Introduction
Plasmablastic lymphoma (PBL) is an uncommon but
aggressive subtype of diffuse large B-cell lymphoma that is
associated with acquired immunodeficiency syndrome and
characterized by its association with Epstein-Barr virus (EBV)
(1). PBL is diagnostically
challenging and has a poor prognosis (2). PBL diagnosis through fine-needle
aspiration cytology is infrequently reported, yet cytomorphologic
features such as hypercellular smears with abundance of
plasmablastic cells may provide an early indicator for diagnosing
PBL (3). The immunophenotype of
PBL is positive for the plasma cell markers CD79a, multiple myeloma
1/interferon regulatory factor 4, B lymphocyte-induced maturation
protein-1, CD38 and CD138, but negative for the B-cell markers
CD19, CD20 and paired box 5(4).
The intensification role of induction chemotherapy is
controversial. Additionally, novel agents, such as bortezomib and
lenalidomide, have shown effectiveness in relapsed cases and serve
a relatively important role in frontline treatment (5,6). PBL
predominantly occurs in immunosuppressed men with a solid organ
transplant. Some case reports have indicated that allogeneic
hematopoietic stem cell transplantation provides long-term survival
opportunities for these patients (2). The present study reported an unusual
case of bilateral renal and duodenal PBL.
Case report
A 71-year-old man presented to the emergency
department of the Tri-Service General Hospital (Taipei, Taiwan) in
July 2020 with a 7-day history of progressive left flank pain and
tarry stool. Their past medical history (14 years ago) included PBL
of the left nasal cavity and paranasal sinuses. The patient
