Introduction
In Japan, uterine cervical cancer (CC) is a frequent
cancer type in females, with 10,978 individuals affected in 2018.
Radical hysterectomy (RH) is selected and performed in Japan,
particularly for stage IB-IIA CC. Japanese CC guidelines indicate
that surgery was performed as the primary treatment in 90, 79, 66
and 59% of patients with stage IB1, IB2, IIA1 and IIA2 CC,
respectively (1). Based on
pathological assessment after RH, gynecologists decide whether
adjuvant therapy should be applied or not. To evaluate the
requirement for adjuvant therapy in early-stage CC with negative
pelvic nodes and negative parametrial invasion, several guidelines
indicated that histopathological assessment must determine tumor
size, depth of cervical stromal invasion and the presence of
lymphovascular invasion as risk factors for recurrence. The
National Comprehensive Cancer Network (NCCN) guidelines version
4.2019 described that if the surgical findings met the Sedlis
criteria, external pelvic radiation was required (2). The British Gynaecological Cancer
Society guidelines defined intermediate-risk factors as follows: i)
Presence of lymphovascular space invasion; ii) tumor maximum
diameter >4 cm at final pathology; and iii) deep cervical
stromal invasion (3). On the other
hand, the Japanese guidelines describe flexible scales of tumor
volume and depth of stromal invasion and there are different
criteria regarding tumor volume and depth of stromal invasion at
each institution (2).
Our group has been using a prognostic risk scoring
system called the Gynecologic Oncology Group (GOG) score to
evaluate the risk of recurrence since 2007. This system, reported
by Delgado et al (4) from
the GOG, comprises multiplying the relative risks assigned for
three factors: Tumor penetration, tumor size and lymph vascular
invasion. A GOG score >120 correlated with a 41% risk of
recurrence without adjuvant therapy after RH. Therefore, adjuvant
therapy for patients according to this criterion is thought to be
justifiable. By reference to the protocol reported by Kridelka
et al (5), the basis of a
GOG score >120 has been adopted by our group as an adjuvant
therapy criterion. In the present study, the validity of the GOG
score as a basis for adjuvant therapy was evaluated in cases of
stage IB-IIA node-negative CC after RH and pelvic
lymphadenectomy.
Patients and methods
Patients
The present study was a retrospective analysis
involving patients diagnosed with stage IB-IIA CC, according to the
International Federation of Gynecology and Obstetrics (FIGO) 2008
classification, admitted to the hospital of the University of
Occupational and Environmental Health (Fukuoka, Japan) between
February 2007 and December 2015. The exclusion criteria were as
follows: Presence of lymph node metastasis, positive surgical
margin and parametrial invasion from postoperative pathological
findings; or the patient received therapies other than radical
surgery as the primary therapy. The medical ethics committee of the
University of Occupational and Environmental Health (Fukuoka,
Japan) approved the present study (reference no. H30-160) and an
opt-out policy was provided on the web. The present study was
performed in accordance with the ethical standards of the 1964
Declaration of Helsinki.
Surgery
To determine the FIGO staging, magnetic resonance
imaging (MRI), computed tomography (CT) and pelvic examination
under anesthesia were performed. All patients were treated with
type C radical surgery and pelvic lymphadenectomy (6). Operations were performed under the
supervision of gynecologic oncologists certified by the Japan
Society of Gynecologic Oncology.
GOG scoring
The requirement for adjuvant therapy was determined
by the GOG score according to preoperative imaging examination and
histopathological findings. The tumor diameter was evaluated by
vaginal speculum examination, preoperative MRI based on the
interpretation of a radiologist and histopathological examination
of the conization specimen. The depth of tumor stromal invasion and
capillary/lymphatic space invasion were evaluated using
histopathological examination performed by two or more
pathologists. Based on the histopathological reports from the
pathology department, the scoring system according to the original
paper by Delgado et al (4)
was calculated in a weekly gynecologic cancer board. The score was
calculated by multiplying the relative risks assigned for the 3
factors according to Delgado's original paper, which are the tumor
size, depth of invasion (DOI) and lymph vascular space invasion
(LVSI). Adjuvant therapy with radiotherapy was performed in
patients with a GOG score >120.
