Introduction
Clinical benefits are derived from agents that bind
to circulating vascular endothelial growth factor (VEGF), a key
factor in vasculogenesis and angiogenesis. Bevacizumab, a humanized
monoclonal antibody that targets VEGF-A, has been widely used for
the treatment of a number of solid tumors, such as metastatic
colon, non-small-cell lung, breast, brain and kidney cancer
(1-3).
Infusion of certain humanized monoclonal antibodies, such as
rituximab and trastuzumab, results in infusion-related
hypersensitivity reactions (HSRs) (4). Bevacizumab contains <10% murine
protein, which may also cause HSRs. Therefore, these antibodies are
initially infused cautiously at a slower rate. To prevent HSRs,
bevacizumab is initially infused for 90 min, with a second infusion
for 60 min and subsequent infusions administered over a period of
30 min. Serious HSRs have not been reported in any major phase II
or III trial (1,5,6).
However, the standard dose of bevacizumab varies, for example, 5 or
7.5 mg/kg for colorectal cancer and 15 mg/kg for lung and brain
cancer (7,8). Bevacizumab has been reported to be
administered at a dose of 15 mg/kg for 90 min, followed by 5 mg/kg
for 30 min at the same infusion rate of 0.166 mg/kg/min.
Furthermore, bevacizumab administered at a dose of 15 mg/kg for 30
min has been infused at a rate of 0.5 mg/kg/min. This demonstrated
that a high bevacizumab infusion rate of up to 0.5 mg/kg/min, which
is three-fold higher than normal, is also safe and does not
increase HSRs (2,3).
Standard bevacizumab infusions over 90, 60 and 30
min, which are widely used in the management of various cancer
types, may be cumbersome and burden patients. Several studies have
reported the use of short bevacizumab infusions in patients with
colorectal cancer without any severe clinical HSRs (9-13).
The safety of short infusions was also reported in the management
of other types of cancer, such as lung, ovarian and breast cancer
(9,14). Regarding other adverse effects,
short bevacizumab infusions did not increase the risk of
proteinuria and hypertension (9).
Although the National Comprehensive Cancer Network guidelines state
that a bevacizumab infusion rate of 0.5 mg/kg/min is safe (15), short bevacizumab infusions have not
been adopted in numerous countries owing to a lack of published
safety and efficacy data. The present prospective, multicenter
study was conducted to evaluate the safety and efficacy of short
bevacizumab infusions in patients with colorectal cancer.
Materials and methods
Study protocol and patients
In this prospective study, patients with untreated,
unresectable, advanced or metastatic colorectal cancer who had not
previously received chemotherapy or bevacizumab were recruited
between June 2017 and March 2019 from four hospitals (Osaka
Metropolitan University Graduate School of Medicine, Osaka; Osaka
City General Hospital, Osaka; Machida Gastrointestinal Hospital,
Osaka; and Osaka Ekisaikai Hospital, Osaka) in Japan. Inclusion
criteria were as follows: An age of ≥20 years, an Eastern
Cooperative Oncology Group performance status (PS) score of 0, 1 or
2, and one or more measurable lesions assessed by an investigator
according to the Response Evaluation Criteria In Solid Tumor
(RECIST; version 1.1) guidelines (16). Exclusion criteria included the
prior use of bevacizumab, brain metastasis and a history of
bleeding or thrombosis. All patients received the first bevacizumab
dose (5 or 7.5 mg/kg) for 30 min. If it was tolerated, the second
bevacizumab infusion rate was 0.5 mg/kg/min (5 mg/kg over 10 min
and 7.5 mg/kg over 15 min). No premedication was required prior to
bevacizumab administration. It was essential to observe the
patients for HSRs (pruritus, flushing, laryngeal edema and
hypertension) at 10 and 30 min after treatment.