received radiotherapy for PBL, which metastasized to the right neck
4 years ago. Their vital signs were normal, and physical
examination showed left costovertebral angle tenderness. The
laboratory tests revealed a blood urea nitrogen level of 38 mg/dl,
serum creatinine level of 1.4 mg/dl, lactic acid dehydrogenase of
3,090 U/l and positive fecal occult blood. Bedside point-of-care
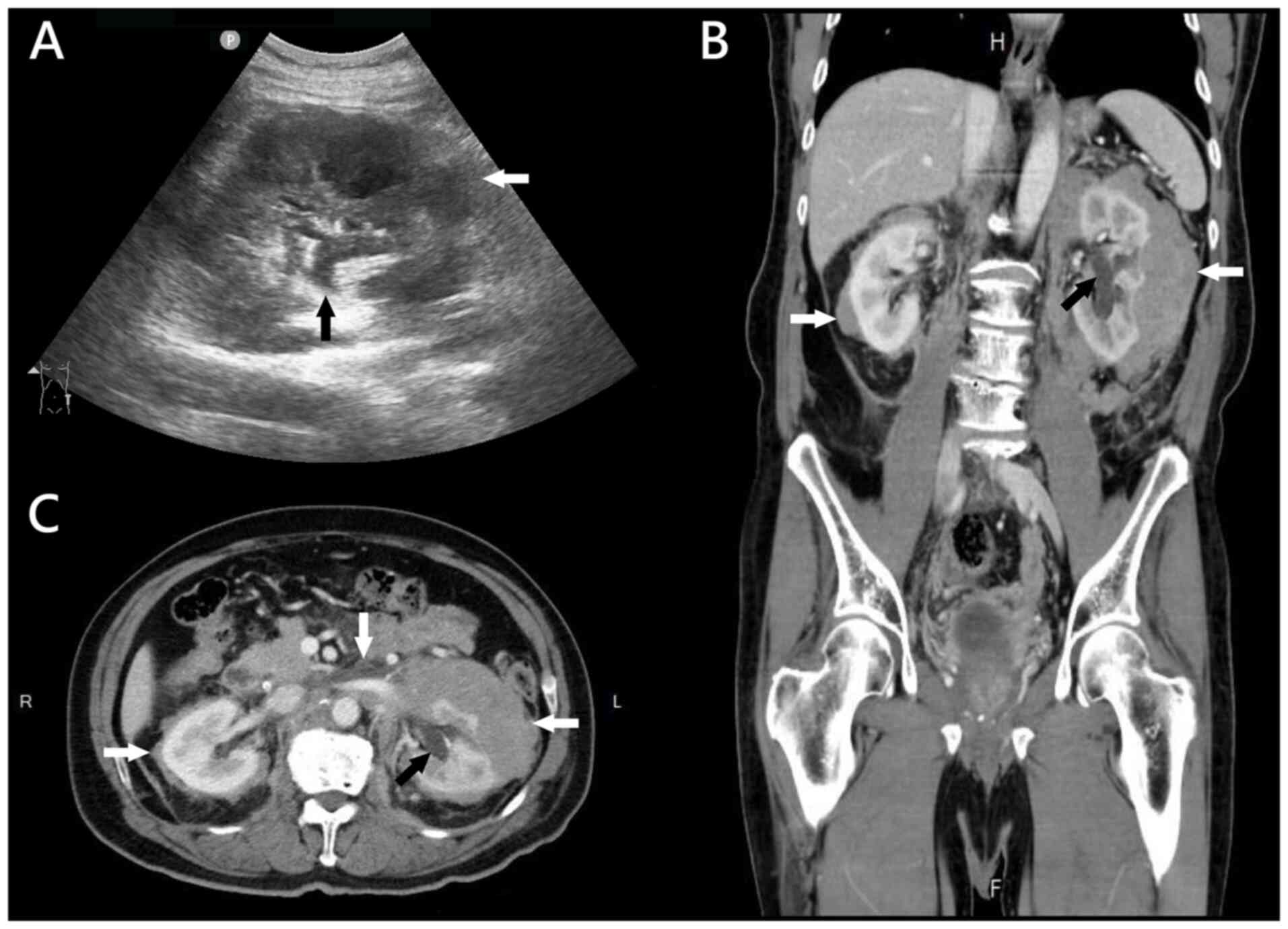
ultrasound of the left kidney revealed lobulated ill-defined
hypoechoic foci in the perirenal spaces with mild hydronephrosis
(Fig. 1A). Subsequent
contrast-enhanced computed tomography (CT) of the abdomen
demonstrated lobulated low-density lesions in the bilateral
perirenal and paraaortic spaces (Fig.
1B and C). The patient was
subsequently admitted to the internal medicine department.
The laboratory findings included mild anemia and the
detection of monoclonal immunoglobulin (Ig)G and κ/λ-type Bence
Jones protein. The patient was negative for human immunodeficiency
virus (HIV) rapid point-of-care test, hepatitis B virus, hepatitis
C virus and EBV viral-capsid antigen (EBV-VCA) IgM (<10.00
U/ml), but positive for EBV-VCA IgG (>750.00 U/ml). Renal and
duodenal biopsies were arranged to rule out malignancy. The tissues
were fixed by perfusion with 4% formaldehyde for at least 6 h at
room temperature, paraffin-embedded at 60˚C, cut into 20-µm-thick
sections. The tissue sections were rehydrated in TBS (25 mM
Tris-HCl, pH 7.4, 137 mM NaCl, 2.7 mM KCl) for 5 min at room
temperature. They were then incubated with the antibody in
‘antibody diluent’ (Dako; Agilent Technologies, Inc.) for 30 min at
