Introduction
Glioblastoma (GBM) is the most frequent intrinsic
brain tumor in adults (1). Despite
intense research, the prognosis is still fatal with a mean overall
survival (OS) of 12 to 16 months (2-4).
Maximal treatment includes gross total tumor resection followed by
concomitant radiochemotherapy and temozolomide (5,6).
Glioblastoma treatments are based on systemic therapeutic
approaches, which highlights the lack of specific targeted
therapies for GBM patients and the need for better molecular
understanding of the underlying mechanisms of glioma genesis and
progression. One of these mechanisms includes
epithelial-mesenchymal transition (EMT), a complex process allowing
cells with epithelial characteristics to gain mesenchymal
properties due to highly regulated changes in gene expression
(7). While epithelial-like cells
show a polarized subtype and are likely to engage in intracellular
adhesion, mesenchymal differentiated cells present with altered
polarization, higher migratory capacity and stem cell properties
(8-10).
This transition is valuable in processes such as embryogenesis and
wound healing, but it leads to increased tumor progression,
chemoresistance and mitotic progression (11). This process can occur in reverse
[i.e., mesenchymal-epithelial transition (MET)], and cells are able
to dynamically shift between transitional stages (12,13).
Cells with a more epithelial phenotype express higher levels of
E-cadherin coded by the cadherin-1 (CDH1) gene, a cellular membrane
glycoprotein that assists cell adhesion and membrane stability
(9,14). A typical mesenchymal marker, in
contrast, is N-cadherin, coded by CDH2, which is associated with
migratory capacity and loss of cell polarity (11). The decrease in E-cadherin
expression, which is linked to increased N-cadherin expression, is
a crucial indicator of EMT called the cadherin switch (11,13).
Previous studies have exhibited that EMT signaling
in various cancer cells (e.g., colorectal carcinoma and breast
cancer) leads to stem-like properties, induced autophagy,
aggressive behavior and metastatic progression (15-17).
As GBMs are of glial rather than epithelial origin, not every
aspect of EMT applies to these tumors. Nonetheless, evidence
suggests that glioma cells can change their morphological phenotype
and genetic signature from a more epithelial-like to a more
mesenchymal-like character (11);
the term ‘EMT-like process’ describes this mechanism. In GBM,
EMT-like behavior is associated with invasion, tumor progression
and therapy resistance (18). Iser
et al (11) discuss a link
between astrocyte-glioma interaction via EMT-inducing factors, and
other studies postulate that glioma cells gain stem cell properties
by transitioning to a mesenchymal state (18).
The transcription factor zinc finger E-box-binding
homeobox 1 (ZEB1) is an important inducer of EMT in several
malignancies, including GBM (19).
Despite heterogeneous reports on the regulatory function of EMT in
GBM, ZEB1 expression is associated with higher grades of
malignancy, tumor progression and invasion (20-22).
Regarding the expression of E-cadherin in human GBM, contradictory
data exists, with some authors describing overexpression in GBM and
others reporting low expression (23,24).
Camand et al (25) reported
lower N-cadherin expression levels in GBM compared to normal brain
tissue (NBT), whereas other studies have described overexpression
and correlation with higher grades of malignancy (26,27).
The present study was conducted to examine the
expression of E-cadherin and N-cadherin, as well as the central
EMT-induction factor ZEB1 and the marker of cell cycle upregulation
cyclin-dependent kinase 1 (CDK1) in human GBM compared to NBT.
Furthermore, the researchers investigated whether the expression of
those genes is related to progression-free survival (PFS) and
OS.
Materials and methods
Patient collective and tissue
specimens
Forty-four patients who underwent tumor resection
for a supratentorial GBM in the neurosurgical department of the
University Hospital of Giessen, Germany between 2006 and 2015 were
included. All patients were diagnosed with GBM, IDH-wild-type. The
Institute of Neuropathology of the University Hospital Giessen
(Germany) provided information on the O6
methylguanine-DNA-methyltransferase (MGMT) promotor methylation
status, as well as paraffin-embedded tissue sections of all
patients. Follow-up records including age at diagnosis, sex, date
of surgery, treatment protocols, magnetic resonance imaging (MRI)
data and time of death were available. For all patients,
intraoperatively obtained tissue from the 5-aminolevulinic acid
(5-ALA)-positive tumor area was stored in frozen nitrogen. As a
control, the researchers used five paraffin-embedded sections of
normal human brain tissue (NBT) provided to their institution by
the Institute of Neuropathology and four kryosample-derived brain
tissue, provided by the Institute of Pathology at the University of
Salzburg, Austria.
The study was conducted in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of the
University Hospital Giessen, Germany (AZ 07/09). Written informed
consent was obtained from all patients before enrollment in the
study.