Adjuvant radiotherapy
The radiation oncologist finalized the treatment
plan involving the use of radiation therapy (RT) and the
gynecologist determined whether concurrent chemotherapy was to be
administered, considering potential complications. Adjuvant RT
started within 2-4 weeks after surgery. The clinical target volume
(CTV) included the common iliac vessels, external and internal
iliac vessels, presacral area, parametrium and upper vagina,
according to the RT Oncology Group CTV guidelines for whole-pelvis
RT (7). The total radiation dose
was 50 or 50.4 Gy in 25 or 28 fractions, respectively (daily
fractions of 1.8 or 2.0 Gy over 5-6 weeks, 5 fractions per week)
except for 1 patient who was treated with boost irradiation to the
primary tumor bed (total dose of 61.2 Gy, daily fraction of 1.8 Gy)
due to dense adhesion of the primary tumor to the bladder. Unless
there was a specific contraindication, concurrent chemotherapy with
cisplatin (40 mg/m2) was administered weekly.
Observation/follow-up
The patients were instructed to visit our hospital
every 1-3 months for the first 1-2 years, then every 3-6 months in
the 3rd year and every 6 months in the 4th and 5th years. Clinical
examinations, such as PAP smear, pelvic examination and tumor
marker detection, were performed at each visit and CT was performed
every 6 months.
Statistical analysis
The primary endpoint of the present study was
overall survival (OS) and recurrence-free survival (RFS) according
to the groups stratified based on the GOG score as follows: Group
A, ≤40; group B, >40 and ≤70; group C, >70 and ≤120; and
group D, >120. The Kaplan-Meier method was used for survival
analysis and statistical significance was determined using the
log-rank test. The age differences were analyzed using Student's
t-test and categorical variables were analyzed with the
χ2 test or Fisher's exact test.
All statistical analyses were performed with EZR ver
4.0.2 (Saitama Medical Center, Jichi Medical University), which is
a graphical user interface for R (The R Foundation for Statistical
Computing), at a significance level of P<0.05(8). More precisely, it is a modified
version of R commander (version 1.51) that was designed to add
statistical functions frequently used in biostatistics.
Results
Patient characteristics
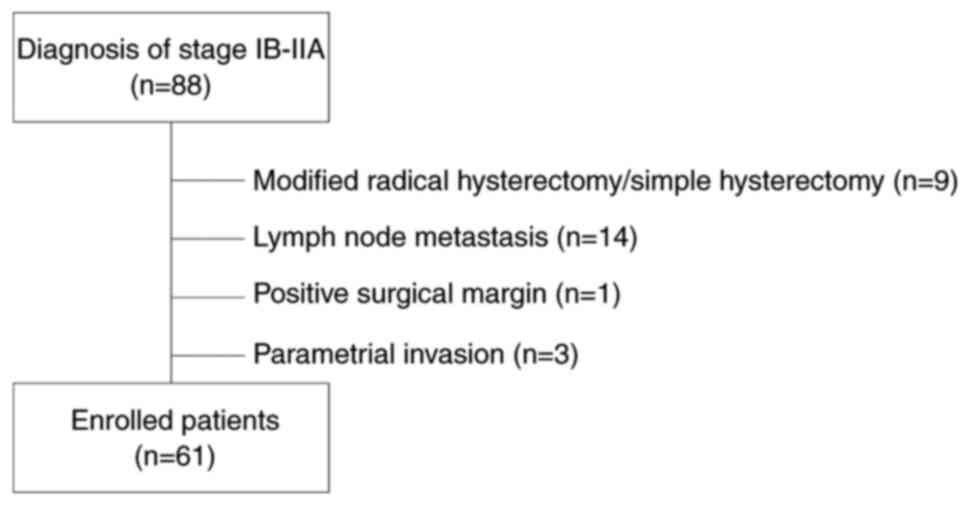
A total of 88 patients diagnosed with stage IB-IIA
CC were identified and preliminarily enrolled based on the
exclusion criteria in Fig. 1.