The primary study endpoint was progression-free
survival (PFS). The secondary endpoints were incidence of HSRs,
toxicities associated with bevacizumab (proteinuria, hypertension,
gastrointestinal perforations, arterial/venous thromboembolic
events and bleeding) and overall response rate (RR). Tumor response
was assessed by computed tomography according to the RECIST ver.
1.1 every 8 weeks. Adverse events were scored at each follow-up
according to the National Cancer Institute Common Terminology
Criteria for Adverse Events (version 4.0) (17). The study was conducted in
accordance with the Declaration of Helsinki and approved by the
Ethics Committee of Osaka City University Graduate School of
Medicine (Osaka, Japan; protocol no. 3666). Written informed
consent was obtained from all patients before enrollment.
Statistical analysis
Continuous variables are presented as the median
(range), and categorical variables are presented as number
(percentage). The Kaplan-Meier method was used to generate the PFS
curve. All statistical analyses were performed using EZR (version
1.34; Saitama Medical Center, Jichi Medical University), which is a
graphical user interface for R (version 3.3.2; The R Foundation for
Statistical Computing).
Results
A total of 23 patients (12 men and 11 women) were
enrolled in the study, with a median age of 70 years (range, 44-80
years). Of these, 13 (57%) patients had an ECOG PS score of 0, and
10 (43%) patients had a PS score of 1. The primary tumor site
included the right side, left side and rectal colon in 8 (35%), 6
(26%) and 9 (39%) patients, respectively. Regarding RAS mutation
status, 7 (30%) and 16 (70%) patients had the RAS wild-type and the
RAS mutant-type, respectively. A total of 18 (78%) patients had
undergone primary tumor resection. Bevacizumab was administered
with SOX (n=11; 48%), XELOX (n=9; 39%), FOLFOX (n=2; 9%) and
FOLFOXIRI (n=1; 4%). Overall, 12 (52%) patients were on
antihypertensive medication, and 1 (4%) patient had proteinuria. In
total, 3 (13%) patients had a history of HSRs. Patient
characteristics are shown in Table
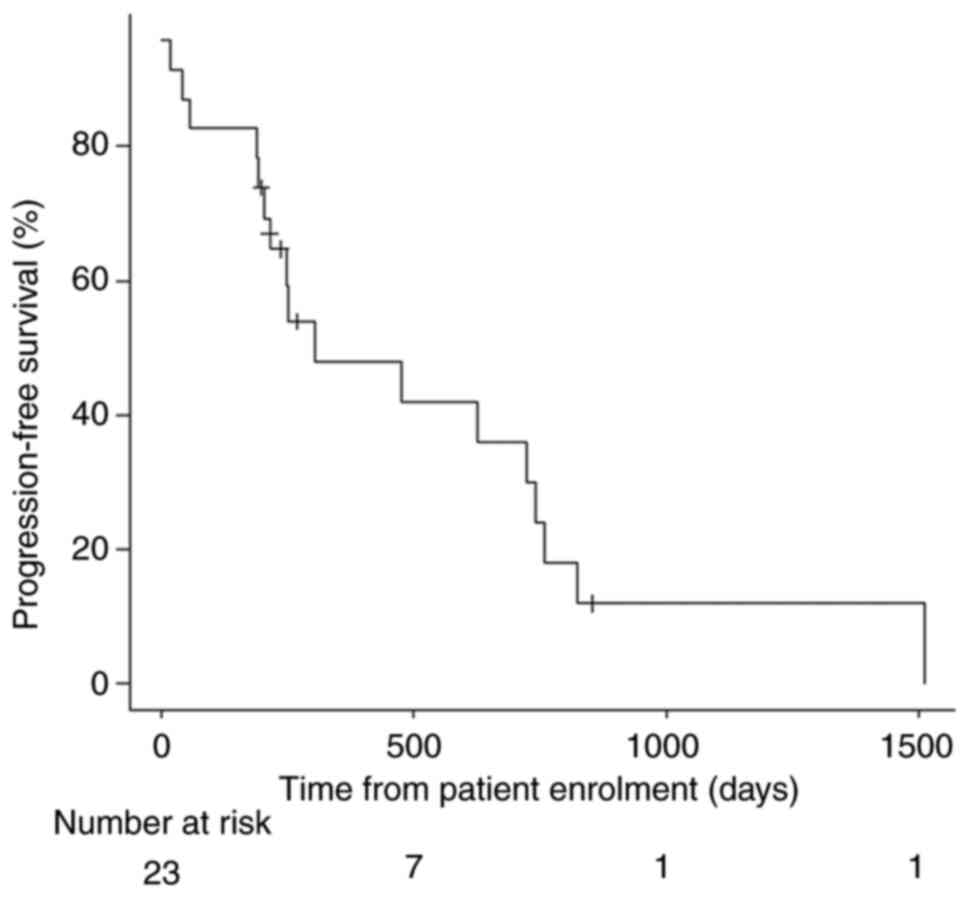
I. The median follow-up period after bevacizumab initiation was
751 days, and the median PFS time was 306 days (interquartile
range, 204-743 days) (Fig. 1). The
protocol treatment was discontinued owing to disease progression in
13 (57%) patients, toxicities unrelated to bevacizumab in 4