Gene expression analysis by qPCR
Tissue was thawed and RNA from the tumor specimen
was isolated using the RNEasy kit (Qiagen GmbH) according to the
manufacturer's protocol. The NanoDrop ND-1000 spectrophotometer was
used to photometrically evaluate the RNA concentration and purity
(Thermo Fisher Scientific, Inc.), and samples with an extinction
ratio of E260/280 >2 were used for further processing.
Transcription to cDNA was performed using the QuantiTect reverse
transcription kit (Qiagen GmbH).
For quantitative real-time polymerase chain reaction
(qPCR), the TaqMan gene expression master mix and gene expression
assays were used according to the manufacturer's protocol. For
amplification, StepOne real-time PCR system (Applied
Biosystems/Thermo Fisher Scientific, Inc.) was used. Analysis was
conducted manually in triplicates, and dimensionless expression
values were calculated via the ΔCT method. The customized TaqMan
gene expression assays were HS00611018_m1 for ZEB1, HS00938778_m1
for CDK1, HS01023894-m1 for CDH1 (E-cadherin), HS00983056-m1 for
CDH2 (N-cadherin) and Hs99999903_Actβ for Actin-β (all from Thermo
Fisher Scientific, Inc.).
Immunostaining
All GBM specimens and five NBT sections were
deparaffined and, after preheating, immunohistochemistry (IHC) was
performed with the DCS SuperVision 2 kit (DCS Innovative
Diagnostik-Systeme), following the manufacturer's instructions. The
researchers used an anti-ZEB1 monoclonal mouse antibody (ab180905)
in a dilution of 1:500, an anti-CDK1 monoclonal rabbit antibody
(ab183550) in a dilution of 1:500, anti-N-cadherin monoclonal mouse
antibodies (ab98952) in a dilution of 1:2,000 and anti-E-cadherin
polyclonal rabbit antibodies (ab15148) in a dilution of 1:100 (all
from Abcam). Human colon sections were used as a positive control
for CDK1, human lung cancer tissue for ZEB1, human skin tissue for
E-cadherin and human heart-sections for N-cadherin.
Regarding quantification staining intensity, ZEB1,
CDK1 and E-cadherin were scored from 0 = no staining to 3 = intense
staining and multiplied by the ratio of stained to unstained cells.
For the cytoplasmic N-cadherin, only the staining intensity was
scored as above. For all specimens, a dimensionless
immunoreactivity score (IRS) defined the staining intensity.
Statistics
Statistical analysis was performed with SPSS
(version 24) (IBM Corp.). A t-test followed by ANOVA, a
Kruskal-Wallis test and a Mann-Whitney U test evaluated expression
analysis. For survival analysis, groups with gene expression above
and below the median gene expression were defined, and OS and PFS
were calculated with the Kaplan-Meier method and log-rank test. A
Pearson test analyzed correlation between the investigated genes.
Results with P<0.05 were defined as significant.
Results
Patient characteristics and
epidemiological data
The study included 44 patients with the diagnosis of
IDH-wildtype GBM. Of the patients, 68.18% (n=30) were male and
31.82% (n=14) were female. Mean age (SD) at diagnosis was 63.8
(±11.5) years. All patients were treated by gross total resection
of the tumor followed by concomitant radiochemotherapy and
temozolomide maintenance therapy according to the Stupp-protocol
(2). In 54.5% (n=24) of the
patients, the MGMT promotor was methylated, and in 45.5% (n=20) of
the patients, it was not methylated. Median progression-free
survival (PFS) in the patient collective was 6.56±) 8.47) months
and the median OS was 15.8 (±20.2) months (Table I).
 | Table IClinical characteristics and
epidemiological data for all 44 investigated patients with GBM. |
Table I
Clinical characteristics and
epidemiological data for all 44 investigated patients with GBM.
