statistical analyses. MMr,
AL and AA managed the sample collection and processing the data and
supervised the place in which all samples were stored. Proofreading
and technical assistance were carried out by BA, IA, MME and wrote
the manuscript. All authors read and approved the manuscript and
confirm the accuracy of the data and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Required ethical approval was obtained from the
Committee of Biomedical Research Ethics in Morocco (no. 3/2018/30
April/2018). All patients consented to participate in the study and
their information was handled as anonymous according to the ethical
standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Blattner M, Lee DJ, O'Reilly C, Park K,
MacDonald TY, Khani F, Turner KR, Chiu YL, Wild PJ, Dolgalev I, et
al: SPOP mutations in prostate cancer across demographically
diverse patient cohorts. Neoplasia. 16:14–20. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
zur Hausen H: Human papillomaviruses and
their possible role in squamous cell carcinomas. Curr Top Microbiol
Immunol. 78:1–30. 1977.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Faridi R, Zahra A, Khan K and Idrees M:
Oncogenic potential of human papillomavirus (HPV) and its relation
with cervical cancer. Virol J. 8(269)2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Paavonen J, Naud P, Salmerón J, Wheeler
CM, Chow SN, Apter D, Kitchener H, Castellsagué X, Teixeira JC,
Skinner SR, et al: Efficacy of human papillomavirus (HPV)-16/18
AS04-adjuvanted vaccine against cervical infection and precancer
caused by oncogenic HPV types (PATRICIA): Final analysis of a
double-blind, randomised study in young women. Lancet. 374:301–314.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McNicol PJ and Dodd JG: Detection of human
papillomavirus DNA in prostate gland tissue by using the polymerase
chain reaction amplification assay. J Clin Microbiol. 28:409–412.
1990.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen AC, Waterboer T, Keleher A, Morrison
B, Jindal S, McMillan D, Nicol D, Gardiner RA, McMillan NA and
Antonsson A: Human papillomavirus in benign prostatic hyperplasia
and prostatic adenocarcinoma patients. Pathol Oncol Res.
17:613–617. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang L, Xie S, Feng X, Chen Y, Zheng T,
Dai M, Zhou CK, Hu Z, Li N and Hang D: Worldwide prevalence of
human papillomavirus and relative risk of prostate cancer: A
meta-analysis. Sci Rep. 5(14667)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ghasemian E, Monavari SH, Irajian GR,
Jalali Nodoshan MR, Roudsari RV and Yahyapour Y: Evaluation of
human papillomavirus infections in prostatic disease: A
cross-sectional study in Iran. Asian Pac J Cancer Prev.
14:3305–3308. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bae JM: Human papillomavirus 16 infection
as a potential risk factor for prostate cancer: An adaptive
meta-analysis. Epidemiol Health. 37(e2015005)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lawson JS and Glenn WK: Evidence for a
causal role by mouse mammary tumour-like virus in human breast
cancer. NPJ Breast Cancer. 5(40)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Resnick RM, Cornelissen MT, Wright DK,
Eichinger GH, Fox HS, ter Schegget J and Manos MM: Detection and
typing of human papillomavirus in archival cervical cancer
specimens by DNA amplification with consensus primers. J Natl
Cancer Inst. 82:1477–1484. 1990.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee SH, Vigliotti VS, Vigliotti JS and
Pappu S: Validation of human papillomavirus genotyping by signature
DNA sequence analysis. BMC Clin Pathol. 9(3)2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Y, Holland JF, Bleiweiss IJ, Melana
S, Liu X, Pelisson I, Cantarella A, Stellrecht K, Mani S and Pogo
BG: Detection of mammary tumor virus env gene-like sequences in
human breast cancer. Cancer Res. 55:5173–5179. 1995.PubMed/NCBI
|
|
15
|
Ford CE, Tran D, Deng Y, Ta VT, Rawlinson
WD and Lawson JS: Mouse mammary tumor virus-like gene sequences in
breast tumors of Australian and Vietnamese women. Clin Cancer Res.
9:1118–1120. 2003.PubMed/NCBI
|
|
16
|
Medel-Flores O, Valenzuela-Rodríguez VA,
Ocadiz-Delgado R, Castro-Muñoz LJ, Hernández-Leyva S,
Lara-Hernández G, Silva-Escobedo JG, Vidal PG and Sánchez-Monroy V:
Association between HPV infection and prostate cancer in a Mexican
population. Genet Mol Biol. 41:781–789. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dillner J, Knekt P, Boman J, Lehtinen M,
Af Geijersstam V, Sapp M, Schiller J, Maatela J and Aromaa A:
Sero-epidemiologal association between human-papillomavirus
infection and risk of prostate cancer. Int J Cancer. 75:564–567.
1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tirosh O, Conlan S, Deming C, Lee-Lin SQ
and Huang X: NISC Comparative Sequencing Program. Su HC, Freeman
AF, Segre JA and Kong HH: Expanded skin virome in DOCK8-deficient
patients. Nat Med. 24:1815–1821. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tsiagbe VK, Yoshimoto T, Asakawa J, Cho
SY, Meruelo D and Thorbecke GJ: Linkage of superantigen-like
stimulation of syngeneic T cells in a mouse model of follicular
center B cell lymphoma to transcription of endogenous mammary tumor
virus. EMBO J. 12:2313–2320. 1993.PubMed/NCBI
|
|
20
|
Sharma P and Schreiber-Agus N: Mouse
models of prostate cancer. Oncogene. 18:5349–5355. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kang JJ, Schwegel T and Knepper JE:
Sequence similarity between the long terminal repeat coding regions
of mammary-tumorigenic BALB/cV and renal-tumorigenic C3H-K strains
of mouse mammary tumor virus. Virology. 196:303–308.
1993.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Uz-Zaman T, Ignatowicz L and Sarkar NH:
Mouse mammary tumor viruses expressed by RIII/Sa mice with a high
incidence of mammary tumors interact with the Vbeta-2-and
Vbeta-8-specific T cells during viral infection. Virology.
314:294–304. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Moore R, Dixon M, Smith R, Peters G and
Dickson C: Complete nucleotide sequence of a milk-transmitted mouse
mammary tumor virus: Two frameshift suppression events are required
for translation of gag and pol. J Virol. 61:480–490.
1987.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Singh N, Josefsson A, Hussain S and
Hugosson J: Human papillomavirus (HPV) infection as an emerging
risk factor in prostate cancer. J Global Oncol. 4 (Suppl
2):2018.
|