Finally, 61 patients matched the inclusion criteria and were
included in the analysis; their characteristics are presented in
Table I. The patients' mean age
was 47.82 years (age range, 22-76 years). The median follow-up
period was 79 (range, 39-149) months, excluding one patient who was
lost to follow-up at 39 months. A total of four patients had
recurrence and two patients died of disease (Table II). The overall relapse rate and
morbidity rate were 6.56% (four out of 61) and 3.28% (two out of
61), respectively. None of the patients had any other malignant
diseases or died of intercurrent diseases.
 | Table IPatients' characteristics (n=61). |
Table I
Patients' characteristics (n=61).
| Item | Total | Recurrence | P-value | Died of disease | P-value |
|---|
| Age, years | | | 0.00816 | | 0.0639 |
|
20-29 | 4 | 0 | | 0 | |
|
30-39 | 15 | 0 | | 0 | |
|
40-49 | 17 | 0 | | 0 | |
|
50-59 | 10 | 0 | | 0 | |
|
60-69 | 9 | 3 | | 2 | |
|
70- | 6 | 1 | | 0 | |
| Stage (FIGO
2008) | | | 1 | | 1 |
|
IB1 | 50 | 4 | | 2 | |
|
IB2 | 5 | 0 | | 0 | |
|
IIA | 6 | 0 | | 0 | |
| Procedure | | | 1 | | 1 |
|
Radical
hysterectomy + PLN | 59 | 4 | | 2 | |
|
Radical
trachelectomy + PLN | 2 | 0 | | 0 | |
| Histology | | | 0.534 | | 0.0705 |
|
SCC | 36 | 2 | | 0 | |
|
Adenocarcinoma | 19 | 1 | | 1 | |
|
Adenosquamous
carcinoma | 6 | 1 | | 1 | |
| Tumor size, cm | | | 0.89 | | 0.83 |
|
<1 | 8 | 0 | | 0 | |
|
≥1,
<2 | 8 | 0 | | 0 | |
|
≥2,
<3 | 13 | 1 | | 0 | |
|
≥3,
<4 | 24 | 3 | | 2 | |
|
≥4,
<5 | 7 | 0 | | 0 | |
|
≥5 | 1 | 0 | | 0 | |
| DOI | | | 0.254 | | 0.76 |
|
Superficial | 20 | 0 | | 0 | |
|
Middle | 22 | 3 | | 1 | |
|
Deep | 19 | 1 | | 1 | |
| LVSI | | | 0.113 | | 0.492 |
|
Positive | 31 | 4 | | 2 | |
|
Negative | 30 | 0 | | 0 | |
| GOG score | | | 1 | | 0.384 |
|
≤120 | 48 | 3 | | 1 | |
|
Patients who
received chemotherapy | 1a | 0 | | 0 | |
|
>120 | 13 | 1 | | 1 | |
|
Patients who
received radiation therapy | 0 | 0 | | 0 | |
|
Patients who
received chemotherapy | 1 | 1 | | 1 | |
|
Patients who
refused adjuvant therapy | 2 | 0 | | 0 | |
 | Table IICharacteristics of the patients with
recurrence. |
Table II
Characteristics of the patients with
recurrence.
| Age, years | Stage | Histology | DOI, mm (invasion/
cervical wall) | LVSI | Site of
recurrence | GOG score | Adjuvant
therapy | Disease-free
interval, months | Survival period,
months | Outcome |
|---|
| 61 | IB1 | Adenosquamous | 10 / 20 | + | Pelvic floor,
PAN | 89.76 | None | 46 | 60 | DOD |
| 73 | IB1 | SCC | 6 / 8 | + | Pelvic floor | 89.76 | None | 20 | 78 | Alive |
| 68 | IB1 | SCC | 10 / 15 | + | Liver | 90.44 | None | 34 | 91 | Alive |
| 62 | IB1 | Adenocarcinoma | 16 / 22 | + | Pelvic floor | 183.6 | TC 3 cycles | 27 | 66 | DOD |
The common decades of life of affected subjects were
the forties (27.9%), thirties (24.6%) and fifties (16.4%). Unlike
the population of the patients, all patients with recurrence or who
died of disease were >60 years of age (4 patients). FIGO stage
IB1 was the most frequent stage (50 of 61 patients; 82.0%) and four
patients had recurrence. RH was performed in 59 patients and
radical trachelectomy was applied in two nulliparous patients to
preserve their fertility. The tumor size in the two patients who
received trachelectomy was 1.6 and 2.4 cm in diameter and the
pathological diagnosis was squamous cell carcinoma (SCC) in both
cases. The common histological types, from most to least, were SCC
(36 of 61 patients; 59%), adenocarcinoma (19 of 61; 31.1%) and
adenosquamous carcinoma (6 of 61; 9.8%). A total of four patients
with recurrent disease were diagnosed with SCC (2 patients),
adenocarcinoma (1 patient) and adenosquamous cell carcinoma (1
patient). Furthermore, two patients with SCC survived after
additional therapy (radiation therapy for intrapelvic recurrence
and chemotherapy with irinotecan and nedaplatin for liver
metastasis) without any evidence of further malignancy during the
follow-up period.