| Patient
characteristics (N=44) | |
|---|
| Mean age (SD) at
diagnosis (years) | |
|
Total | 63.8±11.5 |
|
Males | 64.3±12.4 |
|
Females | 62.9±9.5 |
| Sex n, (%) | |
|
Male | 30 (68.18) |
|
Female | 14 (31.82) |
| Molecular
characteristics, n (%) | |
|
IDH-1-wild-type | 44 |
|
MGMT-promotor
methylated | 24 (54.5) |
|
MGMT-promotor
not methylated | 20 (45.5) |
| Median PFS
(months) | |
|
Total | 6.56±8.47 |
|
MGMT-promotor
methylated | 9.17 |
|
MGMT-promotor
not methylated | 4.73 |
| Median OS
(months) | |
|
Total | 15.8±20.2 |
|
MGMT-promotor
methylated | 13.74 |
|
MGMT-promotor
not methylated | 9.17 |
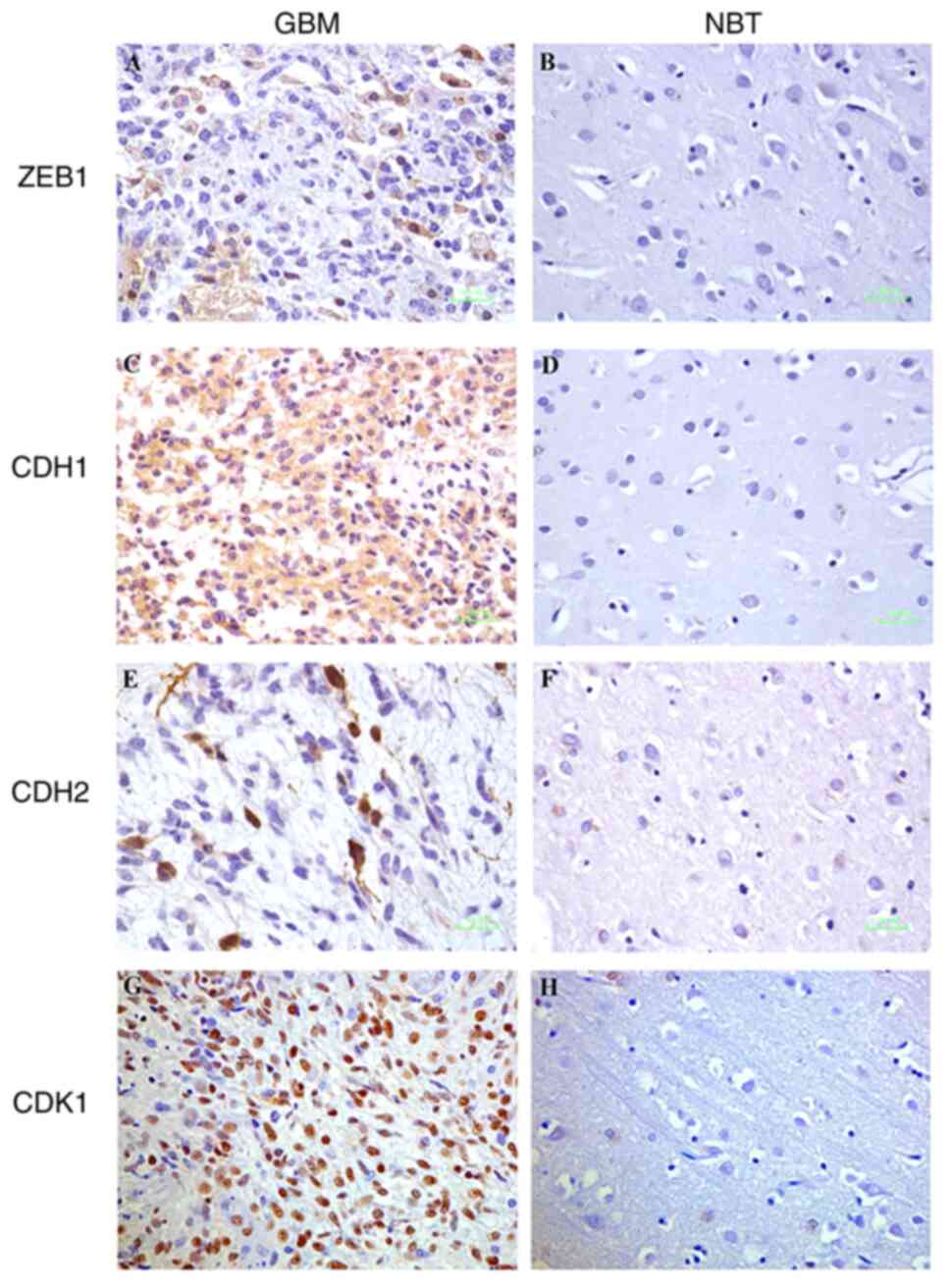
CDH1 (Fig. 1A),
CDH2 (Fig. 1C), CDK1 (Fig. 1E) and ZEB1 (Fig. 1G) were overexpressed in human GBM
compared to these levels in the NBT specimens (Fig. 1B, D, F and
H).
