Introduction
An estimated 19.3 million new cancer cases and 10
million cancer deaths occurred worldwide in 2020(1). About 1.9 million new colorectal
cancer (CRC) cases and 916,000 deaths were estimated to occur,
representing about one in 10 cancer cases and deaths. CRC is the
second most common cancer in women and the third in men and the
second most common cause of cancer-related mortality worldwide
(1). The primary prevalent
metastatic organs are the regional lymph nodes, liver, lungs, and
peritoneum (2). In CRC with
regional lymph node metastasis, intestinal resection with lymph
node dissection is recommended for curative treatment. On the other
hand, in cases with distant (extraregional) lymph node metastasis,
para-aorta lymph node metastasis predominantly occurs and the
resection is performed in selected patients to have potential to
achieve a cure and bring longer survival, although no prospective
comparative clinical trials have a clear therapeutic effect
(2). Mediastinal lymph node
metastasis from CRC uncommonly occurs and is occasionally
recognized with lung metastasis (3). There were few cases of mediastinal
lymph node metastasis with liver or para-aorta lymph node
metastasis (4-9).
Moreover, solitary mediastinal lymph node metastasis from CRC
without any other organ involvement is extremely rare, and the
optimal treatment remains unclear. Here we report a case of
solitary anterior mediastinal lymph node metastasis with
pericardial invasion from transverse colon cancer and review the
relevant literature.
Case report
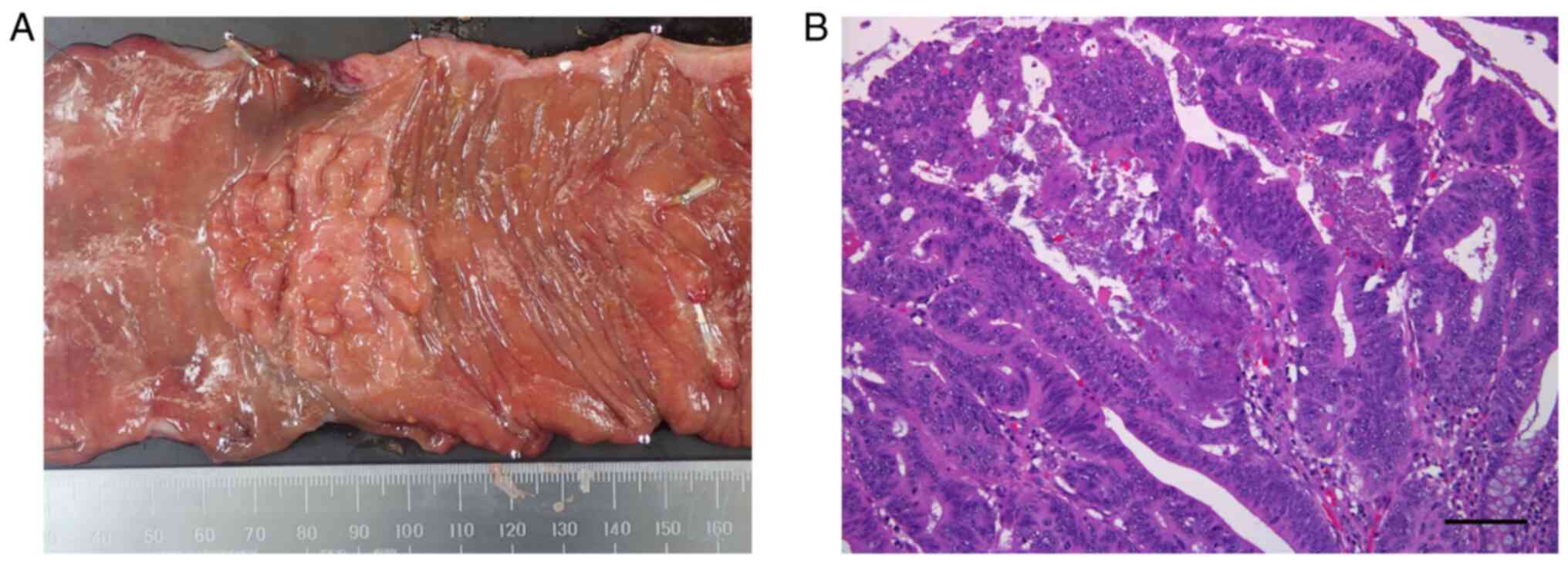
An 82-year-old Japanese woman underwent laparoscopic
right hemicolectomy with regional lymph node dissection for
transverse colon cancer, which was a pathologically
well-differentiated adenocarcinoma classified as T1N1bM0 stage IIIA
in the UICC classification (Fig.