Tumor sizes of ≥3 and <4 cm (39.3%) and ≥2 and
<3 cm (21.3%) were common; furthermore, all four patients with
recurrence were detected in these groups and had intermediate or
deep invasion and LVSI. A total of 48 patients had a GOG score
≤120. Of the 13 patients with a GOG score >120, 10 received
adjuvant RT. The remaining three patients declined, with 1
preferring chemotherapy and two preferring no adjuvant treatment.
Furthermore, three patients with GOG ≤120 had recurrence and one of
them died of disease. All patients with recurrent disease had over
one-half of stromal invasion and had LVSI.
Response and survival analysis
A total of 60 patients, except for one patient with
double cancer, were analyzed using the Kaplan-Meier method and the
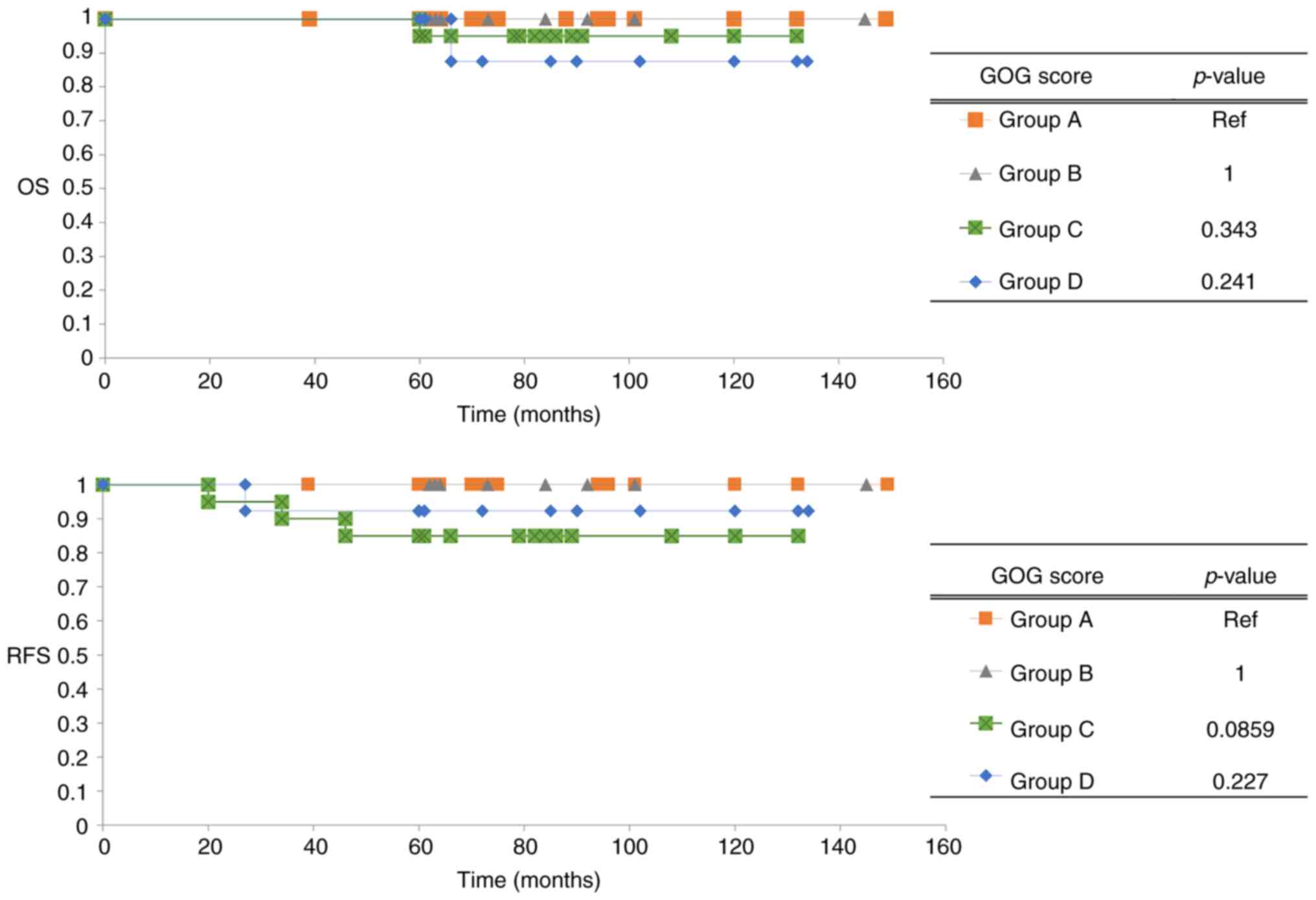
log-rank test. In Fig. 2, OS and
RFS are presented according to stratified groups by GOG score
during the overall follow-up period. In both groups A and B, OS and
RFS of patients involved no recurrence. By contrast, the OS of
group D and the RFS of group C were both low. The log-rank test was
applied to obtain P-values for comparisons of group A (GOG score;
≤40) as a low-score group with the others. The OS and RFS of both
groups C and D were low; however, the log-rank test indicated no
significant differences compared to group A. Table III indicates the 5-year OS rate
and 5-year RFS rate according to the groups. As one patient in
group D died of disease after five years of primary treatment, this
case did not reflect the 5-year OS of group D. In total, the 5-year
OS and the RFS were 98.3 and 93.3%, respectively.
 | Table IIIFive-year OS and RFS rates according
to the groups stratified by GOG score. |
Table III
Five-year OS and RFS rates according
to the groups stratified by GOG score.
| A, OS |
|---|
| Group based on GOG
score | n | 5-year OS | SD | 95% CI |
|---|
| A | 19 | 1 | NA | NA |
| B | 8 | 1 | NA | NA |
| C | 20 | 0.95 | 0.049 | 0.695-0.993 |
| D | 13 | 1 | NA | NA |
| B, RFS |
| Group based on GOG
score | n | 5 year-RFS | SD | 95% CI |
| A | 19 | 1 | NA | NA |
| B | 8 | 1 | NA | NA |
| C | 20 | 0.85 | 0.080 | 0.604-0.949 |