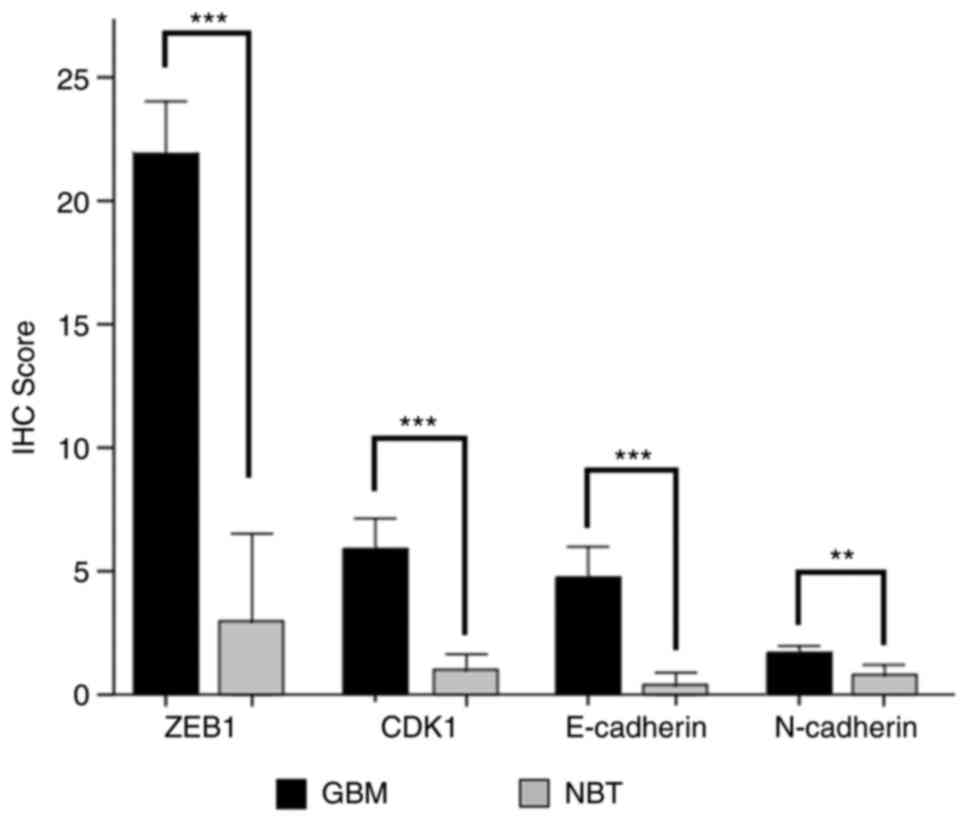
ZEB1, E-cadherin, N-cadherin and CDK1 had
significantly higher protein levels in all tested GBM specimens
compared to NBT specimens. ZEB1 had an IRS of 21.71 (±6.96) in GBM
vs. 3 (±3.94) in NBT specimens (P<0.001) (Fig. 2). The E-cadherin expression level
in GBM was at 4.95 (±4.23) and 0.4 (±0.55) in NBT specimens
(P<0.001). For N-cadherin, the IRS was 1.84 (±0.52) in GBM,
compared to 0.8 (±0.45) in the NBT specimens (P=0.001). CDK1 was
also overexpressed in human GBM with an IRS of 5.84 (±4.1) while
the IRS in the NBT specimens was 1 (±0.71; P<0.001 (Fig. 2).
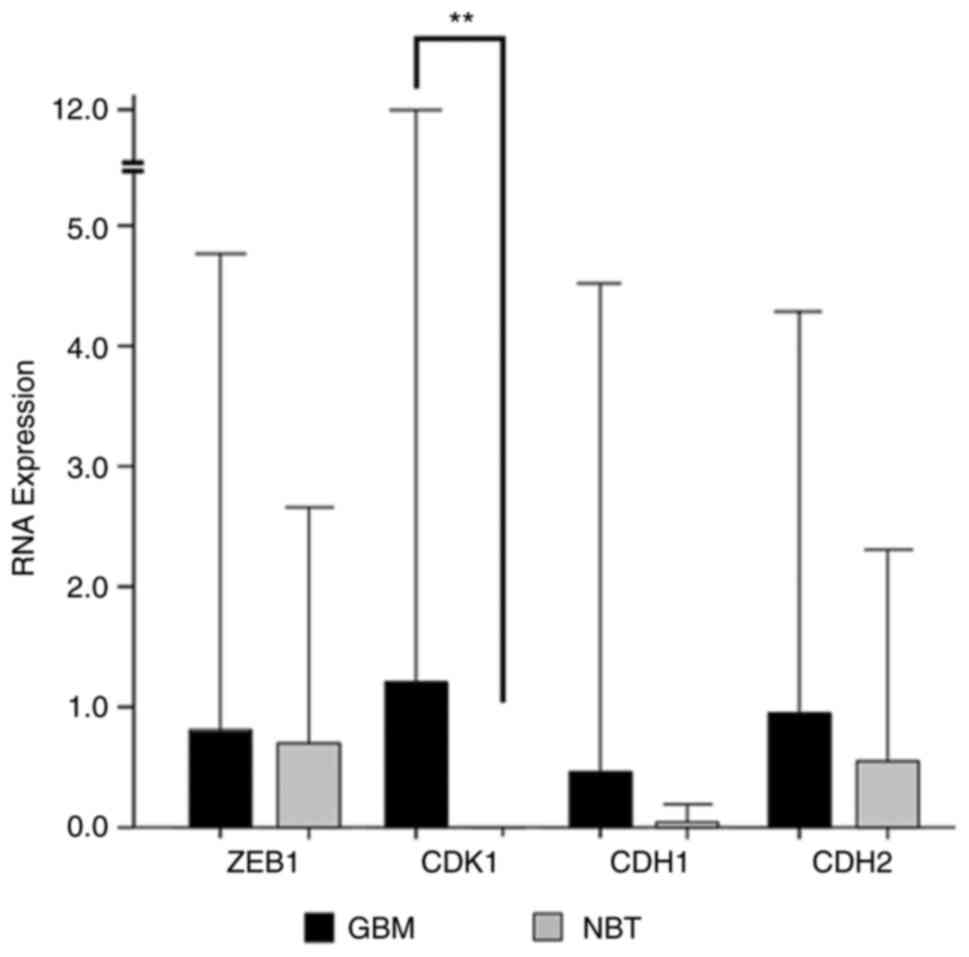
Regarding mRNA levels, similar results were
observed. The gene expression level of ZEB1 was 0.81 (±1.98)
in GBM vs. 0.7 (±0.98) in NBT specimens. The expression level of
CDH1 coding for E-cadherin was 0.46 (±2.03) in GBM and 0.03
(±0.05) in the NBT specimens, while CDH2 coding for
N-cadherin was expressed at a level of 0.95 (±1.67) in GBM vs. 0.55
(±0.87) in the NBT specimens. However, none of these results
reached statistical significance. Only the expression of
CDK1 was significantly higher in GBM tissue with an
expression value of 1.12 (±5.14) in GBM compared to 0.0014
(±0.0005) in the NBT specimens (P=0.001) (Fig. 3).
No difference was observed in the expression level
of CDH1, CDH2, CDK1 or ZEB1, nor in the corresponding proteins when
comparing MGMT methylated and non-methylated tumor specimens.
Survival analysis
For all investigated genes, PFS and OS were analyzed
for all patients and in relation to the MGMT-promotor methylation
status. Neither for the total collective of the investigated GBM
patients nor in the MGMT-positive or GBM-negative patients was a