patients (17%; grade 1 pneumonitis, grade 2 palmar-plantar
erythrodysesthesia syndrome, grade 2 leukoencephalopathy and grade
2 malaise in 1 patient each), toxicities associated with
bevacizumab in 2 patients (9%; grade 4 small intestinal perforation
and grade 3 stroke in 1 patient each), a complete response in 2
patients (9%) and surgery in 1 (4%) patient. The treatment protocol
was continued in 1 patient (data not shown). The responses to the
protocol treatment were complete response, partial response, stable
disease and progressive disease in 2 (9%), 11 (48%), 7 (30%) and 3
(13%) patients, respectively. The overall RR and disease control
rate were 57 and 87%, respectively (Table II). No HSRs were reported in any
of the 23 patients. A total of 6 (26%) patients developed
proteinuria, of whom 3 exhibited grade 1 disease and 3 exhibited
grade 2 disease. Hypertension was observed in 12 (52%) patients, of
whom 3 (13%), 6 (26%) and 3 (13%) patients had grade 1, 2 and 3
disease, respectively. The adverse events associated with
bevacizumab included grade 4 small intestinal perforation and grade
3 arterial/venous thromboembolic event (stroke) in 1 patient each
(Table III). No
treatment-related deaths occurred during the study period. After
the failure of first-line chemotherapy, 17 patients received
second-line chemotherapy. Consequently, 11 (65%) of these patients
were treated with bevacizumab.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Value |
|---|
| Median age (range),
years | 70 (44-80) |
| Sex, n (%) | |
|
Male | 12(52) |
|
Female | 11(48) |
| Median weight
(range), kg | 57 (41-76) |
| Hypertension, n
(%) | |
|
Yes | 12(52) |
|
No | 11(48) |
| Proteinuria, n
(%) | |
|
Yes | 1(4) |
|
No | 22(96) |
| Hypersensitivity
reactions, n (%) | |
|
Yes | 3(13) |
|
No | 20(87) |
| ECOG performance
status, n (%) | |
|
0 | 13(57) |
|
1 | 10(43) |
| Primary tumor site, n
(%) | |
|
Right
colon | 8(35) |
|
Left
colon | 6(26) |
|
Rectum | 9(39) |
| Metastatic organs, n
(%) | |
|
Liver | 13(57) |
|
Lung | 11(48) |
|
Lymph
node | 4(17) |
|
Peritoneum | 4(17) |
| Number of metastatic
organs, n (%) | |
|
1 | 13(57) |
|
2 | 8(35) |
|
3 | 2(9) |
| RAS mutation status,
n (%) | |
|
Wild-type | 7(30) |
|
Mutant-type | 16(70) |
| Resection of primary
tumor, n (%) | |
|
Yes | 5(22) |
|
No | 18(78) |
| Chemotherapy, n
(%) | |
|
SOX | 11(48) |
|
CAPOX | 9(39) |
|
FOLFOX | 2(9) |
|
FOLFOXIRI | 1(4) |
 | Table IIOverall response. |
Table II
Overall response.