| D | 13 | 0.92 | 0.074 | 0.566-0.989 |
Discussion
In the present study, a retrospective analysis of
the prognosis of stage IB-IIA node-negative CC was performed using
the GOG score after RH and trachelectomy. Furthermore, to the best
of our knowledge, the present study was the first to involve the
evaluation of prognosis based on the scoring system in Japan. The
GOG score system that was used was first published by Delgado et
al (4). They provided a
retrospective evaluation of the prognosis of stage IB CC of the
phenotype SCC without any adjuvant therapy. Kridelka et al
(5) determined the criteria for
the management of lymph node-negative stage IB uterine CC after RH
and demonstrated the evaluation of therapeutic outcome based on
these criteria. In contrast to the previous report by Delgado et
al (4), the criteria of
Kridelka et al (5)also
included other pathological types, such as adenocarcinoma. The
criteria indicated that the higher-risk group with a GOG score
>120 must receive small-field pelvic radiation and the
lower-risk group with a GOG score ≤120 must be monitored closely
without adjuvant therapy. At our institute, the GOG score has been
used for the indication of adjuvant therapy after RH since 2007.
The validity of the criteria for the intermediate risk of CC has
been controversial and each institute has used various criteria
independently. Yahata et al (9) reported that their institute defined
intermediate risk as the patient having at least one of the
following: Deep stromal invasion >1/3, lymph vascular space
invasion and bulky tumor >4 cm. Kim et al (10) defined deep stromal invasion as
invasion depth/cervical wall >1/2. They indicated that the
patients with intermediate risk factors of CC require adjuvant
therapy. On the contrary, Cibula et al (11) reported the outcome for patients
with no adjuvant radiotherapy for intermediate risk with lymph
node-negative CC. The recurrence rate was 6.3% (eight out of 127)
and the 5-year disease-specific survival rate was 95.7%. They
performed type C2 RH for all patients without adjuvant radiotherapy
and obtained better outcomes than the present study.
