Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most aggressive malignancies and the fourth leading cause of
cancer-related mortality (1).
Despite advances in diagnostic and therapeutic strategies over the
past two decades, the prognosis for patients with PDAC remains poor
and the 5-year survival rate is ~6% (2,3).
PDAC usually causes no detectable symptoms in early stages and the
majority of patients are diagnosed in advanced stages with poor
survival rates (4). The difficulty
in identifying patients at high risk of metastasis and/or
recurrence highlights the challenge of the unsatisfactory prognosis
of patients with PDAC.
Computed tomography (CT) and magnetic resonance
imaging (MRI) are currently the predominant imaging modalities for
the preoperative diagnosis of patients with PDAC. These imaging
techniques can classify pancreatic cancer into resectable,
borderline resectable, locally advanced and metastatic (5). This radiological classification is
based on local vascular invasion and the presence of parenchymatous
and peritoneal metastases and allows patients to be classified for
either upfront surgery or oncologic treatment (6,7).
Surgical treatment of resectable pancreatic tumor remains the
optimum treatment option for this cancer (8,9).
Therefore, careful and appropriate preoperative staging is
important in patients with pancreatic cancer.
As PDAC is a group of heterogeneous diseases with
different biological and clinical characteristics, the
identification of prognostic and predictive markers is clinically
relevant for improved management of the disease. Some gene and
protein candidates have been reported to be associated with the
disease progression of PDAC (10,11).
These biomarkers could also be useful to clarify molecular PDAC
subtypes that appear anatomically similar to aiding clinical
management (7,12).
In particular, serum tumor markers are non-invasive
additional tools that are increasingly used in various cancers
including PDAC, to advance understanding of cancer pathophysiology,
improve molecular stratification and thus achieve improved
outcomes. Carcinoembryonic antigen (CEA) and carbohydrate antigen
19-9 (CA19-9) are tumor markers associated with both diagnosis and
prognosis of patients with PDAC (13-16).
CEA, a glycoprotein with a molecular weight of 180-200 kDa, was
first isolated from fetal colon and colorectal cancer tissue in
1965(17). CEA is overexpressed in
several cancers, including colon, breast, lung and thyroid cancers
(18-20).
Moreover, serum CEA level is elevated in 30-60% of patients with
PDAC (21,22) and it is even suggested to be an
independent predictor of poor survival rates in patients with PDAC
(23).
CA 19-9 is a tumor-associated antigen with a
half-life of 4-8 days, whose epitope has been shown to be the
sialylated Lewis antigen (24).
CA19-9 is the only biomarker currently recommended in the National
Comprehensive Cancer Network guidelines for clinical use in PDAC
(25). However, when analyzing
serum CA19-9 levels, in a clinical setting, several constraints
should be considered. This is because CA19-9 may not have
sufficient sensitivity and specificity in patients with certain
metastases or obstructive jaundice (26). Moreover, sialylated Lewis
antigen-negative individuals, who comprise ~5-10% of the
population, have low or no CA19-9 secretion (27). Although CA19-9 is not accurate
enough to be used for screening asymptomatic individuals for PDAC,
it is currently the most useful blood test for distinguishing
pancreatic cancer from chronic or recurrent pancreatitis, with a
sensitivity of 70-90% and a specificity of 68-91% (28,29).
It is also one of the most important prognostic factors for both
patients with resectable disease and those with unresectable
disease (30,31). In patients with PDAC, high
expression of CA19-9 was associated with positive nodal status, an
advanced disease and low survival rates (32). Measurement of CA19-9 as a
prognostic factor provides valuable information to support
therapeutic decision making, as patients with high preoperative
CA19-9 levels are expected to have early recurrence. An elevated
tumor marker value also implies a high probability of residual
disease after resection (33).
Preoperative serum levels of CEA and CA19-9 can be helpful to
identify a subgroup of patients with poor outcomes after surgery
(34). Currently, there are no
biomarkers that can be discovered in the time interval between
carcinogenesis and invasion, when the disease is potentially
curable, due to low sensitivity and specificity in early detectable
small tumors (35). CEA and CA19-9
still are the most studied biological markers for establishing both
diagnosis and prognosis in PDAC (16,36,37).
CEA and CA19-9 as biological markers are independent predictive
factors for the presence of advanced PDAC with a positive
predictive value of 91.0% for the combination of CEA (>7.0
ng/ml) and CA 19-9 (>305 U/ml) in predicting the presence of
advanced disease (37). The aim of
this study was to investigate the serum levels of CEA and CA19-9
and their associations with clinicopathological variables and
survival outcomes in Libyan patients with PDAC.
Patients and methods
Clinicopathological data
The study group consisted of 123 patients with PDAC
diagnosed between 2010 and 2018 at the National Cancer Institute in
Misurata, Libya. Biological markers included serum levels of CEA
and CA19-9, were determined in all patients before any treatment.
The present study was conducted under research ethics approval by
ethical committee at the National Cancer Institute, Misurata
(Ethical Approval Number: EAN 6/2021). Written informed consent was
obtained from all patients for surgical treatment, pathologic
examinations and investigations performed according to the
institutional guidelines of the National Cancer Institute,
Misurata, Libya. Complete demographic and clinicopathological data
included age, gender, family history, tumor location and size,
lymph node status, stage, histological grade, type of treatment and
follow-up data. These clinicopathological data were obtained from
the patients' records and are summarized in Table I. The mean age of the patients was
61.2 years (range, 19-90 years). Tumor staging of pancreatic
adenocarcinoma was evaluated according to the TNM classification
(38). Radiological staging by
Computed Tomography (CT) and/or Magnetic Resonance Imaging (MRI)
was performed in all patients to assess tumor resectability. The
extent of the tumor (local and distant) at the time of diagnosis
was confirmed by imaging [CT, MRI, or Positron Emission Tomography
(PET)]. Lymph node status was also assessed radiologically and
positivity for malignancy was confirmed by histopathology of
surgically resected nodes.
 | Table IClinicopathological variables of 123
patients with pancreatic ductal adenocarcinoma. |
Table I
Clinicopathological variables of 123
patients with pancreatic ductal adenocarcinoma.
