Introduction
Uterine cervical cancer has the fourth highest
incidence and the fourth highest mortality rate of all cancers,
with 604,125 new cases and 341,831 deaths in 2020 worldwide
(1). Screening and prevention of
cervical cancer have improved through human papillomavirus typing
and vaccination; however, patients with locally advanced stages
[Federation of Gynecology and Obstetrics (FIGO) stages IB2-ⅣA] are
still commonly diagnosed in clinical practice. Currently, for
locally advanced uterine cervical cancer, especially for stages
IIIB-IVA, concurrent platinum-based chemoradiotherapy (CCRT) is the
standard treatment strategy (2).
However, the 5-year relative survival rate for patients with stage
IIIB-IVB uterine cervical cancer is <60%, which is considerably
lower than that for patients with early-stage uterine cervical
cancer, for whom survival is >90% (3). Neoadjuvant chemotherapy (NAC)
followed by surgery is considered one of the treatment options for
patients with locally advanced uterine cervical cancer, even though
its value remains contested. NAC has the potential to reduce the
tumor size and allow for a hysterectomy for patients with stages
IIIB-IVA uterine cancer in which hysterectomy is normally not
possible, and this course can improve a patient's prognosis
(4-6).
However, if the NAC treatment does not effectively shrink the tumor
size, the patients instead have to undergo radiotherapy, and this
delays the initiation of the core treatment, and thus a worse
prognosis. Therefore, if the effectiveness of NAC for locally
advanced uterine cancer patients can be predicted, it may be
possible to accurately choose the optimal candidate for NAC to
improve outcomes. To this end, there is an urgent need for the
identification of biomarkers that can easily be assessed prior to
initiation of treatment, which can predict the effects of NAC for
locally advanced uterine cancer patients.
The Src family of kinases (SFKs) play crucial roles
in regulating a wide variety of cellular functions such as
proliferation, migration, invasion, differentiation, survival
angiogenesis, and motility in several types of cancers (7). Fyn, a non-receptor tyrosine kinase
that is a member of the SFKs, is a 59 kDa protein, the gene for
which is located on chromosome 6q21(8). Fyn has been reported to phosphorylate
and prevent the breakdown of PI3K enhancer-activating Akt (PIKE-A),
which is an inhibitor of apoptosis (9). The overexpression of Fyn leads to the
promotion of the antiapoptotic activity of Akt (10). Therefore, Fyn is considered an
important molecule that can confer resistance to anti-cancer agents
that exert their effects by inducing apoptosis. This fact can be
applied to the chemotherapy using platinum-based anti-cancer agents
for cervical carcinoma.
However, to the best of our knowledge, there is no
research on whether Fyn affects the outcomes of chemosensitivity to
platinum-based anti-cancer agents in cervical squamous cell
carcinoma. The present study is the first to focus on
chemosensitivity to a platinum-based anti-cancer agent in cervical
squamous cell carcinoma based on Fyn expression, and the results
revealed the value of Fyn expression as a biomarker in predicting
the efficacy of NAC in patients with locally advanced uterine
cervical squamous carcinoma.
Materials and methods
Patients
The present study is a retrospective study in which
a total of 44 patients were enrolled. The inclusion criteria were
as follows: i) Patients diagnosed with FIGO Stage IIIB (FIGO 2008)
uterine cervical cancer whose cancer was pathologically confirmed
by punch biopsy specimens before the initiation of treatment; ii)
patients who underwent NAC using cisplatin at the Osaka City
University Hospital (Osaka, Japan) between April 1996 and April
2010; iii) aged 20-70 years old; and iv) the medical records were
available to analyze. Patients whose medical records were deemed
inadequate for analysis were excluded. Information on the clinical
factors such as FIGO stage, age, the effect of NAC, body mass
index, serum squamous cell carcinoma (SCC) antigen value, and tumor
size were collected before the initiation of treatment.
To compare which factors contributed to NAC
efficacy, the patients were grouped depending on the efficacy of
NAC into two groups: A NAC success group and a NAC failure group.