significant difference noted in the OS or PFS for patients with
tumors with high expression levels of ZEB1, CDH1, CDH2 and
CDK1.
Nevertheless, there was a trend toward a longer PFS
and OS in patients with ZEB1 expression below the median compared
to patients with ZEB1 expression above the median. Patients with
higher CDH1 expression had a trend toward a longer PFS and OS
compared to patients with lower CDH1 expression, but the effect
only reached significance for PFS in the subgroup of
MGMT-notmethylated tumors with a mean PFS of 4.37 and MGMT-negative
tumors with CDH1 expression below the median compared to 5.56
months in MGMT-negative tumors with CDH1 expression above the
median (P=0.005). Regarding CDK1 expression, a slight trend toward
a shorter PFS and OS for patients with CDK1 expression above the
median was observed independently of MGMT-promotor methylation
status.
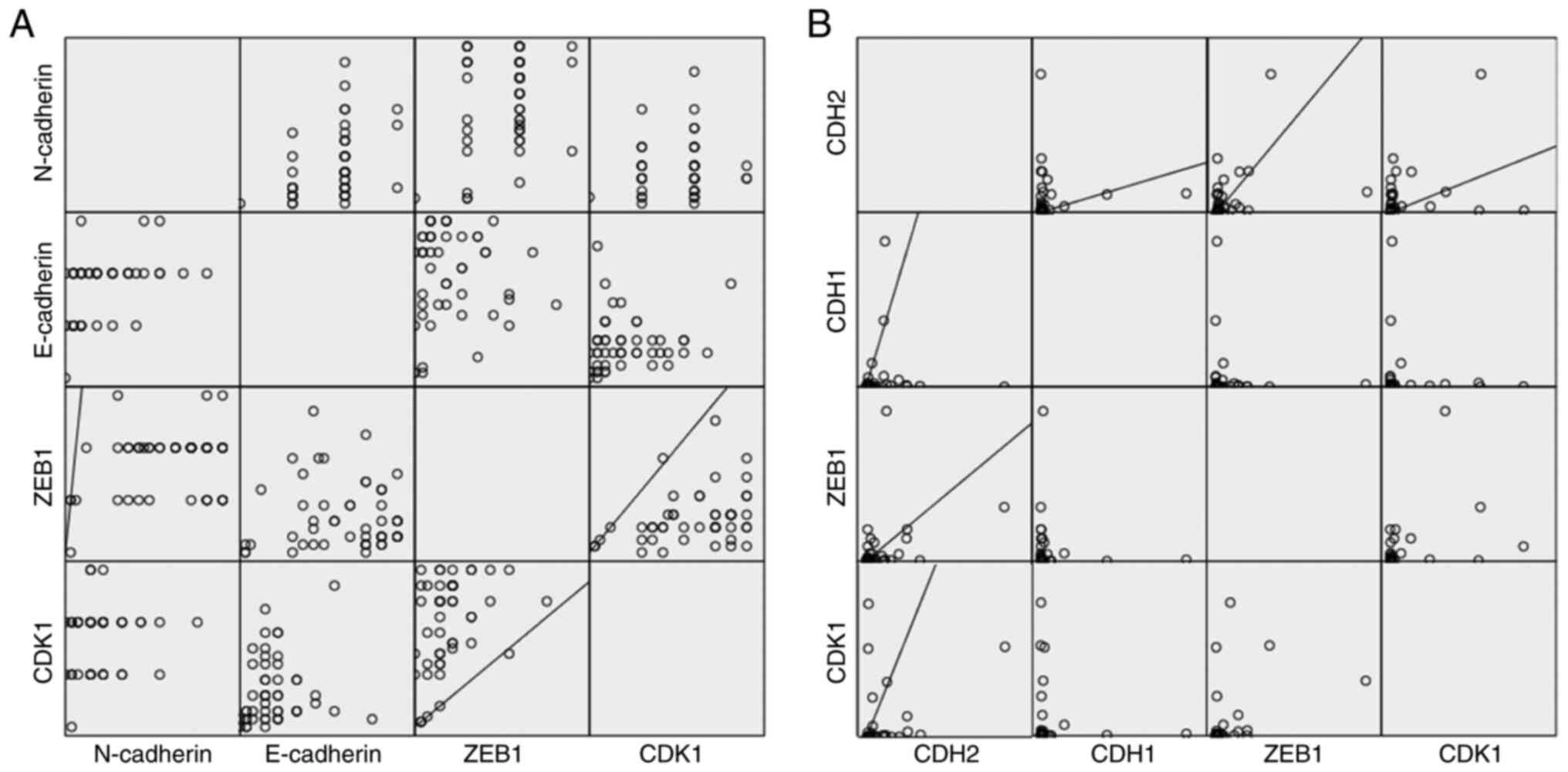
Correlation of expression levels of
the investigated genes and proteins
To investigate whether there is a correlation
between the expression levels of the proteins, Pearson's analysis
was performed. Overexpression of ZEB1 was associated with higher
expression of CDH2/N-cadherin at the mRNA level with r=0.347
(P=0.03) and at the protein level with r=0.349 (P=0.01), suggesting
a linear correlation. Similar high expression levels of ZEB1
corresponded to high CDK1 protein expression levels (r=0.363;
P=0.01), although this could not be confirmed at the mRNA level.
High CDK1 expression was associated with higher CDH2/N-cadherin
mRNA expression (r=0.336; P=0.04). Only for the mRNA level was high
CDH1 expression correlated with higher CDH2 expression (r=0.345;
P=0.015) (Fig. 4).
Discussion
The present study demonstrated that zinc finger