| Variables | Number of patients
(n=123) | Percent (%) |
|---|
| Age (years) | | |
|
<50 | 24 | 19.5 |
|
≥50 | 99 | 80.5 |
| Sex | | |
|
Male | 73 | 59.3 |
|
Female | 50 | 40.7 |
| Family history | | |
|
Positive | 6 | 4.9 |
|
Negative | 117 | 95.1 |
| Tumor site | | |
|
Head | 96 | 78.0 |
|
Tail | 15 | 12.2 |
|
Body | 12 | 9.8 |
| Surgical
resectability | | |
|
Resectable | 24 | 19.5 |
|
Unresectable | 99 | 80.5 |
| Tumor size | | |
|
T1 | 4 | 3.3 |
|
T2 | 13 | 10.6 |
|
T3 | 14 | 11.4 |
|
T4 | 1 | 0.8 |
|
Cannot be
assessed | 91 | 74.4 |
| Lymph node
status | | |
|
Negative | 12 | 9.8 |
|
Positive | 14 | 11.4 |
|
Cannot be
assessed | 97 | 78.9 |
| M | | |
|
M0 | 27 | 22.0 |
|
M1 | 96 | 78.0 |
| Histology
grade | | |
|
G1 | 6 | 4.9 |
|
G2 | 30 | 24.4 |
|
G3 | 87 | 70.7 |
| Stage | | |
|
Stage 1 | 12 | 9.8 |
|
Stage 2 | 12 | 9.8 |
|
Stage 3 | 3 | 2.4 |
|
Stage 4 | 96 | 78.0 |
| Systemic
treatment | | |
|
Adjuvant
chemotherapy | 20 | 16.3 |
|
Palliative
chemotherapy | 81 | 65.9 |
|
No
treatment | 22 | 17.9 |
|
(supportive
therapy) | | |
The overall time to recurrence was estimated as the
interval between the date of surgery and the date of recurrence
(local and/or distant). Disease recurrence (local and distant) was
confirmed by imaging (CT, MRI, or PET) performed when clinical
symptoms suggestive of disease recurrence were present.
Disease-free survival was defined as the time between initial
treatment and last follow-up that patients survive without disease
recurrence.
Treatment and follow-up
Approximately 21 patients were treated by
pancreaticoduodenectomy (Whipple procedure), while palliative
surgery was performed in three patients and no surgery was
performed in 99 patients who had metastases at the time of
diagnosis. However, fine needle aspiration biopsy with image
guidance and endoscopic retrograde cholangiopancreatography were
performed in these patients for histopathological diagnosis. In the
National Cancer Institute in Misurata the following guidelines were
established: Adjuvant combined chemotherapy (gemcitabine and
oxaliplatin) was given to 18 patients while 2 patients received
combined chemotherapy based on FOLFIRINOX (folinic acid,
fluorouracil, irinotecan and oxaliplatin) and 81 patients received
palliative chemotherapy with capecitabine and/or gemcitabine. In
addition, 22 patients were not eligible for chemotherapy, so these
patients did not receive chemotherapy. Patients were followed-up
until death or the end of the observation period (until October
2018). The median duration of follow-up was 6 months (range 1-41
months). At the end of the follow-up period, 91 patients (74%) had
succumbed to pancreatic cancer.
CEA and CA19-9 measurement
Prior to each treatment, approximately 5 ml of
peripheral fasting blood was drawn from the forearm veins. The
blood was immediately taken to the central laboratory of the
National Cancer Institute in Misurata and then routinely
centrifuged for 10 min at speed of 1,792 x g at 20-22˚C
temperature. The serum samples were first stored at 4˚C. Then they
were placed in polypropylene vials and stored at -80˚C. The
concentrations of CEA and CA19-9 in serum were determined using an
electrochemiluminescence immunoassay (double antibody sandwich
ELISA, cat. nos. TM E-4131 and TM E-4531 respectively; Labor
Diagnostika Nord GmbH & CoKG) on a Roche cobas e 602 modules
(Roche Diagnostics). This technology uses a sandwich
chemiluminescence immunoassay; Chemibeads contain a
chemiluminescent dye and Sensibeads contain a photosensitizer dye.
Biotinylated antibodies (1:100) and Chemibeads form sandwiches and
immune complexes are formed by further addition of Sensibeads. A
chemiluminescence reaction is initiated at 680 nm and finally the
signal is detected at 612 nm (according to the manufacturer's
instructions). The accuracy of internal and external quality
controls was determined according to the guidelines of RiliBAeK
(17). The detection limit and
blank limit were as follows: CEA: 0.2 and 0.12 ng/ml, CA19-9: 2.0
and 1.0 U/ml, CA15-3: 1.0 and 0.3 U/ml, respectively. Roche's
original ancillary reagents were used, including
streptavidin-coated magnetic beads, anti-CEA monoclonal antibody
and biotinylated anti-CA19-9 and anti-CEA monoclonal antibody and
Ru-labelled anti-CA19-9.
Statistical analysis
Continuous variables were calculated using SPSS 26.0
for Windows (IBM Corp.). Frequency tables were analyzed using the
χ2 or Fisher's exact tests to evaluate the power of
association between categorical variables. Kaplan-Meier curves were
constructed for survival rate analysis and differences between
curves were analyzed using the log-rank test. Multivariate survival
analysis for the outcome [overall survival and disease-free
survival (DFS)] was performed using the proportional hazard Cox
model in a backward stepwise manner with the log-likelihood ratio
(L-R) significance test, using standard values for the entry and
exclusion criteria. The cut-off point for CEA of 5 ng/ml and for
CA19-9 of 400 U/ml was used to distinguish between high-expression
and low-expression tumors as it provided the best results for
prognosis prediction in this and other studies (39,40).
The assumption of proportional hazards was controlled by
log-minus-log (LML) survival plots. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient demographic and clinicopathologic
variables. The demographic and clinicopathologic variables are
shown in Table I. The mean age of
the patients was 61.2 years (range, 19-90 years) and the majority
of patients (80.5%) were >50 years old. Regarding sex
distribution, PDAC was commoner in men (59.3%). A total of 4.9% of
patients had a family history of pancreatic cancer and in 96
patients the tumors were located in the head of the pancreas (87%).
The majority of patients (80.5%) had distant metastases or locally
advanced unresectable disease. The most common T stage was Tx
(74.4%), followed by T3, T2, T1 and T4 in decreasing frequency
(11.4, 10.6 and 83.3 and 0.8%, respectively). A total of 14
patients (11.4%) had positive lymph nodes and negative lymph nodes
were detected in 12 patients, while lymph node status could not be
assessed in the majority of patients (78.9%). Most patients had
high-grade tumors (70.7%). According to the AJCC staging system, 99
patients were at stage IV (78%), 3 patients were at stage III, 12
patients were at stage II and 12 patients were at stage I (38). Regarding treatment, 24 patients
were treated by radical surgery, while palliative surgery was
performed in three patients and no surgical intervention was
performed in 99 patients. Adjuvant chemotherapy was performed in 20
patients, 81 patients received palliative chemotherapy and 22
patients were not eligible for chemotherapy.