1A and B). The patient was
followed up postoperatively without adjuvant chemotherapy. At 18
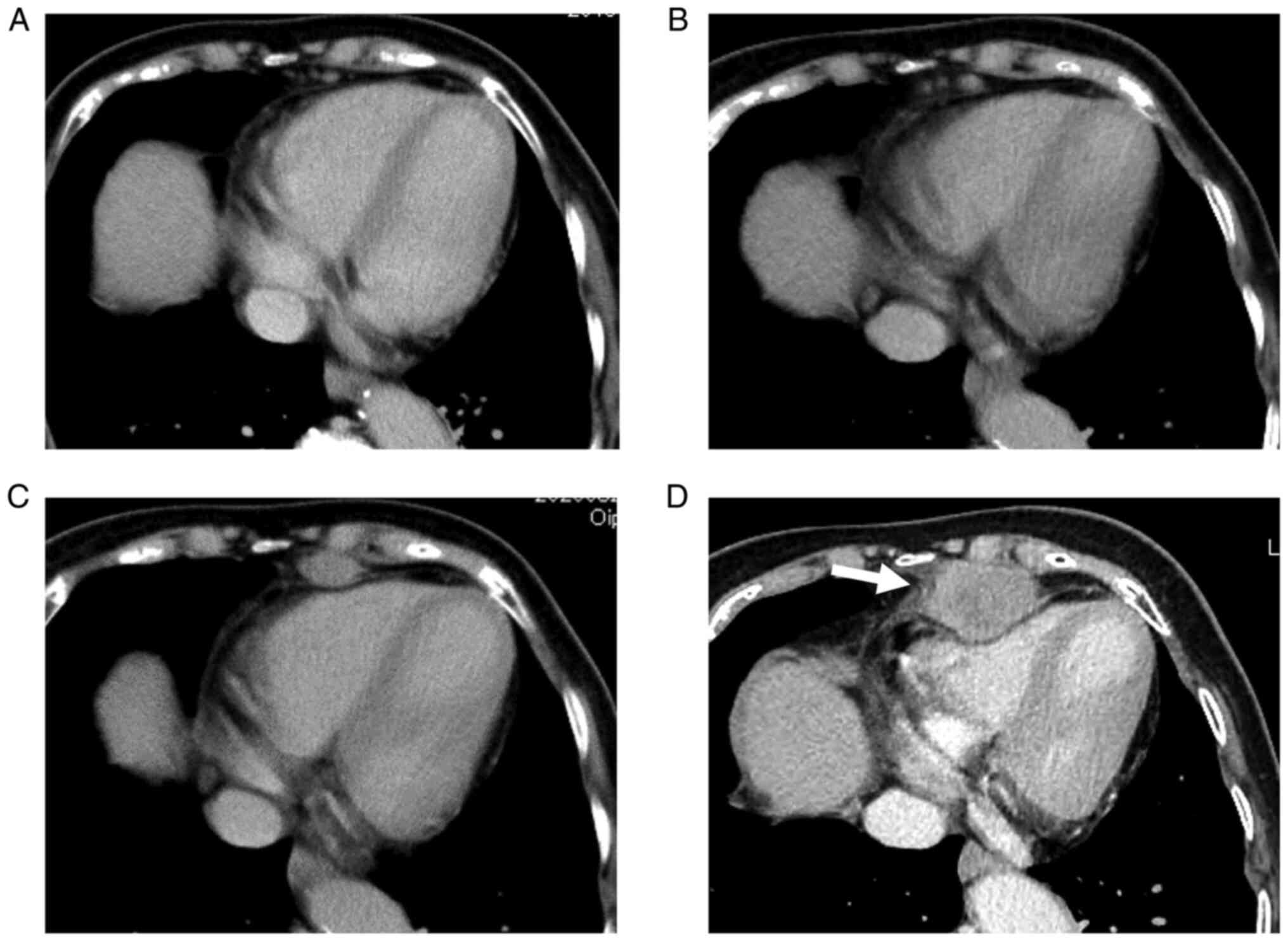
months post-colectomy, the patient had no symptoms, but follow-up
contrast-enhanced computed tomography (CECT) revealed a mediastinal
tumor that rapidly increased (Fig.