| Parameter | n (%) |
|---|
| Complete
response | 2(9) |
| Partial response | 11(48) |
| Stable disease | 7(30) |
| Progressive
disease | 3(13) |
| Overall response
rate | 13(57) |
| Disease control
rate | 20(87) |
 | Table IIIAdverse events. |
Table III
Adverse events.
| | Grade |
|---|
| Adverse event | 1 | 2 | 3 | 4 |
|---|
| Hypersensitivity
reaction, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Proteinuria, n
(%) | 3(13) | 3(13) | 0 (0) | 0 (0) |
| Hypertension, n
(%) | 3(13) | 6(26) | 3(13) | 0 (0) |
| Gastrointestinal
perforations, n (%) | 0 (0) | 0 (0) | 0 (0) | 1(4) |
| Arterial/venous
thromboembolic events, n (%) | 0 (0) | 0 (0) | 1(4) | 0 (0) |
| Bleeding, n
(%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Discussion
The current prospective multicenter study
investigated the safety and efficacy of short bevacizumab infusions
in patients with colorectal cancer. The findings suggested that a
shorter bevacizumab infusion was as effective as the standard
infusion schedule and did not increase the risk of HSRs or
associated severe adverse events. To the best of our knowledge,
this is the first prospective study to evaluate the efficacy of
short bevacizumab infusions. The infusion duration affects the
efficacy of certain drugs, such as 5-fluorouracil, as they have a
short half-life. The >20-day half-life of bevacizumab, which
implies that the infusion duration may not affect its efficacy,
could explain the absence of reports correlating its efficacy and
the infusion duration.
In the present study, the PFS time and RR was 306
days and 57%, respectively. Previous studies of
oxaliplatin-containing regimens combined with bevacizumab showed a
median PFS time of 9-10 months and an RR of 46-52% (1,5,6,18).
The present study appears comparable to previous studies in terms
of efficacy results. Besides HSRs, previous clinical studies have
reported adverse events such as gastrointestinal perforation
(0.3-2.0%) and stroke (0.3-5.0%) associated with bevacizumab
infusion (1,5,6,18).
In the present study, 1 patient (4%) had a grade 4 small intestinal
perforation and another (4%) had a grade 3 stroke. Therefore, the
results are comparable to those reported previously.
The incidence rates of severe proteinuria and
hypertension were similar to those in other clinical studies
(0.3-1.0% and 3.5-15%, respectively) (1,5,6,18). A
higher incidence of all grade hypertension, comparable to that
reported previously (22.4-43%) (1,5,6,18),
was observed in the present study (52%). However, the previous
studies failed to report the number of patients receiving active
antihypertensive medication. In the present study, a relatively
large number of patients (52%) had a history of hypertension.
Therefore, they were considered to have elevated blood
pressure.
Recently, bevacizumab has shown promise as an
adjunct therapy that enhances the effects of immune checkpoint
inhibitors in the management of non-small cell lung cancer
(IMpower150 study) and renal cell cancer (IMmotion150 study)
(19,20). In the field of colorectal cancer,
phase II and III studies of bevacizumab plus immune checkpoint
inhibitors plus cytotoxic chemotherapy have reported promising
results (21-23).
In future, the use of chemotherapy with bevacizumab is expected to
increase, resulting in more infusions, and making short bevacizumab
infusions increasingly important to reduce the infusion rate
waiting times and increase patient convenience. Therefore, short
bevacizumab infusions are meaningful.