General description of CEA and CA19-9
expression profiles
CEA and CA19-9 expressions at cut point of (5 ng/ml
and 400 U/ml, respectively) are shown in Table II. The median CEA expression was 8
ng/ml (mean 22.8; range 1.1-377 ng/ml). CEA expression was low in
46 samples (<5 ng/ml) and high in 77 samples (≥5 ng/ml). The
median value of CA19-9 was 389 U/ml (mean 762.4; range 1-10050
U/ml). CA19-9 was low in 64 samples (<400 U/ml) and high in 59
samples (≥400 U/ml). CEA expression was more frequent in tumors
with high CA19-9 than in patients with low CA19-9
(P<0.0001).
 | Table IICEA and CA19-9 expression in Libyan
pancreatic ductal adenocarcinoma. CEA level at cut point of 5 ng/ml
and CA 19.9 level at cut point of 400 U/ml. |
Table II
CEA and CA19-9 expression in Libyan
pancreatic ductal adenocarcinoma. CEA level at cut point of 5 ng/ml
and CA 19.9 level at cut point of 400 U/ml.
| Biological
variables | Number of patients
(n=123) | Percent (%) |
|---|
| CEA level
(ng/ml) | | |
|
<5 | 46 | 37.4 |
|
≥5 | 77 | 62.6 |
| CA 19-9 level
(U/ml) | | |
|
<400 | 64 | 52.0 |
|
≥400 | 59 | 48.0 |
Correlation of CEA expression with
clinicopathological variables
The significant correlations between CEA expression
(<5 ng/ml vs. ≥5 ng/ml) and clinicopathological variables are
shown in Table III. High CEA
expression was significantly associated with surgically
unresectable tumor (P<0.020), unevaluable lymph node status
(P<0.007), advanced stages (P<0.0001) and distant metastases
(P<0.002). However, age at diagnosis, gender, family history,
tumor site, tumor size and histological grade showed no significant
relationship with CEA expression.
 | Table IIIThe association between CEA and
CA19-9 expressions with clinicopathological variables in pancreatic
adenocarcinoma cancer (n=123). Comparison between low CEA and
CA19-9 expression group and high expression group. |
Table III
The association between CEA and
CA19-9 expressions with clinicopathological variables in pancreatic
adenocarcinoma cancer (n=123). Comparison between low CEA and
CA19-9 expression group and high expression group.
| | CEA expression
(%) | | CA19-9 expression
(%) | |
|---|
| Clinicopathological
variable | Number | <5 ng/ml | ≥5 ng/ml | P-value | | | P-value |
|---|
| Age /years | | | | 0.91 | | | 0.25 |
|
<50 | 24 | 37.5 | 62.5 | | 62.5 | 37.5 | |
|
≥50 | 99 | 37.4 | 62.6 | | 49.5 | 50.5 | |
| Sex | | | | 0.38 | | | 0.91 |
|
Male | 73 | 34.2 | 65.8 | | 52.1 | 47.9 | |
|
Female | 50 | 42.0 | 58.0 | | 52.0 | 48.0 | |
| Family history | | | | 0.07 | | | 0.08 |
|
Positive | 6 | 16.7 | 83.3 | | 33.3 | 66.7 | |
|
Negative | 117 | 38.5 | 61.5 | | 53.0 | 47.0 | |
| Site | | | | 0.96 | | | 0.37 |
|
Head | 96 | 37.5 | 62.5 | | 54.2 | 45.8 | |
|
Elsewhere | 27 | 37.0 | 63.0 | | 44.4 | 55.6 | |
| Surgical
resectability | | | | 0.020 | | | 0.020 |
|
Resectable | 24 | 58.3 | 41.7 | | 75.0 | 25.0 | |
|
Unresectable | 99 | 32.3 | 67.7 | | 46.5 | 53.5 | |
| T stage | | | | 0.092 | | | 0.091 |
|
T1 | 4 | 50.0 | 50.0 | | 50.0 | 50.0 | |
|
T2 | 13 | 69.2 | 30.8 | | 76.9 | 23.1 | |
|
T3 | 14 | 42.9 | 57.1 | | 71.4 | 28.6 | |
|
T4 | 1 | 0.00 | 100 | | 0.00 | 100 | |
|
Tx | 91 | 31.9 | 68.1 | | 46.2 | 53.8 | |
| Lymph node
status | | | | 0.007 | | | 0.001 |
|
Positive | 12 | 50.0 | 50.0 | | 85.7 | 14.3 | |
|
Negative | 14 | 83.3 | 16.7 | | 66.7 | 33.3 | |
|
Nx | 97 | 29.9 | 70.1 | | 45.4 | 54.6 | |
| Histological
grade | | | | 0.26 | | | 0.62 |
|
G1 | 6 | 33.3 | 66.7 | | 50.0 | 50.0 | |
|
G2 | 30 | 50.0 | 50.0 | | 60.0 | 40.0 | |
|
G3 | 87 | 33.3 | 66.7 | | 49.4 | 50.6 | |
| Stage | | | |
<0.0001 | | |
<0.0001 |
|
Early
stage | 24 | 70.8 | 29.2 | | 83.3 | 16.7 | |
|
Late
stage | 99 | 29.3 | 70.7 | | 44.4 | 55.6 | |
| Metastasis | | | | 0.002 | | | 0.002 |
|
M0 | 27 | 63.0 | 37.0 | | 77.8 | 22.2 | |
|
M1 | 96 | 30.2 | 69.8 | | 44.8 | 55.2 | |
Correlation of CA19-9 expression with
clinicopathological variables
The correlations between CA19-9 expression at the
cut point of 400 U/ml and clinicopathological variables are shown
in Table III. High CA19-9
expression was more common in patients with surgically unresectable
tumor (P<0.02), unevaluable lymph node status (P<0.001),
advanced stage (P<0.0001) and distant metastases (P<0.002).
However, age at diagnosis, gender, family history, tumor site,
tumor size and histological grade showed no significant association
with CA19-9 expression.