The most well-known criteria were reported by Sedlis
et al (12) in the GOG
study #92 trial and they determined the systematic eligibility
criterion, which requires at least 2 of the following risk factors:
>1/3 stromal invasion, LVSI and large clinical tumor diameter of
4 cm or more. Adjuvant radiotherapy for the eligible patients
reduced the risk of recurrence and prolonged progression-free
survival in the follow-up study (13). The outcome of this follow-up study
is referred to as the Sedlis criteria, according to which adjuvant
radiotherapy is adopted for negative nodes, negative margins and
negative parametrium of CC in the NCCN Guidelines version 4.2019 CC
(4). On the other hand, additional
opinions to judge the intermediate risk of patients with CC after
surgery were published. Using more simplified criteria, Cao et
al (14) reported that the
patients who were defined as having intermediate-risk CC based on
the criteria did not necessarily require adjuvant therapy to
prevent recurrence. They emphasized that LVSI was the only
independent prognostic factor. On the contrary, when the criteria
were examined in more detail, Chu et al (15) reported on the validation of risk
stratification using a machine learning algorithm in addition to
the Sedlis criteria. This risk stratification consisted of age,
LVSI, stromal invasion, size and type of adjuvant therapy, and was
able to indicate expected OS and disease-free survival (DFS) 2 and
5 years after surgery (15). These
expected OS and DFS rates were derived based on the time-dependent
receiver operating characteristic (ROC) curves and the area under
the ROC curve. In the present study, it was hypothesized that the
risk stratification is able to easily predict the expected OS and
DFS; however, this was difficult to use as an indication for
detecting low risk or intermediate risk of CC after surgery.
Regarding the outcomes of the present study,
prognoses differed according to the different categories of the GOG
score. The outcome of group B (GOG score >40 and ≤70) was not
different from that of group A (GOG score ≤40). Based on the above
findings, a GOG score ≤70 may require no other adjuvant therapy.
When comparing the Sedlis criteria to the GOG scoring system,
certain criteria, namely positivity for LVSI, superficial stromal
invasion and clinical tumor diameter of ≥5, were associated with a
considerably low GOG score. Evaluation of a GOG score ≤70 may imply
that the use of the Sedlis criteria leads to overtreatment. By
contrast, 5-year RFS of group C (GOG score >70 and ≤120) was
worse than that of group D (GOG score >120). Although 1 patient
in group D died of disease after adjuvant chemotherapy, other
patients who received adjuvant radiotherapy were still alive
without any evidence of disease. In group D, 5-year OS and 5-year
RFS were 100 and 92.3%, respectively. Kridelka et al
(5) reported that the recurrence
rate after small-field pelvic radiation for patients with a GOG
score of 120 or higher was 4% (one out of 25). Yeo et al
(16) evaluated the outcome of
patients with a GOG score >40 and ≤120, and those with GOG
>120 received small-field radiotherapy and standard-field
radiotherapy (whole pelvic radiation). The recurrence rates in the
groups with a GOG score >40 and ≤120, and >120 were 2.78%
(one out of 36) and 4% (one out of 25), respectively. No patient
died of CC. Compared to these studies, the outcome of the present
study in group D followed a similar trend. On the other hand, the
outcome of the patients in group C (GOG score, >70 and ≤120) was
not significantly different from that of group A. However, in group
C, three patients had recurrence and one patient died of disease.
Two patients with SCCs survived after additional therapy and one
patient with adenosquamous carcinoma died of disease progression
after recurrence. Regarding recurrent cases from group C, the GOG
score ranged from 89.76 to 90.44. It was not possible to clarify
any appropriate standard, which may exist for the range of group C
(GOG score, >70 and ≤120). Ideally, each factor should be
analyzed after accumulation and review of patients from group
C.
Of note, four cases of recurrence had several
features in common, such as an age of sixty years or more,
positivity for LVSI, >1/2 stromal invasion and clinical tumor
diameter of 2 cm or more. The pathological types were SCC (2
cases), adenocarcinoma (1 case) and adenosquamous carcinoma (1
case). The first three cases in Table
II belonged to group C (GOG score >70 and ≤120) and their
GOG score ranged from 89.76 to 90.44. In group C, eleven cases
other than three recurrent cases had higher GOG scores than those
of these three. It was hypothesized that the probability of
recurrence may not depend on a high GOG score. Based on other
criteria, these cases were indicated to require adjuvant therapy.
Nakamura et al (17)
defined three factors (positivity for LVSI, >1/2 stromal
invasion and clinical tumor diameter of ≥2 and <4) as
intermediate risk factors and these cases satisfied all factors.