The present study has several limitations. First,
this was a single-arm study, not a randomized controlled trial, and
had a small sample size. Therefore, the conclusions of this study
cannot be applied to clinical practice. However, being a
prospective evaluation of the safety and efficacy of the approach
in a multicenter setting, this study reflects real-world clinical
practice. Second, PFS was used as the primary endpoint. In general,
overall survival (OS) is used to show efficacy in clinical trials
and PFS is used as a surrogate endpoint for OS in patients with
metastatic colorectal cancer. However, when the sample size is
small, PFS has been reported to be inadequate as a surrogate
endpoint of OS, which also affects effectiveness. Thus, there is a
likelihood of bias pertaining to PFS due to other confounding
factors (24). Finally,
anti-epidermal growth factor receptor inhibitors plus cytotoxic
chemotherapy are used as first-line treatment for RAS wild-type
left-sided colorectal cancer, as they are more effective than
anti-VEGF inhibitors (25). The
present study was conducted before this evidence was established.
In the present study, 5 patients (22%) had RAS wild-type left-sided
colorectal cancer. Additionally, BRAF mutation status was not
evaluated in this study, as it was not required at that time.
In conclusion, the present results suggest that a
short bevacizumab infusion involving an initial infusion for 30 min
followed by infusion at a rate of 0.5 mg/kg/min is safe and
efficacious. Short bevacizumab infusions are expected to improve
the patient's satisfaction and the tolerability, and reduce the
burden on healthcare providers. As the study sample size was too
small, further studies with larger sample sizes are required to
validate the safety and efficacy of short bevacizumab infusions in
patients with colorectal cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KT, AK and AN were responsible for the
conceptualization of the study. KT, KA, SO, HM and TI performed the
formal analysis. KT performed the investigation. Data curation was
provided by KT, KA, SO, HM, TI, AK and AN. YNad, MO, SF, KO, SH,
FT, NK were involved in the data analysis. KT wrote the original
draft. KT, YNad, MO, SF, KO, SH, FT, NK, YNag and YF reviewed and
edited the manuscript. KT, YNag and YF supervised the study. KT, FT
and NK were responsible for project administration. FT, NK, YNag
and YF confirm the authenticity of all the raw data. All authors
have read and agreed to the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
the Osaka City University Graduate School of Medicine (Osaka,
Japan; protocol no. 3666). Written informed consent was obtained
from all patients involved in the study.
Patient consent for publication
Written informed consent for publication was
obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Ziger A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkier T, Cella D and Davidson NE:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dillman RO: Infusion reactions associated
with the therapeutic use of monoclonal antibodies in the treatment
of malignancy. Cancer Metastasis Rev. 18:465–471. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kabbinavar FF, Schulz J, McCleod M, Patel
T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B and Novotny WF:
Addition of bevacizumab to bolus fluorouracil and leucovorin in
first-line metastatic colorectal cancer: Results of a randomized
phase II trial. J Clin Oncol. 23:3697–3705. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
National Comprehensive Cancer Network
(NCCN): NCCN Guidelines Central Nervous System: Cancers. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
Accessed July 13, 2022.
|
|
8
|
National Comprehensive Cancer Network
(NCCN): NCCN Guidelines Central Nervous System: Cancers. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
Accessed July 13, 2022.
|
|
9
|
Shah SR, Gressett Ussery SM, Dowell JE,
Marley E, Liticker J, Arriaga Y and Verma U: Shorter bevacizumab
infusions do not increase the incidence of proteinuria and
hypertension. Ann Oncol. 24:960–965. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Terazawa T, Nishitani H, Kato K, Hashimoto
H, Akiyoshi K, Iwasa S, Nakajima TE, Hamaguchi T, Yamada Y and
Shimada Y: The feasibility of a short bevacizumab infusion in
patients with metastatic colorectal cancer. Anticancer Res.
34:1053–1056. 2014.PubMed/NCBI
|
|
11
|
Makris G, Kantzioura A, Beredima M,
Karampola M and Emmanouilides C: Feasibility of rapid infusion of
the initial dose of bevacizumab in patients with cancer. J BUON.