Correlation of serum CEA and CA 19-9
expression patterns with patient survival outcomes
Univariate survival analyzes (survival rates) with
CEA expression at a cut-off point of 5 ng/ml and CA19-9 at a
cut-off point of 400 U/ml are shown in Table IV. The survival rate was 28.6% in
patients with low CEA expression and 24.7% in patients with high
expression profile (P<0.016). The low CA 19-9 expression group
had an improved survival rate than the high expression group (31.3
and 20.3%, respectively) (P<0.014).
 | Table IVUnivariate survival according to
analysis of CEA expression (cut point of 5 ng/ml) and CA19-9 (cut
point of 400 U/m) in Libyan pancreatic ductal adenocarcinoma
(n=123). |
Table IV
Univariate survival according to
analysis of CEA expression (cut point of 5 ng/ml) and CA19-9 (cut
point of 400 U/m) in Libyan pancreatic ductal adenocarcinoma
(n=123).
| | Survival
analysis | |
|---|
| Variables | Threshold | No of patients | Median survival
(months) | Mean survival
(months) | Survival rate
(percent) | P-value |
|---|
| All patients | | 123 | 6.00 | 8.55 | 26.0 | |
| CEA level | | | | | | 0.016 |
| | <5 | 46 | 8.00 | 11.02 | 28.6 | |
| | ≥5 | 77 | 5.00 | 7.27 | 24.7 | |
| CA19-9 level | | | | | | |
| | <400 | 64 | 7.00 | 9.89 | 31.3 | 0.014 |
| | ≥400 | 59 | 5.00 | 7.10 | 20.3 | |
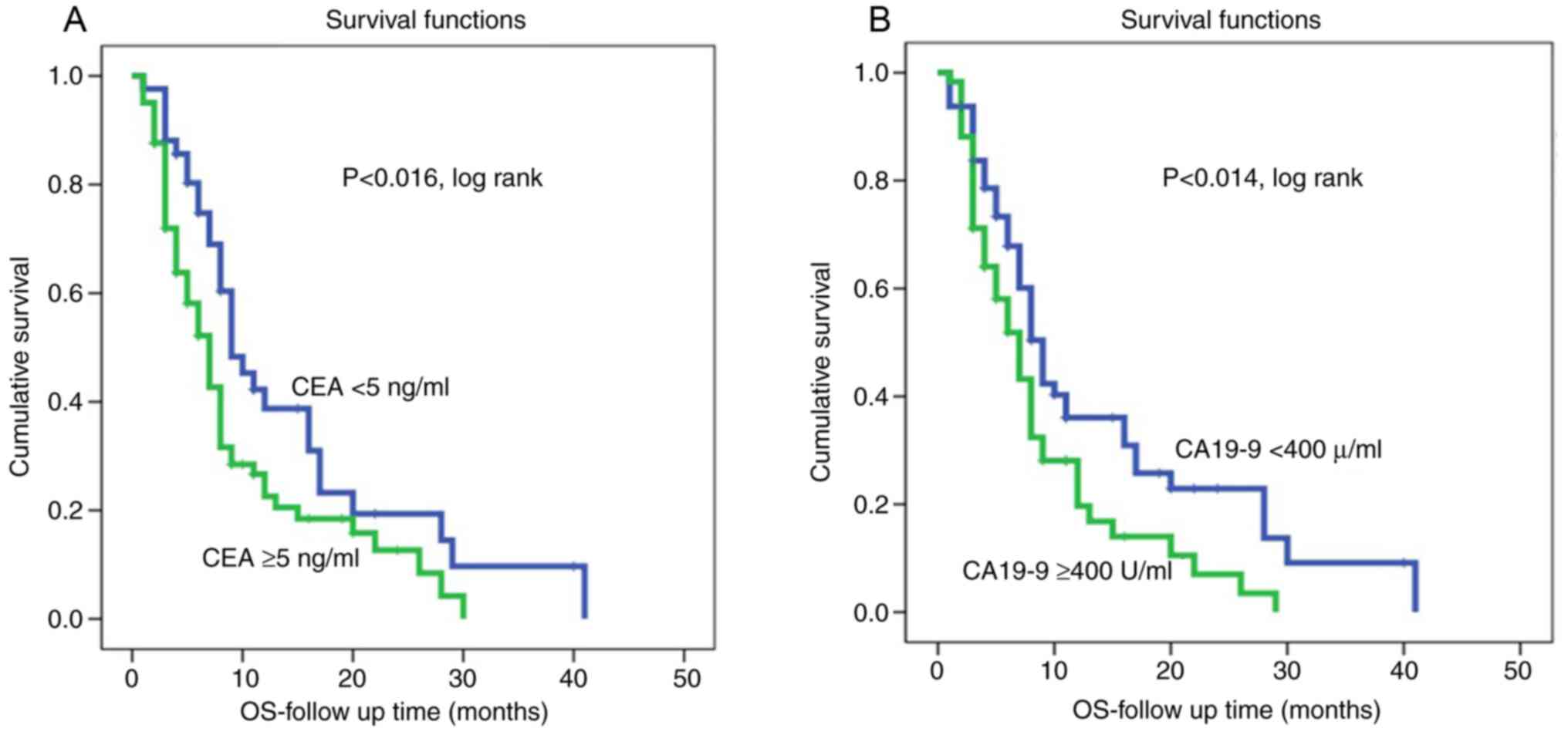
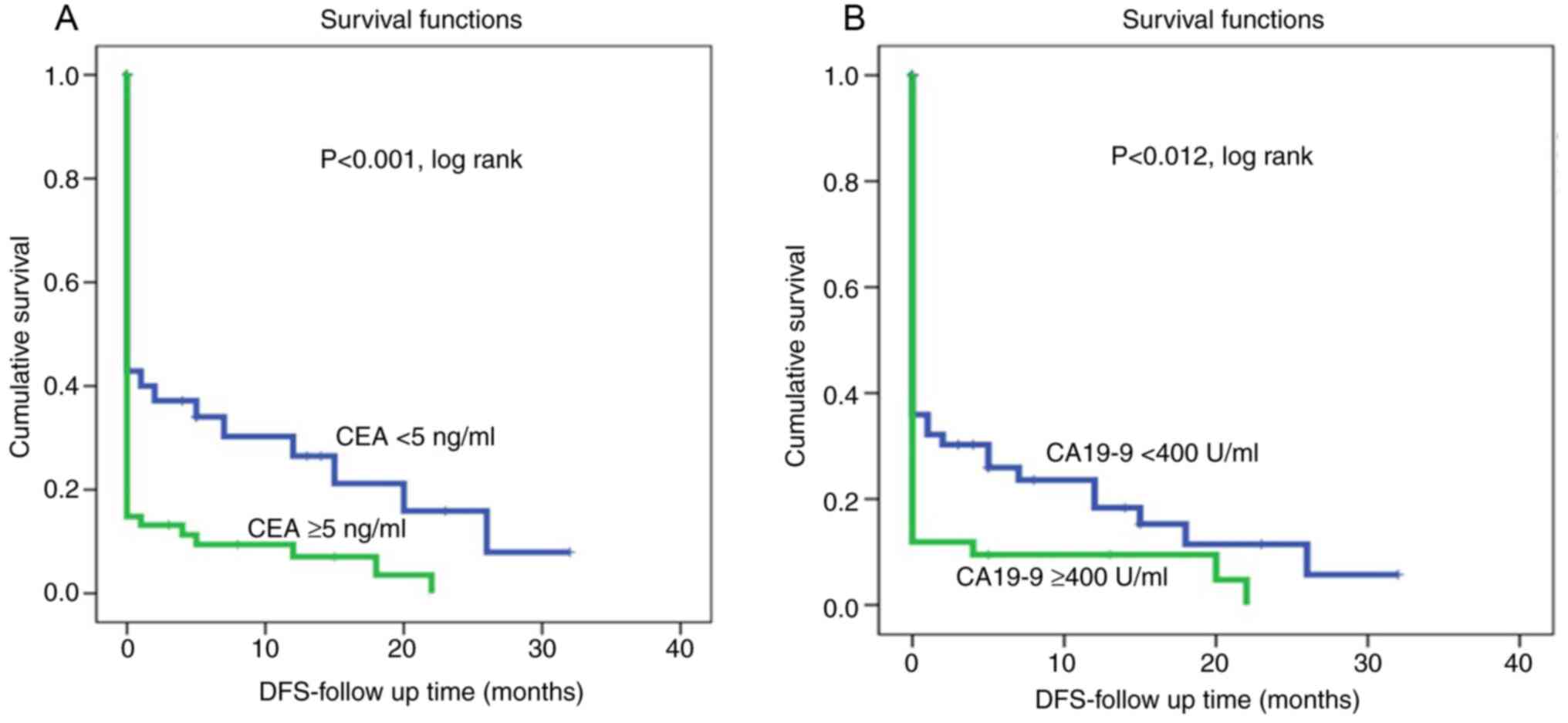
Kaplan-Meier survival curves for both CEA and CA19-9
levels showed that shorter survival was associated with high CEA
and high CA19-9 levels (Fig. 1).
On the other hand, patients with low CEA and CA19-9 levels were
associated with a lower recurrence rate and therefore had longer
disease-free survival (P<0.002 and P<0.0001, log rank,
respectively, (Fig. 2). However,
multivariate Cox regression analysis revealed that both serum CEA
and CA19-9 levels were not independent markers of patient survival
in relation to patient age, lymph node status, tumor grade and
tumor stage.
In addition, the correlation of surgical and/or
systemic treatment with clinicopathologic variables and patient
outcomes was observed in the present study, as shown in Table V. Patients who underwent radical
surgical treatment were associated with small tumor size
(P<0.0001), negative lymph node status (P<0.0001), early
stage (P<0.0001), low expression of CEA level (P=0.020) and low
expression of CA19-9 level (P=0.020). Survival was 37.5% in
patients with resectable tumor and 23.2% in patients with
non-resectable tumor (P<0.0001). Analysis using Kaplan-Meier
survival curves for surgical resectability showed that shorter