E-box-binding homeobox 1 (ZEB1), E-cadherin and N-cadherin were
overexpressed in human glioblastoma (GBM) compared to normal brain
tissue (NBT) specimens, and that their expression was independent
from the MGMT promotor methylation status. In the patient
collective, no differences in overall survival (OS) or
progression-free survival (PFS) related to the expression of ZEB1,
CDH1, CDH2 or CDK1 were observed. However, higher ZEB1 expression
was correlated with higher levels of CDH2/N-cadherin and CDK1,
suggesting a link between ZEB1 overexpression and a more
mesenchymal phenotype with aberrant cell cycle processing.
ZEB1 belongs to a family of transcription factors
characterized by two zinc finger clusters and a homeodomain that
enable the molecule to bind specific DNA sequences. Interacting
with several binding partners (i.e., co-transcription factors),
ZEB1 is able to upregulate and downregulate the transcription of
several genes. Via this mechanism, ZEB1 leads to downregulation of
cadherin-1 (CDH1) and upregulation of cadherin-2 (CDH2) (i.e.,
cadherin shift) a central hallmark of epithelial-mesenchymal
transition (EMT) in carcinoma cells (28).
In the literature, there is evidence that ZEB1 is
involved in EMT induction, tumor progression and therapy resistance
in GBM (18). Siebzehnrubl et
al report that ZEB1 knockdown in a GBM mouse model led to
downregulation of EMT signaling and increased chemosensitivity,
leading to improved survival (22). Additionally, irradiation was
reported to decrease ZEB1 expression and thereby EMT-related gene
expression, leading to better patient survival rates (29). Previous studies have shown that
ZEB1 promotes EMT by inducing the cadherin shift in human GBM and
other cancers (11,30-33).