2A-D). The tumor was located at the anterior inferior
mediastinum and compressed the heart (Fig. 2D). The results of the tumor marker
test were within the normal range, with the carcinoembryonic
antigen and carbohydrate antigen 19-9 levels of 2.7 ng/ml and 4.14
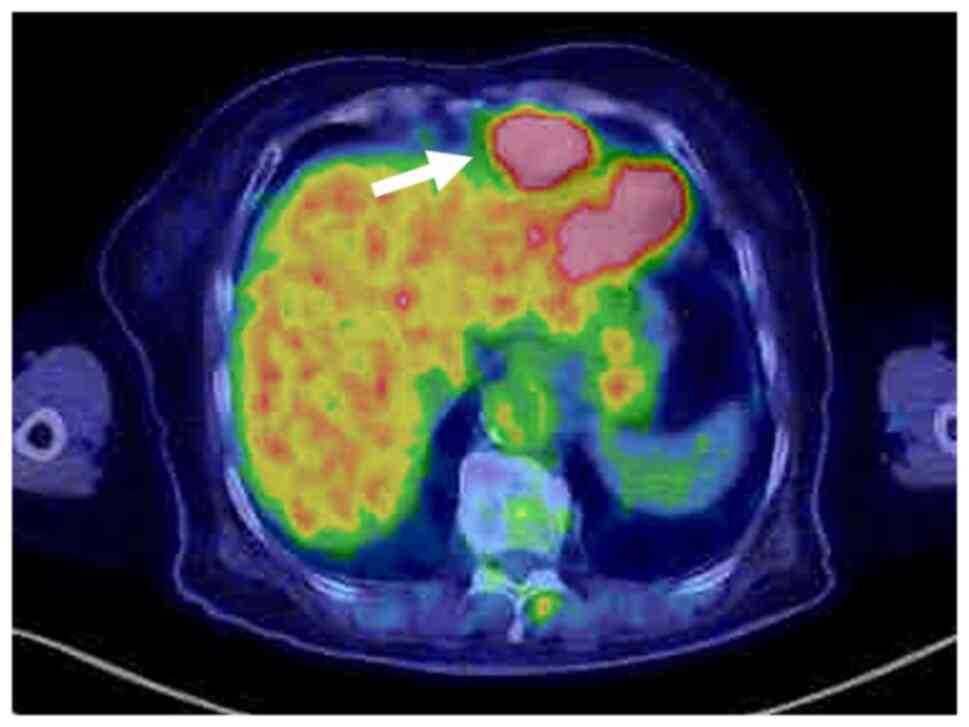
U/ml, respectively. Positron emission tomography revealed that the
tumor showed fluorodeoxyglucose uptake with a standardized uptake
value of 16.5 (Fig. 3). Further,
the liver adjacent to the surgical site of the previous
cholecystectomy showed fluorodeoxyglucose uptake but did not show
any abnormal findings on CECT scan. Mediastinal lymph node
metastasis from CRC was considered a preoperative diagnosis. Thus,
surgical resection of the tumor would be an appropriate method as
the rapidly growing tumor may cause fatal complications in the
future.