survival was associated with non-resectable tumor patients
(P<0.0001). The best survival was observed in patients receiving
adjuvant chemotherapy compared with patients receiving palliative
chemotherapy or supportive care only (35.0, 27.3 and 23.5%,
respectively).
 | Table VSurgical resectability accordance to
demographic, clinicopathological and biological features in Libyan
pancreatic ductal adenocarcinoma (n=123). |
Table V
Surgical resectability accordance to
demographic, clinicopathological and biological features in Libyan
pancreatic ductal adenocarcinoma (n=123).
| | Surgical
resectability | |
|---|
| Clinicopathological
variable | Number | Resectable | Unresectable | P-value |
|---|
| Age /years | | | | 0.25 |
|
<50 | 24 | 37.5 | 62.5 | |
|
≥50 | 99 | 37.4 | 49.5 | |
| Sex | | | | 0.72 |
|
Male | 73 | 20.5 | 79.5 | |
|
Female | 50 | 18.0 | 82.0 | |
| Family history | | | | 0.08 |
|
Positive | 6 | 0.00 | 100.0 | |
|
Negative | 117 | 20.5 | 79.5 | |
| Site | | | | |
|
Head | 96 | 19.8 | 80.2 | 0.88 |
|
Elsewhere | 27 | 18.5 | 81,5 | |
| T stage | | | |
<0.0001 |
|
T1 | 4 | 100.0 | 0.00 | |
|
T2 | 13 | 69.2 | 30.8 | |
|
T3 | 14 | 57.1 | 42.9 | |
|
T4 | 1 | 0.00 | 100.0 | |
|
Tx | 91 | 3.3 | 96.7 | |
| N | | | |
<0.0001 |
|
Positive | 12 | 21.4 | 78.6 | |
|
Negative | 14 | 83.3 | 3.1 | |
|
Nx | 97 | 3.1 | 96.9 | |
| Histological
grade | | | | 0.62 |
|
G1 | 6 | 83.3 | 16.7 | |
|
G2 | 30 | 36.7 | 63.3 | |
|
G3 | 87 | 9.2 | 90.8 | |
| Stage | | | |
<0.0001 |
|
Early
stage | 24 | 79.2 | 20.8 | |
|
Late
stage | 99 | 5.1 | 94.9 | |
| CEA level
(ng/ml) | | | | 0.020 |
|
<5 | 46 | 30.4 | 69.6 | |
|
≥5 | 77 | 13.0 | 87.0 | |
| CA19-9 level
(U/ml) | | | | 0.020 |
|
<400 | 64 | 28.1 | 71.9 | |
|
≥400 | 59 | 10.2 | 89.8 | |
Discussion
PDAC is an aggressive heterogenous malignancy
associated with a very poor prognosis and imposes an enormous
burden on patients, their families and the healthcare system as a
whole. Therefore, the identification and evaluation of diagnostic,
prognostic and predictive markers is urgently needed for clinicians
to achieve improved outcomes. Unfortunately, most patients are
often diagnosed with metastatic disease or locally advanced,
unresectable tumor (2-4,41).
Although newer techniques have been used and target groups have
been rigorously selected, screening programs are still ineffective
for a large group and may be harmful, as noted in 2019(42). Therefore, regular screening with
endoscopic ultrasound and MRI/CT imaging is recommended for
individuals who are at high risk for genetic diseases (43,44).