In contrast to other malignancies, the typical hallmark of EMT
(i.e., the switch from higher E-cadherin/CDH1 expression in an
epithelial phenotype to higher N-cadherin/CDH2 expression in a
mesenchymal phenotype) is not necessarily observed in GBM (11). In this study's dataset, a linear
correlation between CDH1 and CDH2 expression was observed, although
ZEB1 expression was only correlated to CDH2 expression. Various
reports support this observation that the classical cadherin shift
does not apply to gliomas (10,12,25).
Due to the non-epithelial origin of glial tumors having a different
gene expression pattern, authors have proposed the term ‘EMT-like’
or ‘glial to mesenchymal transition’ (GMT) (11).
Many malignancies harbor mutations that lead to
aberrant cell cycle progression and thereby proliferation and tumor
progression (34).
Cyclin-dependent kinase 1 (CDK1) is a serine/threonine protein
kinase that regulates the entry in mitosis. It is more highly
expressed in cells with aberrant proliferation patterns and
overexpressed in human GBM (35).
Cancer cells with CDK1 overexpression tend to undergo faster tumor
progression and higher proliferation rates (35,36).
In alignment with the literature, the CDK1
expression at the protein level was significantly higher in GBM
than in NBT specimens in this study. The researchers also observed
a linear correlation between N-cadherin/CDH2, ZEB1 and CDK1
expression. These findings imply that higher N-cadherin expression,
which is characteristic for a mesenchymal phenotype, is also
associated with aberrant proliferation and progression.
In conclusion, ZEB1, E-cadherin, N-cadherin and CDK1
are overexpressed in human GBM compared to NBT. ZEB1 expression
levels correlate with N-cadherin and CDK1 expression levels. These
findings lead to the conclusion that ZEB1 is a relevant regulator
of EMT and cadherin shift in human GBM. This corresponds to CDK1
overexpression, which indicates aberrant cell cycle progression and
is associated with an aggressive tumor phenotype. ZEB1, as a
promotor of this cell signaling, is therefore a relevant target for
further research and specific therapeutic approaches for GBM.
Acknowledgements
Professor Daniel Neureither of the Department of
Pathology at the University Hospital Salzburg (Austria) kindly
provided the normal brain tumor specimens.
Funding
Funding: This research did not receive external funding.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author.
Authors' contributions
Conceptualization of the study was accomplished by
MAK. JN, ST and FPS were responsible for the methodology, software
and statistical analyses. Investigation and data curation were
accomplished by FH, HG, ST and EU. Writing and original draft
preparation was the responsibility of HG. Writing, review and
editing were conducted by MAK, EU and FPS. Supervision and project
administration were the responsibility of MAK. All authors have
read and agreed to the published version of the manuscript. HG and
MAK guarantee the authenticity of the raw data collected in the
study.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of
University Hospital Giessen (AZ 07/09).
Patient consent for publication
Patient consent for publication was obtained.
Competing interests
The authors declare that they have no competing or
conflicting interests. MAK, HG, JN, FPS, FH, EU and ST confirm
disclosing all financial and non-financial competing interests for
myself and on behalf of my co-authors.
References
|
1
|
Ostrom QT, Gittleman H, Liao P,
Vecchione-Koval T, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and other central nervous
system tumors diagnosed in the United States in 2010-2014. Neuro
Oncol. 19 (Suppl 5):v1–v88. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Delgado-López PD and Corrales-García EM:
Survival in glioblastoma: A review on the impact of treatment
modalities. Clin Transl Oncol. 18:1062–1071. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Weller M, van den Bent M, Tonn JC, Stupp
R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E,
Balana C, Chinot O, et al: European Association for Neuro-Oncology
(EANO) guideline on the diagnosis and treatment of adult astrocytic
and oligodendroglial gliomas. Lancet Oncol. 18:e315–e329.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stupp R and Roila F: ESMO Guidelines
Working Group. Malignant glioma: ESMO clinical recommendations for
diagnosis, treatment and follow-up. Ann Oncol. 20 (Suppl
4):S126–S128. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Pala A, Karpel-Massler G, Rainer C and
Eric M: Epithelial to Mesenchymal transition and progression of
glioblastoma. In: Clinical Management and Evolving Novel
Therapeutic Strategies for Patients with Brain Tumors. Lichtor T
(ed). InTech, ISBN 978-953-51-1058-3, 2013.
|
|
9
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Lee JK, Joo KM, Lee J, Yoon Y and Nam DH:
Targeting the epithelial to mesenchymal transition in glioblastoma:
The emerging role of MET signaling. Onco Targets Ther. 7:1933–1944.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Iser IC, Lenz G and Wink MR: EMT-like
process in glioblastomas and reactive astrocytes. Neurochem Int.