20:923–927. 2015.PubMed/NCBI
|
|
12
|
Yanmaz MT, Guner SI, Satılmıs B, Akyol H
and Aydın MA: Thirty-minutes infusion rate is safe enough for
bevacizumab; no need for initial prolong infusion. Med Oncol.
31(276)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Reidy DL, Chung KY, Timoney JP, Park VJ,
Hollywood E, Sklarin NT, Muller RJ and Saltz LB: Bevacizumab 5
mg/kg can be infused safely over 10 minutes. J Clin Oncol.
25:2691–2695. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mir O, Alexandre J, Coriat R, Ropert S,
Boudou-Rouquette P, Bui T, Chapron J, Durand JP, Dusser D and
Goldwasser F: Safety of bevacizumab 7.5 mg/kg infusion over 10
minutes in NSCLC patients. Invest New Drugs. 30:1756–1760.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
National Comprehensive Cancer Network
(NCCN): NCCN Guidelines Central Nervous System: Cancers. https://www.nccn.org/professionals/physicio_gls/pdf/colon.pdf.
Accessed July 13, 2022.
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
National Cancer Institute. Common
Terminology Criteria for Adverse Events (CTCAE) v4.0 https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fctep.cancer.gov%2Fprotocoldevelopment%2Felectronic_applications%2Fdocs%2FCTCAE_4.03.xlsx&wdOrigin=BROWSELINK.
|
|
18
|
Yamazaki K, Nagase M, Tamagawa H, Ueda S,
Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T,
et al: Randomized phase III study of bevacizumab plus FOLFIRI and
bevacizumab plus mFOLFOX6 as first-line treatment for patients with
metastatic colorectal cancer (WJOG4407G). Ann Oncol. 27:1539–1546.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McDermott DF, Huseni MA, Atkins MB, Motzer
RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, et
al: Clinical activity and molecular correlates of response to
atezolizumab alone or in combination with bevacizumab versus
sunitinib in renal cell carcinoma. Nat Med. 24:749–757.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Antoniotti C, Borelli B, Rossini D,
Pietrantonio F, Morano F, Salvatore L, Lonardi S, Marmorino F,
Tamberi S, Corallo S, et al: AtezoTRIBE: A randomised phase II
study of FOLFOXIRI plus bevacizumab alone or in combination with
atezolizumab as initial therapy for patients with unresectable
metastatic colorectal cancer. BMC Cancer. 20(683)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Damato A, Bergamo F, Antonuzzo L, Nasti G,
Iachetta F, Romagnani A, Gervasi E, Larocca M and Pinto C:
FOLFOXIRI/Bevacizumab plus nivolumab as first-line treatment in
metastatic colorectal cancer RAS/BRAF mutated: Safety run-in of
phase II NIVACOR trial. Front Oncol. 11(766500)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mettu NB, Ou FS, Zemla TJ, Halfdanarson
TR, Lenz HJ, Breakstone RA, Boland PM, Crysler OV, Wu C, Nixon AB,
et al: Assessment of capecitabine and bevacizumab with or without
atezolizumab for the treatment of refractory metastatic colorectal
cancer: A randomized clinical trial. JAMA Netw Open.
5(e2149040)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tang PA, Bentzen SM, Chen EX and Siu LL:
Surrogate end points for median overall survival in metastatic
colorectal cancer: Literature-based analysis from 39 randomized
controlled trials of first-line chemotherapy. J Clin Oncol.
25:4562–4568. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aljehani MA, Morgan JW, Guthrie LA, Jabo
B, Ramadan M, Bahjri K, Lum SS, Selleck M, Reeves ME, Garberoglio C
and Senthil M: Association of primary tumor site with mortality in
patients receiving bevacizumab and cetuximab for metastatic
colorectal cancer. JAMA Surg. 153:60–67. 2018.PubMed/NCBI View Article : Google Scholar
|