Therefore, treatment plans are based on the extent of the tumor,
performance status of patients and various other clinical factors.
However, not all patients benefit from conventional cancer therapy
(45). Despite marked advances in
treatment strategies, advanced PDAC cancer remains incurable and
the goals of therapy range from relief of symptoms to prolonging
survival. Of note, surgical treatment of a resectable tumor remains
the best treatment option for this cancer (8,9). A
number of studies suggest that multiple genes may correlate with
PDAC patient outcomes (10,11).
Moreover, tumor biology is heterogeneous in patients with PDAC with
similar tumor anatomy (12,13).
This highlights the importance of appropriate preoperative
radiological PDAC staging and molecular and genetic
stratification/classification to understand the biological behavior
of this highly aggressive malignancy. Numerous studies have
investigated the efficacy of various biological markers as
diagnostic, predictive and prognostic markers in PDAC (22,23,34,38,46).
Among them, CEA and CA19-9 are the most commonly used tumor
biomarkers.
The present study performed a detailed retrospective
analysis of 123 patients with PDAC diagnosed and treated at the
National Cancer Institute, Misurata, Libya. CEA and CA19-9
expression levels at cut-off points (5 ng/ml and 400 U/ml,
respectively) were found to be the most promising discriminators of
both clinicopathological variables and survival outcomes.
In the cohort of present study, the median value of
CEA in serum was 8 ng/ml and high CEA expression was detected in
63% of patients. The median value of CA19-9 in serum was 389 U/ml
and high CA19-9 expression was detected in 48% of patients. In
addition, the present study showed that the majority of Libyan
patients had higher malignancy grade, such as locally advanced
inoperable tumors, poorly undifferentiated tumors and distant
metastases. Tumor size and lymph node status could not be
determined in 75 and 79% of tumors, respectively. On the other
hand, only 20% of patients had resectable tumors and 24 patients
had early-stage PDAC at the time of diagnosis. These figures are
consistent with other published data, which also reported that only
20% of patients diagnosed with PDAC were eligible for surgical
resection and the majority of patients had advanced disease stage
at the time of diagnosis (42,47-50).
Patients with high expression of CEA and/or CA19-9
are often associated with a worse prognosis. Therefore, increased
expression of CEA and CA19-9 might indicate that the tumors are
already at an advanced stage. Moreover, tumor progression was
associated with higher levels of these tumor markers. Consistent
with these findings, Lee et al (45) report that expression of CEA and
CA19-9 is closely associated with tumor differentiation degree,
lymph node metastasis and tumor progression. The aggressive
behavior of PDAC, reflected in both unclear clinical symptoms at
presentation and genetic heterogeneity of the tumor, may explain
the advanced stages of the disease at diagnosis (41,45).
The present study also showed that the highly
aggressive malignant phenotype of PDAC, manifested by surgically
unresectable tumors, unevaluable lymph nodes, advanced stage and
distant metastases, was significantly associated with high serum
CEA and CA19-9 expression profiles. On the other hand, surgically
resectable tumors, negative lymph nodes, early stage and low risk
of metastasis were more common in the group with low serum CEA and
CA19-9 expression. CEA expression was more frequent in tumors with
high CA19-9 expression compared with those with low CA19-9
expression (P<0.0001). The most important finding of the
present study was undoubtedly the significant correlation of CEA
and CA19-9 expression with disease progression, especially overall
survival and disease-free survival. The median follow-up time of
the cohort study was six months and ~74% of patients had died of
PDAC at the end of the follow-up period. Patients with low serum
CEA and CA19-9 levels had a lower recurrence rate and lived longer
than their counterparts with high serum levels. Analysis using
Kaplan-Meier curves also showed that short survival was more common
in the group with high CEA and CA19-9 levels, while the group with
low CEA and CA19-9 levels had longer disease-free survival
(P<0.002 and P<0.0001, respectively). These findings are
consistent with the results of other previous studies (34,38,46),
which indicated that Libyan patients with high expression of CEA
and CA19-9 were associated with poor prognosis. Although
associations between serum CEA and CA19-9 levels and treatment
outcomes are found in numerous studies, some discrepancies are also
reported. While some studies agreed with the findings of the
present study and suggest that overexpression of CEA is associated
with poor prognosis (6,38,46),
others such as the study by Poruk et al (39) reported that CA19-9 is a useful
biological marker in PDAC to predict disease extent, surgical
resectability, disease progression and response to treatment. The
present study showed that the combination of CEA and CA19-9 is more
successful for prognosis prediction than a single biological marker
(47-49).
Therefore, Xu et al (50)
showed that elevated CEA levels in combination with CA19-9 can
significantly increase the prognostic efficacy of CA19-9. The
present study confirmed this association and found that CEA
expression was more frequent in tumors with high CA19-9 compared
with patients with PDAC with low CA19-9.
The present study showed that the prognostic value
of CEA and CA19-9 was almost the same and both markers did not to
act as independent prognosticators for the outcome of patients with
PDAC. However, van Manen et al (37) reported that both biological markers
(CEA and CA19-9) were independent predictors for an advanced PDAC
cohort from the Netherlands and that the predictive power of CEA
was higher than that of CA19-9. These discrepancies could be due to
several factors, including cohort size, diagnostic techniques
(imaging and/or biopsies), genomic background of the cohort
(ethnicity) and/or stage at diagnosis. However, other reports have
found that elevated CEA and advanced stage are independent factors
for poor survival of patients with PDAC (51,52).
The present study confirmed the role of both CEA
(>8 ng/ml) and CA19-9 (>389 U/ml) serum levels as key markers
for multidisciplinary management of Libyan patients with PDAC to
aid decision making. Despite their significant associations with
survival outcomes, neither marker was informative enough to serve
as an independent predictor for advanced patients with PDAC.