122:139–143. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Saitoh M: Involvement of partial EMT in
cancer progression. J Biochem. 164:257–264. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21(965)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pal A, Barrett TF, Paolini R, Parikh A and
Puram SV: Partial EMT in head and neck cancer biology: A spectrum
instead of a switch. Oncogene. 40:5049–5065. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kahlert UD, Joseph JV and Kruyt FAE: EMT-
and MET-related processes in nonepithelial tumors: Importance for
disease progression, prognosis, and therapeutic opportunities. Mol
Oncol. 11:860–877. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Edwards LA, Li A, Berel D, Madany M, Kim
NH, Liu M, Hymowitz M, Uy B, Jung R, Xu M, et al: ZEB1 regulates
glioma stemness through LIF repression. Sci Rep.
7(69)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Madany M, Thomas T and Edwards LA: The
curious case of ZEB1. Discoveries (Craiova). 6(e86)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Siebzehnrubl FA, Silver DJ, Tugertimur B,
Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT,
Kupper MD, Neal D, et al: The ZEB1 pathway links glioblastoma
initiation, invasion and chemoresistance. EMBO Mol Med.
5:1196–1212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schwechheimer K, Zhou L and Birchmeier W:
E-cadherin in human brain tumours: Loss of immunoreactivity in
malignant meningiomas. Virchows Arch. 432:163–167. 1998.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Howng SL, Wu CH, Cheng TS, Sy WD, Lin PC,
Wang C and Hong YR: Differential expression of Wnt genes,
beta-catenin and E-cadherin in human brain tumors. Cancer Lett.
183:95–101. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Camand E, Peglion F, Osmani N, Sanson M
and Etienne-Manneville S: N-cadherin expression level modulates
integrin-mediated polarity and strongly impacts on the speed and
directionality of glial cell migration. J Cell Sci. 125(Pt
4):844–857. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Utsuki S, Sato Y, Oka H, Tsuchiya B,
Suzuki S and Fujii K: Relationship between the expression of E-,
N-cadherins and beta-catenin and tumor grade in astrocytomas. J
Neurooncol. 57:187–192. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Asano K, Duntsch CD, Zhou Q, Weimar JD,
Bordelon D, Robertson JH and Pourmotabbed T: Correlation of
N-cadherin expression in high grade gliomas with tissue invasion. J
Neurooncol. 70:3–15. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tian Y, Xie Q, He J, Luo X, Zhou T, Liu Y,
Huang Z, Tian Y, Sun D and Yao K: Radioactive (125)I seeds inhibit
cell growth and epithelial-mesenchymal transition in human
glioblastoma multiforme via a ROS-mediated signaling pathway. BMC
Cancer. 15(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vandewalle C, van Roy F and Berx G: The
role of the ZEB family of transcription factors in development and
disease. Cell Mol Life Sci. 66:773–787. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aigner K, Dampier B, Descovich L, Mikula
M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist
P, et al: The transcription factor ZEB1 (deltaEF1) promotes tumour
cell dedifferentiation by repressing master regulators of
epithelial polarity. Oncogene. 26:6979–6988. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eger A, Aigner K, Sonderegger S, Dampier
B, Oehler S, Schreiber M, Berx G, Cano A, Beug H and Foisner R:
DeltaEF1 is a transcriptional repressor of E-cadherin and regulates
epithelial plasticity in breast cancer cells. Oncogene.
24:2375–2385. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Qi S, Song Y, Peng Y, Wang H, Long H, Yu
X, Li Z, Fang L, Wu A, Luo W, et al: ZEB2 mediates multiple
pathways regulating cell proliferation, migration, invasion, and
apoptosis in glioma. PLoS One. 7(e38842)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Otto T and Sicinski P: Cell cycle proteins
as promising targets in cancer therapy. Nat Rev Cancer. 17:93–115.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen H, Huang Q, Zhai DZ, Dong J, Wang AD
and Lan Q: CDK1 expression and effects of CDK1 silencing on the
malignant phenotype of glioma cells. Zhonghua Zhong Liu Za Zhi.
29:484–488. 2007.PubMed/NCBI(In Chinese).
|
|
36
|
André S, Kojima S, Yamazaki N, Fink C,
Kaltner H, Kayser K and Gabius HJ: Galectins-1 and -3 and their
ligands in tumor biology. Non-uniform properties in cell-surface
presentation and modulation of adhesion to matrix glycoproteins for
various tumor cell lines, in biodistribution of free and
liposome-bound galectins and in their expression by breast and
colorectal carcinomas with/without metastatic propensity. J Cancer
Res Clin Oncol. 125:461–474. 1999.PubMed/NCBI View Article : Google Scholar
|