Similarly, these cases also satisfied indications for adjuvant
therapy according to the Sedlis criteria (18). It should be noted that cases with a
GOG score ≤120 may include cases with indications for adjuvant
therapy based on other criteria.
There were certain limitations to the present study.
First, the sample size of the present study was too small to
evaluate the prognosis using the GOG score for stage IB-IIA
node-negative CC precisely, as this was a single institutional
study. At the beginning of the present study, it was intended to
evaluate the validity of a GOG score ≥120 as an indication of
adjuvant therapy. Eventually, only the prognosis of the four groups
stratified by the GOG score was compared. Furthermore, a single
institutional study has a problem of guaranteeing surgical outcomes
if the institute has a low surgical volume. Matsuo et al
(19) reported that the prognosis
for early-stage CC after RH was associated with the institutional
surgical volume. In addition, the original study reported by
Delgado et al (4) indicated
that a GOG score >120 correlated with a 40% risk of recurrence.
The study was designed to evaluate the prognosis of only the SCC
type of CC. However, in the present study, the indications,
including the other pathological types, were determined by
reference to the criteria reported by Kridelka et al
(5), which suggested that patients
with a GOG score ≥120 receive small-field external beam
radiotherapy. Several multivariate analyses indicated that
adenocarcinoma had a poorer prognosis than SCC (20-22).
It is worth acknowledging that prognosis should ideally be analyzed
using samples divided into each pathological type.
In conclusion, a GOG score ≤70 was suggestive of no
recurrence without adjuvant therapy. However, the risk of a GOG
score >70 differed by reference to risk factors that constitute
the GOG score. The current cutoff for adjuvant therapy (GOG score
>120) should be further discussed.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All the datasets generated or analyzed during the
present study are included in this published article.
Authors' contributions
YK, YM and KY conceptualized this study. YK
performed the data analysis. TO, from the perspective of a
radiologist, advised the gynecologists on adjuvant therapy. YA, MM,
KH, HH, TU, TK and SK acquired the clinical data. YK and YM drafted
the manuscript. YK and YM checked and approved the authenticity of
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current retrospective study was approved by the
Ethics Committee of Medical Research, University of Occupational
and Environmental Health (Fukuoka, Japan; approval reference no.
H30-160). Information about the study and how patients are able to
opt out was posted on the website of the hospital.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Ebina Y, Mikami M, Nagase S, Tabata T,
Kaneuchi M, Tashiro H, Mandai M, Enomoto T, Kobayashi Y, Katabuchi
H, et al: Japan society of gynecologic oncology guidelines 2017 for
the treatment of uterine cervical cancer. Int J Clin Oncol.
24:1–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Koh W, Abu-Rustum NR, Bean S, Bradley K,
Campos SM, Cho KR, Chon HS, Chu C, Clark R, Cohn D, et al: Cervical
cancer, version 3.2019, NCCN clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 17:64–84. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Reed N, Balega J, Barwick T, Buckley L,
Burton K, Eminowicz G, Forrest J, Ganesan R, Harrand R, Holland C,
et al: British gynaecological cancer society (BGCS) cervical cancer
guidelines: Recommendations for practice. Eur J Obstet Gynecol
Reprod Biol. 256:433–465. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Delgado G, Bundy B, Zaino R, Sevin BU,
Creasman WT and Major F: Prospective surgical-pathological study of
disease-free interval in patients with stage IB squamous cell
carcinoma of the cervix: A gynecologic oncology group study.
Gynecol Oncol. 38:352–357. 1990.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kridelka FJ, Berg DO, Neuman M, Edwards
LS, Robertson G, Grant PT and Hacker NF: Adjuvant small field
pelvic radiation for patients with high risk, stage IB lymph node
negative cervix carcinoma after radical hysterectomy and pelvic
lymph node dissection. A pilot study. Cancer. 86:2059–2065.
1999.PubMed/NCBI
|
|
6
|
Querleu D and Morrow CP: Classification of
radical hysterectomy. Lancet Oncol. 9:297–303. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chino J, Annunziata CM, Beriwal S,
Bradfield L, Erickson BA, Fields EC, Fitch K, Harkenrider MM,
Holschneider CH, Kamrava M, et al: Radiation therapy for cervical
cancer: Executive summary of an ASTRO clinical practice guideline.