The median serum levels of CEA and CA19-9 for all
PDAC tumors were 8 ng/ml and 389 U/ml, respectively. Tumors with
higher serum CEA and CA19-9 levels were found in 62.6 and 48% of
PDAC cases, respectively. Significantly, patients with PDAC with
higher CEA and CA19-9 serum levels had aggressive tumor grade,
higher recurrence rate and shorter survival time and should be
treated carefully. The prognostic value of CEA and CA19-9 was
almost equivalent but not sufficient to be an independent
prognosticator of patient outcome. An extended multinational study
with a larger cohort is needed to confirm the prognostic value of
both serum levels and tissue expression of these two promising
markers.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EE performed statistical analysis, designed the
present study and drafted manuscript. MEd designed the study and
collected demographic and clinicopathologic data and performed
laboratory work and data analysis. OA, MEl and AJ collected and
analyzed data and drafted the manuscript. MAS and MA performed data
interpretation and analysis, drafting and proof reading and
discussions. AB prepared the figures, reviewed the study,
interpretated data and aided in drafting and proof reading of the
manuscript. All authors critically reviewed and approved the final
version of the manuscript. EE and MEl confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The present study was conducted under research
ethics approval by ethical committee at the National Cancer
Institute, Misurata (Ethical Approval Number: EAN 6/2021). Written
informed consent was obtained from all patients for surgical
treatment, pathologic examinations and investigations performed
according to the institutional guidelines of the National Cancer
Institute, Misurata, Libya.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Petrushnko W, Gundara JS, De Reuver PR,
O'Grady G, Samra JS and Mittal A: Systematic review of
peri-operative prognostic biomarkers in pancreatic ductal
adenocarcinoma. HPB (Oxford). 18:652–663. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Board PATE: Pancreatic Cancer Treatment
(PDQ@): Health professional version. National Cancer Institute,
2019.
|
|
5
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bockhorn M, Uzunoglu FG, Adham M, Imrie C,
Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley
RM, et al: Borderline resectable pancreatic cancer: A consensus
statement by the international study group of pancreatic surgery
(ISGPS). Surgery. 155:977–988. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Isaji S, Mizuno S, Windsor JA, Bassi C,
Fernández-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW,
Kishiwada M, et al: International consensus on definition and
criteria of borderline resectable pancreatic ductal adenocarcinoma
2017. Pancreatology. 18:2–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hidalgo M, Cascinu S, Kleeff J, Labianca
R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL and Heinemann V:
Addressing the challenges of pancreatic cancer: Future directions
for improving outcomes. Pancreatology. 15:8–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Eberlin LS, Margulis K, Planell-Mendez I,
Zare RN, Tibshirani R, Longacre TA, Jalali M, Norton JA and
Poultsides GA: Pancreatic cancer surgical resection margins:
Molecular assessment by mass spectrometry imaging. PLoS Med.
13(e1002108)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Piciucchi M, Capurso G, Valente R, Larghi
A, Archibugi L, Signoretti M, Stigliano S, Zerboni G, Barucca V, La
Torre M, et al: Early onset pancreatic cancer: Risk factors,
presentation and outcome. Pancreatology. 15:151–155.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pontén F, Schwenk JM, Asplund A and
Edqvist PH: The human protein atlas as a proteomic resource for
biomarker discovery. J Intern Med. 270:428–446. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tzeng CW, Fleming JB, Lee JE, Xiao L,
Pisters PW, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR,
Fogelman DR, et al: Defined clinical classifications are associated
with outcome of patients with anatomically resectable pancreatic
adenocarcinoma treated with neoadjuvant therapy. Ann Surg Oncol.
19:2045–2053. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Asaoka T, Miyamoto A, Maeda S, Tsujie M,
Hama N, Yamamoto K, Miyake M, Haraguchi N, Nishikawa K, Hirao M, et
al: Prognostic impact of preoperative NLR and CA19-9 in pancreatic
cancer. Pancreatology. 16:434–440. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sugiura T, Uesaka K, Kanemoto H, Mizuno T,
Sasaki K, Furukawa H, Matsunaga K and Maeda A: Serum CA19-9 is a
significant predictor among preoperative parameters for early
recurrence after resection of pancreatic adenocarcinoma. J
Gastrointest Surg. 16:977–985. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Waraya M, Yamashita K, Katagiri H, Ishii
K, Takahashi Y, Furuta K and Watanabe M: Preoperative serum CA19-9
and dissected peripancreatic tissue margin as determiners of
long-term survival in pancreatic cancer. Ann Surg Oncol.
16:1231–1240. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Meng Q, Shi S, Liang C, Liang D, Xu W, Ji
S, Zhang B, Ni Q, Xu J and Yu X: Diagnostic and prognostic value of
carcinoembryonic antigen in pancreatic cancer: A systematic review
and meta-analysis. Onco Targets Ther. 10:4591–4598. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gold P and Freedman SO: Specific
carcinoembryonic antigens of the human digestive system. J Exp Med.
122:467–481. 1965.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Molina R, Barak V, van Dalen A, Duffy MJ,
Einarsson R, Gion M, Goike H, Lamerz R, Nap M, Sölétormos G and
Stieber P: Tumor markers in breast cancer-European group on tumor
markers recommendations. Tumour Biol. 26:281–293. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Grunnet M and Sorensen JB:
Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung
Cancer. 76:138–143. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Juweid M, Sharkey RM, Behr T, Swayne LC,
Rubin AD, Herskovic T, Hanley D, Markowitz A, Dunn R, Siegel J, et
al: Improved detection of medullary thyroid cancer with
radiolabeled antibodies to carcinoembryonic antigen. J Clin Oncol.
14:1209–1217. 1996.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nazli O, Bozdag AD, Tansug T, Kir R and
Kaymak E: The diagnostic importance of CEA and CA 19-9 for the
early diagnosis of pancreatic carcinoma. Hepatogastroenterology.
47:1750–1752. 2000.PubMed/NCBI
|
|
22
|
Satake K, Chung YS, Yokomatsu H, Nakata B,
Tanaka H, Sawada T, Nishiwaki H and Umeyama K: A clinical
evaluation of various tumor markers for the diagnosis of pancreatic
cancer. Int J Pancreatol. 7:25–36. 1990.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Imaoka H, Mizuno N, Hara K, Hijioka S,
Tajika M, Tanaka T, Ishihara M, Hirayama Y, Hieda N, Yoshida T, et
al: Prognostic impact of carcinoembryonic antigen (CEA) on patients
with metastatic pancreatic cancer: A retrospective cohort study.
Pancreatology. 16:859–864. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rhodes JM and Ching CK: Serum diagnostic
tests for pancreatic cancer. Baillieres Clin Gastroenterol.
4:833–852. 1990.PubMed/NCBI View Article : Google Scholar
|
|
25
|
National Comprehensive Cancer Network:
NCCN clinical practive guideline in oncology (NCCN
Guidelines®): Pancreatic adenocarcinoma. Version 1.2021,
October, 2021.
|
|
26
|
Hata S, Sakamoto Y, Yamamoto Y, Nara S,
Esaki M, Shimada K and Kosuge T: Prognostic impact of postoperative
serum CA 19-9 levels in patients with resectable pancreatic cancer.