The patient underwent resection of the anterior
mediastinal tumor. Intraoperative findings suggested tumor invasion
to the adjacent pericardium, and therefore, pericardiectomy and
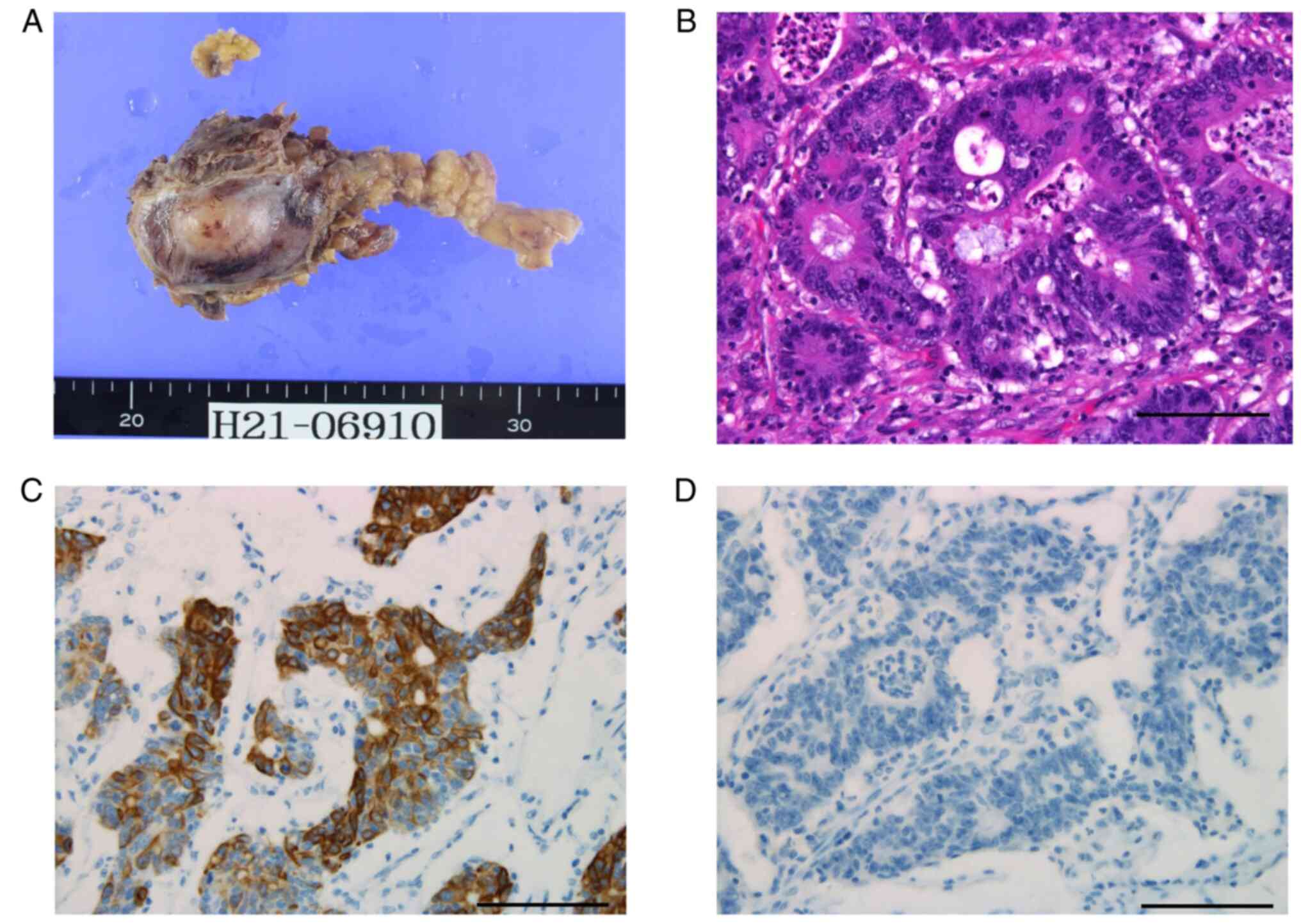
pericardial reconstruction were performed. The resected mediastinal
tumor was 35x25 mm in size (Fig.
4A). Microscopic examination revealed that the tumor was an
adenocarcinoma (Fig. 4B).
Immunohistochemical analysis (refer to Supplementary data for method) revealed
that the cells expressed CK20 (Fig.
4C) and CDX2 but not CK7 (Fig.
4D) and TTF-1. These pathological findings were consistent with
the diagnosis of mediastinal metastasis originating from the
previous transverse colon cancer. Additionally, the cancer was
pathologically identified to have invaded the adjacent pericardium
and diaphragm. However, no cancer cells were detected in the
surrounding lymph node, thymus, and pericardial fluid. Furthermore,
the molecular mutation status of the primary transverse colon
cancer was examined retrospectively. The tumor expressed BRAF V600E
mutations but did not express RAS mutations and microsatellite
instability.
The patient was postoperatively treated with
thoracic drainage for pleural effusion and was discharged on
postoperative day 28. The patient refused additional chemotherapy.