room temperature. Immunohistochemical staining was performed using
the following antibodies: anti-CD20 (cat. no. SM3140B; 1:300;
OriGene Technologies, Inc.), anti-CD79a (cat. no. TA351934; 1:300;
OriGene Technologies, Inc.), anti-CD3 (cat. no. 14-0032-82; 1:100;
BD Biosciences), anti-CD138 (cat. no. 36-2900; 1:500; Thermo Fisher
Scientific, Inc.), anti-CD38 (cat. no MA5-14413; 1:500; Thermo
Fisher Scientific, Inc.) and anti-CD10 (cat. no. TA327616; 1:60;
OriGene Technologies, Inc.), placed horizontally on a thermal plate
at 37˚C. The sections were then rinsed three times in TBS and
subjected to the detection reaction. Briefly, the slides were
incubated with HRP-conjugated DISCOVERY® Universal
Secondary Antibody (cat. no. 760-4205, Ventana Medical Systems;
Roche Tissue Diagnostics), and detected by the Discovery DAB Map
Kit (Ventana Medical Systems; Roche Tissue Diagnostics) for 30 min
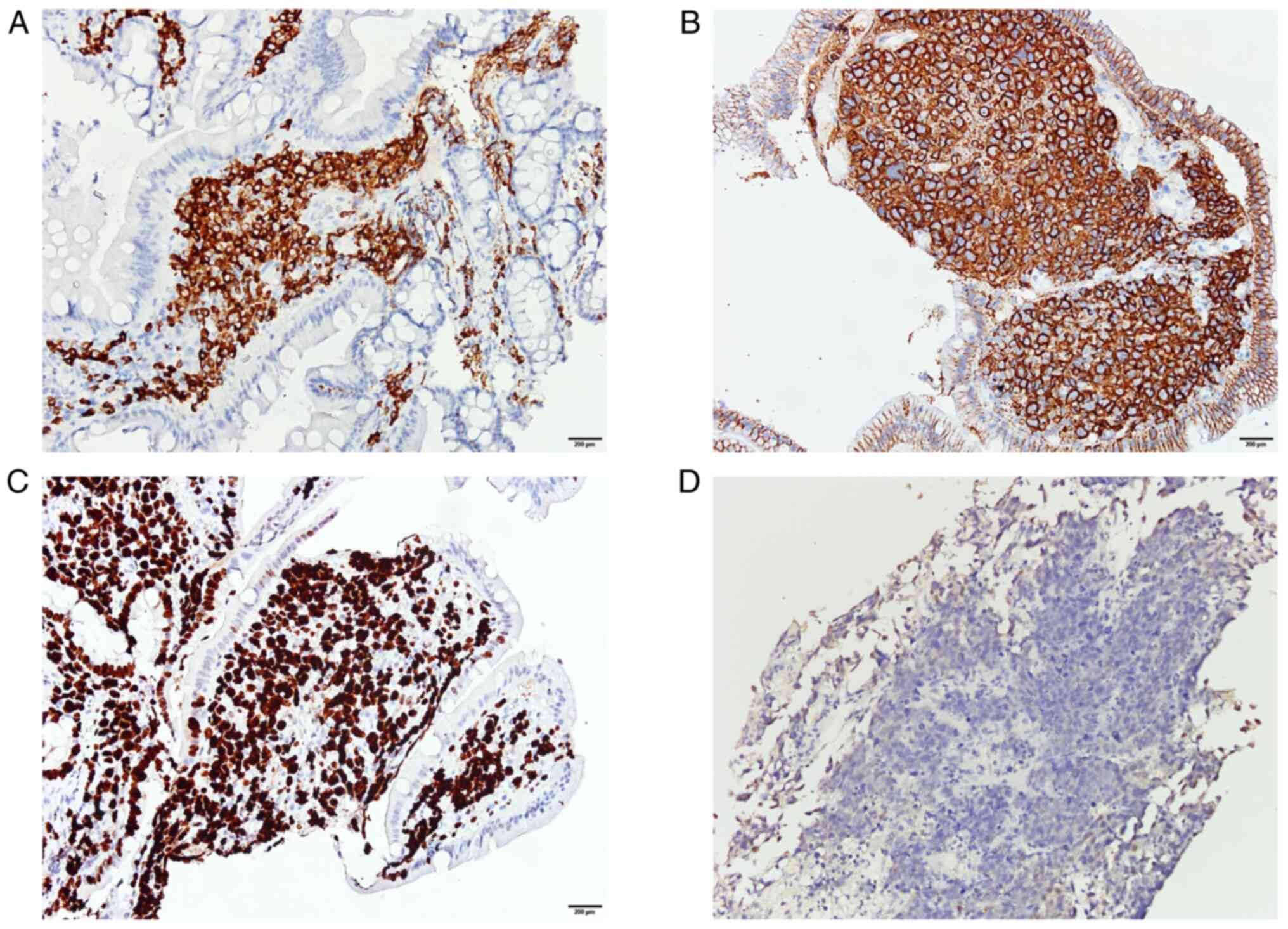
at room temperature. The duodenal biopsies were positive for CD79a
(Fig. 2A), CD138 (Fig. 2B) and CD45, and the renal biopsies
were positive for CD79a, CD138, EBV-encoded RNA (EBER; Fig. 2D), CD30 and epithelial membrane
antigen. The Ki-67 proliferation index was 99% for duodenal
biopsies (Fig. 2C), and both renal
and duodenal biopsies were negative for CD20. Ki-67 index is
calculated visually by counting the total number of
positive-stained tumor cells and dividing that by the total number
of tumor cells in each high-powered field (7). The immunohistochemical features were
comparable with those of PBL.
Bone marrow aspirate showed no increase in the
number of plasma cells, no clonality and normal cytogenetics.
Fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT was
immediately performed, and FDG uptake was observed in the right
submandibular regions, duodenum, bilateral perirenal space,
aortocaval space and peritoneum. Therefore, the patient was
diagnosed with recurrent PBL in the intra-abdominal lymph nodes,
bilateral kidneys and duodenum associated with a bleeding duodenal
ulcer. Systemic chemotherapy was initiated following the diagnosis
with intravenous (i.v.) administration of etoposide (80 mg/day on
days 1-4), vincristine (0.64 mg/day on days 1-4), doxorubicin
hydrochloride (16 mg/day on days 1-4), and cyclophosphamide (1,200
mg/day for 60 min on day 5). Chemotherapy dosages were based on the
guideline suggestions and adjusted according to the side effects of
the drugs experienced by each patient individually. Between
November 2020 and April 2021, the patient received six cycles of
etoposide, prednisone, vincristine, cyclophosphamide, and
doxorubicin hydrochloride (EPOCH) chemotherapy regimens. After six
cycles of chemotherapy, PET/CT was repeated and a regressive change
in previously noted FDG-uptake regions was observed.
Discussion
The present report described a case of an unusual
presentation of flank pain and tarry stool caused by recurrent PBL
in intra-abdominal lymph nodes, bilateral kidneys and duodenum. The
clinical manifestation of PBL varies with extranodal masses in the
head, neck and oral cavity the most common presentation (8). The median overall survival (OS) time
is ~8 months (9). Once considered
a malignancy that largely affected HIV-infected individuals,
Castillo et al (10)
analyzed 71 cases of HIV-negative PBL reported prior to August 2009
and revealed that these cases had distinct clinicopathological
features, such as older age, high Ki-67 expression and negative for
CD20. Moreover, Liu et al (11) indicated that EBV infection was
common, being positive in 58.70% of patients with HIV-negative PBL,
whereas 92.45% were negative for herpesvirus-8 (HHV-8). However,
Morscio et al (9) reported
that ~70% of HIV-positive PBL cases express EBER in malignant cells
and 72% of cases express HHV-8; EBER detection is a sensitive
technique for detecting EBV infection.
The patient in the current case report presented a
rare clinical profile of flank pain and tarry stool, rather than
head and neck masses. Additionally, the point-of-care ultrasound of
the bilateral kidneys and subsequent contrast-enhanced abdominal CT
indicated non-calculus hydronephrosis. Clinically, physicians
should keep urothelial cell carcinoma, transitional cell carcinoma,
retroperitoneal tumors and kidney cancers in mind when non-calculus
hydronephrosis is found by abdominal CT. Serum viral tests showed
positive for EBV-VCA IgG, but negative for EBV-VCA IgM and HIV
rapid point-of-care test. A systematic review of 76 patients with
HIV-negative PBL reported a median OS time of 9 months with a
2-year OS rate of 10% (12).
Castillo et al (10)
reported that patients with HIV-negative PBL had a poorer
chemotherapy response compared with those with HIV-positive PBL. It
was also reported that patients with HIV-negative PBL and Ki-67
expression >80% had a worse outcome, showing a poorer
chemotherapy response and worse OS rate. Saraceni et al
(13) reported a case of
HIV-negative PBL with complete remission for 4 years, but the
manifestations were in the usual sites of head and neck. In
addition, Brahmania et al (14) reported a case of HIV-negative and
EBER-positive PBL in the anorectal junction with complete remission
after adequate concurrent chemoradiation therapy; however, Ki-67
expression in the anorectal tumor cells was <80%, indicating a
better prognosis. In the present case, Ki-67 expression in duodenal
biopsies was 99%. Moreover, Cao et al (15) reported a case of HIV- and
EBER-negative duodenal PBL with Ki-67 expression of ~80%, wherein
chemotherapy regimens such as CHOP included i.v. treatment of
cyclophosphamide (750 mg/m2; day 1), doxorubicin
hydrochloride (50 mg/m2; day 1), vincristine (1.4
mg/m2; day 1) and oral prednisone (100 mg; days 1-50).
However, the patient's disease progressed, and it was concluded
that CHOP is not an optimal treatment regimen; thus, more intensive
regimens are required. Conversely, the patient in the present case
received six cycles of EPOCH chemotherapy regimens, and the repeat
PET/CT showed a regressive change in previously noted FDG-uptake
regions.