Ann Surg Oncol. 19:636–641. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Takasaki H, Uchida E, Tempero MA, Burnett
DA, Metzgar RS and Pour PM: Correlative study on expression of CA
19-9 and DU-PAN-2 in tumor tissue and in serum of pancreatic cancer
patients. Cancer Res. 48:1435–1438. 1988.PubMed/NCBI
|
|
28
|
Aoki H, Ohnishi H, Hama K, Ishijima T,
Satoh Y, Hanatsuka K, Ohashi A, Wada S, Miyata T, Kita H, et al:
Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and
ERK-dependent pathways in rat pancreatic stellate cells. Am J
Physiol Cell Physiol. 290:C1100–C1108. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tanaka M, Chari S, Adsay V, Fernandez-del
Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K and Matsuno
S: International Association of Pancreatology. International
consensus guidelines for management of intraductal papillary
mucinous neoplasms and mucinous cystic neoplasms of the pancreas.
Pancreatology. 6:17–32. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Goonetilleke KS and Siriwardena AK:
Systematic review of carbohydrate antigen (CA 19-9) as a
biochemical marker in the diagnosis of pancreatic cancer. Eur J
Surg Oncol. 33:266–270. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kobayashi T, Kawa S, Tokoo M, Oguchi H,
Kiyosawa K, Furuta S, Kanai M and Homma T: Comparative study of
CA-50 (time-resolved fluoroimmunoassay), Span-1, and CA19-9 in the
diagnosis of pancreatic cancer. Scand J Gastroenterol. 26:787–797.
1991.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Palmquist C, Dehlendorff C, Calatayud D,
Hansen CP, Hasselby JP and Johansen JS: Prediction of
unresectability and prognosis in patients undergoing surgery on
suspicion of pancreatic cancer using carbohydrate antigen 19-9,
interleukin 6, and YKL-40. Pancreas. 49:53–61. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tian F, Appert HE, Myles J and Howard JM:
Prognostic value of serum CA 19-9 levels in pancreatic
adenocarcinoma. Ann Surg. 215:350–355. 1992.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J,
Cen P, Xu J, Liu C, Long J, et al: A preoperative serum signature
of CEA+/CA125+/CA19-9 ≥ 1000 U/ml indicates poor outcome to
pancreatectomy for pancreatic cancer. Int J Cancer. 136:2216–2227.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chari ST, Kelly K, Hollingsworth MA,
Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto
M, Cleeter DF, et al: Early detection of sporadic pancreatic
cancer: Summative review. Pancreas. 44:693–712. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Giannis D, Moris D and Barbas AS:
Diagnostic, predictive and prognostic molecular biomarkers in
pancreatic cancer: An overview for clinicians. Cancers (Basel).
13(1071)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
van Manen L, Groen JV, Putter H,
Vahrmeijer AL, Swijnenburg RJ, Bonsing BA and Mieog JSD: Elevated
CEA and CA19-9 serum levels independently predict advanced
pancreatic cancer at diagnosis. Biomarkers. 25:186–193.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Poruk KE, Gay DZ, Brown K, Mulvihill JD,
Boucher KM, Scaife CL, Firpo MA and Mulvihill SJ: The clinical
utility of CA 19-9 in pancreatic adenocarcinoma: Diagnostic and
prognostic updates. Curr Mol Med. 13:340–351. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ni XG, Bai XF, Mao YL, Shao YF, Wu JX,
Shan Y, Wang CF, Wang J, Tian YT, Liu Q, et al: The clinical value
of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of
pancreatic cancer. Eur J Surg Oncol. 31:164–169. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
US Preventive Services Task Force. Owens
DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Curry
SJ, Doubeni CA, Epling JW Jr, et al: Screening for pancreatic
cancer: US preventive services task force reaffirmation
recommendation statement. JAMA. 322:438–444. 2019.
|
|
43
|
He XY and Yuan YZ: Advances in pancreatic
cancer research: Moving towards early detection. World J
Gastroenterol. 20:11241–11248. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Okano K and Suzuki Y: Strategies for early
detection of resectable pancreatic cancer. World J Gastroenterol.
20:11230–11240. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lee KJ, Yi SW, Chung MJ, Park SW, Song SY,
Chung JB and Park JY: Serum CA 19-9 and CEA levels as a prognostic
factor in pancreatic adenocarcinoma. Yonsei Med J. 54:643–649.
2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Koprowski H, Steplewski Z, Mitchell K,
Herlyn M, Herlyn D and Fuhrer P: Colorectal carcinoma antigens
detected by hybridoma antibodies. Somatic Cell Genet. 5:957–971.
1979.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Reitz D, Gerger A, Seidel J, Kornprat P,
Samonigg H, Stotz M, Szkandera J and Pichler M: Combination of
tumour markers CEA and CA19-9 improves the prognostic prediction in
patients with pancreatic cancer. J Clin Pathol. 68:427–433.
2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kim YC, Kim HJ, Park JH, Park DI, Cho YK,
Sohn CI, Jeon WK, Kim BI and Shin JH: Can preoperative CA19-9 and
CEA levels predict the resectability of patients with pancreatic
adenocarcinoma? J Gastroenterol Hepatol. 24:1869–1875.
2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mehta J, Prabhu R, Eshpuniyani P,
Kantharia C and Supe A: Evaluating the efficacy of tumor markers CA
19-9 and CEA to predict operability and survival in pancreatic
malignancies. Trop Gastroenterol. 31:190–194. 2010.PubMed/NCBI
|
|
50
|
Xu HX, Liu L, Xiang JF, Wang WQ, Qi ZH, Wu
CT, Liu C, Long J, Xu J, Ni QX and Yu XJ: Postoperative serum CEA
and CA125 levels are supplementary to perioperative CA19-9 levels
in predicting operative outcomes of pancreatic ductal
adenocarcinoma. Surgery. 161:373–384. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Salmiheimo A, Mustonen H, Stenman UH,
Puolakkainen P, Kemppainen E, Seppänen H and Haglund C: Systemic
inflammatory response and elevated tumour markers predict worse
survival in resectable pancreatic ductal adenocarcinoma. PLoS One.
11(e0163064)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Distler M, Pilarsky E, Kersting S and
Grützmann R: Preoperative CEA and CA 19-9 are prognostic markers
for survival after curative resection for ductal adenocarcinoma of
the pancreas-a retrospective tumor marker prognostic study. Int J
Surg. 11:1067–1072. 2013.PubMed/NCBI View Article : Google Scholar
|