Follow-up CECT and gadolinium ethoxybenzyl diethylenetriamine
pentaacetic acid-enhanced magnetic resonance imaging showed no
recurrent metastases in any organ. The patient has been alive
without recurrence 8 months after the surgery for mediastinal
metastasis.
Discussion
Metachronous metastatic sites after curative
resection of CRC are the liver, occurring in 7.1% of patients, lung
in 5.5%, peritoneum in 2.0%, and local lesion in 2.0% of patients
(2). Saito et al reported
that 14% of patients who underwent lung resection for metastatic
CRC had mediastinal or hilar lymph node metastasis (3). Some mediastinal lymph node metastases
have been identified in patients with liver, para-aorta lymph node,
or thyroid metastases (4-10).
From these reports, mediastinal lymph node metastasis from CRC is
considered re-metastasis from concurrent or previously metastasized
organs. Conversely, solitary mediastinal lymph node metastasis from
CRC without any other organ involvement is extremely rare, and to
the best of our knowledge, only two patients, including our case,
have been reported (11). In
retrospect, the mediastinal tumor was not detected 12 months after
colectomy; therefore, we should have taken more care when examining
the mediastinum even in the cases where other organ metastases were
absent during follow-up.
The mechanism of mediastinal lymph node metastasis
depends on the presence or absence of intrathoracic lesions.
Mediastinal metastasis can occur following lung metastasis through
the lymphatic drainage system (3).
In patients without intrathoracic lesions, mediastinal metastasis
is presumed to be primarily caused by the thoracic duct (4,12,13).
Most previous reports showed a middle or posterior mediastinal
metastasis (5-9,11)
as the anterior mediastinum does not directly communicate with the
thoracic duct (12). In contrast,
it is hypothesized that hematogenous metastasis via the
paravertebral venous plexus exists in exceptional cases with
ovarian metastasis (14). In our
case, the mediastinal tumor had no lymph node structure
pathologically. However, the metastasis was considered lymphogenous
because primary colon cancer had regional lymph node metastasis and
CECT showed an increase in mediastinal lymph node metastasis over
time. The uniqueness of our case could be attributed to solitary
metastases and its location in the anterior mediastinum. Vetto
et al reported a metastatic form via lymphatic drainage from
the liver to the anterior mediastinum through the right diaphragm,
caval foramen, and esophageal hiatus (4). Therefore, the patient was followed up
with careful attention to latent cancer metastasis, primarily the
liver, after complete resection of anterior mediastinal metastasis.
Notably, to the best of our knowledge, this is the first study that
reported the molecular mutation status expressing BRAF V600E
mutations but not RAS mutations and microsatellite instability.
BRAF mutant CRC is widely known to have a different pattern of
metastatic spread compared with wild-type CRC (15). Distant lymph node and peritoneal
metastases in BRAF mutant CRC are observed more frequently and lung
metastases are observed less frequently than those in wild-type CRC
(15). Additionally, it has been
reported that the rate of distant lymph node metastases was not
different between tumors expressing microsatellite instability and
those with stable microsatellite (15). Thus, oncogenic mutations in BRAF
might be involved in mediastinal lymph node metastasis from CRC and
further studies are needed for a deeper understanding.
The borders of the anterior mediastinum are the
sternum anteriorly, the pericardium posteriorly, the thoracic inlet
superiorly, and the diaphragm inferiorly (16). Anatomically, the anterior
mediastinal tumor can cause direct pericardial invasion, which may
lead to carcinomatous pericarditis (17). Carcinomatous pericarditis can
develop cardiac tamponade and has a poor prognosis with a median
survival of 3-5 months (18-20).
Our patient showed rapid tumor growth and pathological pericardial
invasion, although no cancer cells were found in the pericardial
fluid. Resection of the mediastinal metastasis was considered
effective in preventing possible fatal complications.
CRC treatment progression has created more
opportunities for even patients with metastasis to undergo
surgeries, including hepatectomy and pneumonectomy. The prognosis
after hepatectomy and pneumonectomy is favorable, with a 5-year
survival rate of 35-58 and 30-68%, respectively (21-26).