Notably, this unusual presentation of flank pain and
tarry stool caused by recurrent PBL highlighted that genitourinary
or gastrointestinal manifestations can occur in cases of PBL
recurrence, as well as head and neck manifestations. Additionally,
more intensive chemotherapy regimens, such as EPOCH instead of
CHOP, may be necessary for patients with HIV-negative PBL with
>80% Ki-67 expression, a diagnostic and therapeutically
challenging malignancy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YCL and YTS were major contributors in writing the
manuscript. CKH, YCT, YCC and PFL were involved in critically
revising the manuscript for important intellectual content. YCL and
PFL confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The institutional review board of the Tri-Service
General Hospital (Taipei, Taiwan) approved this study (IRB No.
B-202105167). Written informed consent was obtained from the
patient. All procedures were performed according to the World
Medical Association's Declaration of Helsinki.
Patient consent for publication
The patient provided written informed consent
regarding the publication of all case details and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Delecluse HJ, Anagnostopoulos I,
Dallenbach F, Hummel M, Marafioti T, Schneider U, Huhn D,
Schmidt-Westhausen A, Reichart PA, Gross U and Stein H:
Plasmablastic lymphomas of the oral cavity: A new entity associated
with the human immunodeficiency virus infection. Blood.
89:1413–1420. 1997.PubMed/NCBI
|
|
2
|
Rong C, Sheng L, Wu A, Sun Y and Ouyang G:
Allogeneic hematopoietic stem cell transplantation in a patient
with HIV-negative recurrent plasmablastic lymphoma: A case report.
Medicine. 100(e24498)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Elyamany G, Fouly A, Alqahtani A, Alrumeh
A, Asiri S, Faifi SA and Alshieban S: Cytological diagnosis of
plasmablastic lymphoma involving the parotid gland: A case report
with review of the literature. Case Rep Oncol. 14:244–248.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Castillo JJ, Bibas M and Miranda RN: The
biology and treatment of plasmablastic lymphoma. Blood.
125:2323–2330. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Al-Malki MM, Castillo JJ, Sloan JM and Re
A: Hematopoietic cell transplantation for plasmablastic lymphoma: A
review. Biol Blood Marrow Transplant. 20:1877–1884. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lopez A and Abrisqueta P: Plasmablastic
lymphoma: Current perspectives. Blood Lymphat Cancer. 8:63–70.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kinra P and Malik A: Ki 67: Are we
counting it right? Indian J Pathol Microbiol. 63:98–99.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dong HY, Scadden DT, de Leval L, Tang Z,
Isaacson PG and Harris NL: Plasmablastic lymphoma in HIV-positive
patients: An aggressive Epstein-Barr virus-associated
extramedullary plasmacytic neoplasm. Am J Surg Pathol.
29:1633–1641. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morscio J, Dierickx D, Nijs J, Verhoef G,
Bittoun E, Vanoeteren X, Wlodarska I, Sagaert X and Tousseyn T:
Clinicopathologic comparison of plasmablastic lymphoma in
HIV-positive, immunocompetent, and posttransplant patients:
Single-center series of 25 cases and meta-analysis of 277 reported
cases. Am J Surg Pathol. 38:875–886. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Castillo JJ, Winer ES, Stachurski D, Perez
K, Jabbour M, Milani C, Colvin G and Butera JN: Clinical and
pathological differences between human immunodeficiency
virus-positive and human immunodeficiency virus-negative patients
with plasmablastic lymphoma. Leuk Lymphoma. 51:2047–2053.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu M, Liu B, Liu B, Wang Q, Ding L, Xia C
and Dong L: Human immunodeficiency virus-negative plasmablastic
lymphoma: A comprehensive analysis of 114 cases. Oncol Rep.
33:1615–1620. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Castillo JJ, Winer ES, Stachurski D, Perez
K, Jabbour M, Milani C, Colvin GA and Butera JN: HIV-negative
plasmablastic lymphoma: Not in the mouth. Clin Lymphoma Myeloma
Leuk. 11:185–189. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saraceni C, Agostino N, Cornfield DB and
Gupta R: Plasmablastic lymphoma of the maxillary sinus in an
HIV-negative patient: A case report and literature review.
Springerplus. 2(142)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brahmania M, Sylwesterowic T and Leitch H:
Plasmablastic lymphoma in the ano-rectal junction presenting in an
immunocompetent man: A case report. J Med Case Rep.
5(168)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cao C, Liu T, Lou S, Liu W, Shen K and
Xiang B: Unusual presentation of duodenal plasmablastic lymphoma in
an immunocompetent patient: A case report and literature review.
Oncol Lett. 8:2539–2542. 2014.PubMed/NCBI View Article : Google Scholar
|