Pract Radiat Oncol. 10:220–234. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yahata H, Sonoda K, Inoue S, Yasutake N,
Kodama K, Yagi H, Yasunaga M, Ohgami T, Onoyama I, Kaneki E, et al:
Is adjuvant therapy necessary for patients with intermediate-risk
cervical cancer after open radical hysterectomy? Oncology.
98:853–858. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim K, Kang SB, Chung HH, Kim JW, Park NH
and Song YS: Comparison of chemoradiation with radiation as
postoperative adjuvant therapy in cervical cancer patients with
intermediate-risk factors. Eur J Surg Oncol. 35:192–196.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cibula D, Abu-Rustum NR, Fischerova D,
Pather S, Lavigne K, Slama J, Alektiar K, Ming-Yin L, Kocian R,
Germanova A, et al: Surgical treatment of ‘intermediate risk’ lymph
node negative cervical cancer patients without adjuvant
radiotherapy-A retrospective cohort study and review of the
literature. Gynecol Oncol. 151:438–443. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sedlis A, Bundy BN, Rotman MZ, Lentz SS,
Muderspach LI and Zaino RJ: A randomized trial of pelvic radiation
therapy versus no further therapy in selected patients with stage
IB carcinoma of the cervix after radical hysterectomy and pelvic
lymphadenectomy: A gynecologic oncology group study. Gynecol Oncol.
73:177–183. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rotman M, Sedlis A, Piedmonte MR, Bundy B,
Lentz SS, Muderspach LI and Zaino RJ: A phase III randomized trial
of postoperative pelvic irradiation in stage IB cervical carcinoma
with poor prognostic features: Follow-up of a gynecologic oncology
group study. Int J Radiat Oncol Biol Phys. 65:169–176.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cao L, Wen H, Feng Z, Han X, Zhu J and Wu
X: Role of adjuvant therapy after radical hysterectomy in
intermediate-risk, early-stage cervical cancer. Int J Gynecol
Cancer. 31:52–58. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chu R, Zhang Y, Qiao X, Xie L, Chen W,
Zhao Y, Xu Y, Yuan Z, Liu X, Yin A, et al: Risk stratification of
early-stage cervical cancer with intermediate-risk factors: Model
development and validation based on machine learning algorithm.
Oncologist. 26:e2217–e2226. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yeo RM, Chia YN, Namuduri RP, Yap SP,
Soong YL, Yam PK, Lim TY and Khoo-Tan HS: Tailoring adjuvant
radiotherapy for stage IB-IIA node negative cervical carcinoma
after radical hysterectomy and pelvic lymph node dissection using
the GOG score. Gynecol Oncol. 123:225–229. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nakamura K, Kitahara Y, Satoh T, Takei Y,
Takano M, Nagao S, Sekiguchi I and Suzuki M: Analysis of the effect
of adjuvant radiotherapy on outcomes and complications after
radical hysterectomy in FIGO stage IB1 cervical cancer patients
with intermediate risk factors (GOTIC study). World J Surg Oncol.
14(173)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
National Comprehensive Cancer Network.
Cervical Cancer (version 4.2019). https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1426
(accessed September 2019).
|
|
19
|
Matsuo K, Shimada M, Yamaguchi S, Matoda
M, Nakanishi T, Kikkawa F, Ohmichi M, Okamoto A, Sugiyama T and
Mikami M: Association of radical hysterectomy surgical volume and
survival for early-stage cervical cancer. Obstet Gynecol.
133:1086–1098. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takeda N, Sakuragi N, Takeda M, Okamoto K,
Kuwabara M, Negishi H, Oikawa M, Yamamoto R, Yamada H and Fujimoto
S: Multivariate analysis of histopathologic prognostic factors for
invasive cervical cancer treated with radical hysterectomy and
systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol
Scand. 81:1144–1151. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Biewenga P, van der Velden J, Mol BW,
Stalpers LJ, Schilthuis MS, van der Steeg JW, Burger MP and Buist
MR: Prognostic model for survival in patients with early stage
cervical cancer. Cancer. 117:768–776. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yoneoka Y, Kato MK, Tanase Y, Uno M,
Ishikawa M, Murakami T and Kato T: The baseline recurrence risk of
patients with intermediate-risk cervical cancer. Obstet Gynecol
Sci. 64:226–233. 2021.PubMed/NCBI View Article : Google Scholar
|