However, Saito et al reported that patients with lung and
mediastinal lymph node metastases had a poor prognosis, and
therefore, surgery might not be indicated (3). Conversely, the prognosis of patients
with solitary mediastinal lymph node metastasis remains unknown
because of its rarity and unclear optimal treatment. In the
Japanese Society for Cancer of Colon and Rectum guidelines,
surgical treatment is indicated when a recurrent tumor is observed
in a single organ and when complete surgical resection is possible
(2). Within the guideline, the
resection of solitary mediastinal lymph node metastasis was
performed, resulting in a satisfactory outcome without recurrence 8
months postoperatively. Our patient has undergone short-term
postoperative follow-up every 1-2 months because early
postoperative recurrence may occasionally occur (10).
In conclusion, a patient with a rare incidence of
recurrent CRC was reported with a solitary anterior mediastinal
lymph node metastasis, suggesting that clinicians should consider
the metastasis to mediastinum during follow-up in patients with
CRC. The anterior mediastinal lymph node metastasis from CRC can
cause fatal complications because of the direct pericardial
invasion. In the case of solitary anterior mediastinal metastasis,
surgery may be the most reliable treatment. A large number of cases
must be accumulated to establish optimal management.
Supplementary Material
Tissues were fixed with 10% neutral
buffered formalin for 96 h at room temperature (RT). H&E
staining was performed with Tissue-Tek DRS 2000 automated slide
stainer (Sakura Finetek Japan Co., Ltd.). Slides were stained with
hematoxylin for 3 min and with eosin for 2 min at RT.
Immunohistochemistry was performed with Benchmark XT immunostainer
(Roche Diagnostics). The specimens were incubated for 32 min at
37˚C with the following primary antibodies (Abs): anti-CK7 Ab
(790-4462; Roche Diagnostics), anti-CK20 Ab (790-4431; Roche
Diagnostics), anti-CDX2 Ab (760-4380; Roche Diagnostics) and anti-
TTF-1 Ab (790-4756; Roche Diagnostics). The processes of blocking
and labeled streptavidin biotin method were performed using I-View
DAB universal kit (760-041; Roche Diagnostics). All the protocols
including reagent dilution were performed according to manufacturer
instructions. The blocking and incubation time with the
biotinylated second antibody was 8 min at RT, respectively. Images
of stained specimens were acquired with the BX53 microscope system
(Olympus). The PCR mutation analysis experiments including BRAF
V600E mutations were performed by an external laboratory (SRL,
Inc.).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW drafted the manuscript and provided original
images. RS, MK and MH participated in treating the patient and
revising the manuscript. All authors read and approved the final
manuscript. YW and RS confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this article and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saito Y, Omiya H, Kohno K, Kobayashi T,
Itoi K, Teramachi M, Sasaki M, Suzuki H, Takao H and Nakade M:
Pulmonary metastasectomy for 165 patients with colorectal
carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg.
124:1007–1013. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vetto JT and Cohen AM: Isolated spread of
hepatic metastatic disease to a mediastinal lymph node. Report of a
case and review of pertinent anatomy and literature. Dis Colon
Rectum. 34:1128–1130. 1991.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sano A, Murakawa T, Morota T and Nakajima
J: Resection of a posterior mediastinal metastasis of colon cancer.
Ann Thorac Surg. 92:353–354. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Iwata T, Chung K, Hanada S, Toda M, Nakata
K, Kato T and Miura T: Solitary bulky mediastinal lymph node
metastasis from colon cancer. Ann Thorac Cardiovasc Surg.
19:313–315. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nomura T, Katsumata K, Hara T, Sonoda I,
Kasahara K, Kuwabara H, Shigoka M, Enomoto M, Ishizaki T, Sumi T
and Tsuchida A: Long-Term survival of a patient with mediastinal
lymph node metastasis treated with chemo-radiotherapy after surgery
for cecal cancer. Gan To Kagaku Ryoho. 44:1698–1700.
2017.PubMed/NCBI(In Japanese).
|
|
8
|
El-Halabi MM, Chaaban SA, Meouchy J, Page
S and Salyers WJ Jr: Colon cancer metastasis to mediastinal lymph
nodes without liver or lung involvement: A case report. Oncol Lett.
8:2221–2224. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Matsuda Y, Yano M, Miyoshi N, Noura S,
Ohue M, Sugimura K, Motoori M, Kishi K, Fujiwara Y, Gotoh K, et al:
Solitary mediastinal lymph node recurrence after curative resection
of colon cancer. World J Gastrointest Surg. 6:164–168.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yamamoto Y, Kodama K, Ide Y and Takeda M:
Thymic and mediastinal lymph node metastasis of colon cancer. Ann
Thorac Surg. 103:e13–e15. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Musallam KM, Taher AT, Tawil AN,
Chakhachiro ZI, Habbal MZ and Shamseddine AI: Solitary mediastinal
lymph node metastasis in rectosigmoid carcinoma: A case report.
Cases J. 1(69)2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Baltaxe HA and Constable WC: Mediastinal
lymph node visualization in the absence of intrathoracic disease.
Radiology. 90:94–98. 1968.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
McLoud TC, Kalisher L, Stark P and Greene
R: Intrathoracic lymph node metastases from extrathoracic
neoplasms. AJR Am J Roentgenol. 131:403–407. 1978.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kuba H, Sato N, Uchiyama A, Nakafusa Y,
Mibu R, Yoshida K, Kuroiwa K and Tanaka M: Mediastinal lymph node
metastasis of colon cancer: Report of a case. Surg Today.
29:375–377. 1999.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tran B, Kopetz S, Tie J, Gibbs P, Jiang
ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O and Desai J: . Impact of
BRAF mutation and microsatellite instability on the pattern of
metastatic spread and prognosis in metastatic colorectal cancer.
Cancer. 117:4623–4632. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tecce PM, Fishman EK and Kuhlman JE: CT
evaluation of the anterior mediastinum: spectrum of disease.
Radiographics. 14:973–990. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sawada H, Toyota K, Hakoda K, Kajiwara R,
Hotta R, Inoue M, Ohmori I, Miyamoto K, Sadamoto S and Takahashi T:
A case of stage II ascending colon cancer with cardiac tamponade
due to pericardial metastasis. Am J Case Rep.
22(e932239)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dequanter D, Lothaire P, Berghmans T and
Sculier JP: Severe pericardial effusion in patients with concurrent
malignancy: A retrospective analysis of prognostic factors
influencing survival. Ann Surg Oncol. 15:3268–3271. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dosios T, Theakos N, Angouras D and
Asimacopoulos P: Risk factors affecting the survival of patients
with pericardial effusion submitted to subxiphoid pericardiostomy.
Chest. 124:242–246. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsang TS, Seward JB, Barnes ME, Bailey KR,
Sinak LJ, Urban LH and Hayes SN: Outcomes of primary and secondary
treatment of pericardial effusion in patients with malignancy. Mayo
Clin Proc. 75:248–253. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Martin LW and Warren RS: Current
management of colorectal liver metastases. Surg Oncol Clin N Am.
9:853–876; discussion 877-8. 2000.PubMed/NCBI
|
|
22
|
Abdalla EK, Vauthey JN, Ellis LM, Ellis V,
Pollock R, Broglio KR, Hess K and Curley SA: Recurrence and
outcomes following hepatic resection, radiofrequency ablation, and
combined resection/ablation for colorectal liver metastases. Ann
Surg. 239:818–825; discussion 825-7. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nordlinger B, Sorbye H, Glimelius B,
Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole
ET, Finch-Jones M, et al: Perioperative FOLFOX4 chemotherapy and
surgery versus surgery alone for resectable liver metastases from
colorectal cancer (EORTC 40983): Long-term results of a randomised,
controlled, phase 3 trial. Lancet Oncol. 14:1208–1215.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Watanabe K, Nagai K, Kobayashi A, Sugito M
and Saito N: Factors influencing survival after complete resection
of pulmonary metastases from colorectal cancer. Br J Surg.
96:1058–1065. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Iida T, Nomori H, Shiba M, Nakajima J,
Okumura S, Horio H, Matsuguma H, Ikeda N, Yoshino I, Ozeki Y, et
al: Prognostic factors after pulmonary metastasectomy for
colorectal cancer and rationale for determining surgical
indications: A retrospective analysis. Ann Surg. 257:1059–1064.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kanemitsu Y, Kato T, Hirai T and Yasui K:
Preoperative probability model for predicting overall survival
after resection of pulmonary metastases from colorectal cancer. Br
J Surg. 91:112–120. 2004.PubMed/NCBI View
Article : Google Scholar
|