NAC success was defined as a decrease in tumor size to that
expected in stage I or II, and for whom a hysterectomy was made
possible, and as failure when NAC failed to decrease the tumor size
and a hysterectomy was not made possible, and thus radiation
therapy had to be performed. NAC was performed using cisplatin. The
total amount of cisplatin (Bristol Myers Squibb) administered was
50, 75, or 100 mg/m2 (dependent on the renal function).
Cisplatin was injected intra-arterially using balloon-occluded
arterial infusion over 30 min three times every 4 weeks (4). In the NAC success group, a
hysterectomy and consecutive radiation therapy were performed after
NAC and in the NAC failure group, radiation therapy alone was
performed after NAC.
All patients enrolled in this study provided written
informed consent for the treatment and the use of their samples in
this research prior to the initiation of NAC and the Institutional
Review Board of Osaka City University Hospital approved this study
(approval no. 2019-01).
Immunohistochemical (IHC) staining and
scoring
For IHC, 4 µm thick sections generated from the
paraffin-embedded tissue blocks were used. Before IHC staining,
tissues were deparaffinized and the endogenous peroxidase activity
of the samples was quenched using 3% hydrogen peroxide in methanol,
after which antigen retrieval was performed by immersing the
samples in Target Retrieval Solution, pH 9.0 (cat. no. S2367;
Agilent Technologies, Inc.) and heating in an autoclave at 121˚C
for 20 min. The DAKO LSAB2 Peroxidase kit (cat. no. K0675; Agilent
Technologies, Inc.) was used for IHC according to the
manufacturer's protocol. A rabbit monoclonal anti-Fyn antibody
(cat. no. ab184276; 1:250 dilution) was used as the primary
antibody at 4˚C overnight and biotinylated goat immunoglobulin G
antibodies included in the DAKO LSAB2 Peroxidase kit were used as
secondary antibodies at room temperature for 10 min. The slides
were immersed in DAB solution to develop the stain at room
temperature for 10 min and were counterstained with hematoxylin and
0.3% ammonia water at room temperature for 1 min.
Fyn expression was quantified using an established
weighted scoring method (11). In
this method, two independent factors were employed to generate the
score, multiplying the score of the stained tumor cell percentage
and the score of the staining intensity to obtain a weighted score
for each sample. The score of the stained tumor cells was based on
the average percentage of stained tumor cells and assigned as
follows, 0 (<5%), 1 (5-25%), 2 (25-50%), 3 (50-75%), 4
(>75%). The score of the staining intensity was based on the
intensity of staining and assigned as follows, 1 (weak), 2
(moderate), and 3 (intense).
Cell culture
CaSki cells (human papillomavirus-related cervical
squamous cell carcinoma, cat. no. IFO50007) were incubated in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS and 1% penicillin in a humidified
incubator at 37˚C with 5% CO2.
Fyn knockdown and cell survival
assays
Fyn siRNA transfections were performed using
Lipofectamine® RNAiMax (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A
Fyn-specific siRNA (Fyn siRNA: cat. no. sc-29321; Santa Cruz
Biotechnology, Inc.) or control siRNA (control siRNA-A: cat. no.
sc-37007; Santa Cruz Biotechnology, Inc.) were used. The sense
sequence of Fyn siRNA is CAUCGAGCGCAUGAAUUAU, and the antisense
sequence was AUAAUUCAUGCGCUCGAUG which are provided in 5'→
3'orientation. The sequence of control siRNA is confidential. CaSki
cells were incubated in 96-well plates (2x103
cells/well) and divided into two groups: A treated group, in which
Fyn siRNA transfection was performed, and a control group, in which
control siRNA was transfected. After cell adhesion, in the treated
group, cells were incubated with fresh medium containing Fyn siRNA
transfection complexes, and in the control group, cells were
incubated with fresh medium containing control siRNA at 37˚C for 24
h. Next, the cells in both groups were incubated for 24 h at 37˚C
in fresh medium containing 10, 25, or 50 µM cisplatin. To determine
cell viability, 10 µl Cell Counting Kit-8 solution (Dojindo
Molecular Technologies, Inc.) and 100 µl RPMI-1640 medium were
added to each well of both groups, and the cells were incubated at
37˚C for 2 h. The absorbance of each well was measured at a
wavelength of 450 nm using a microplate reader (Corona Electric
Co., Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed to verify the successful
knockdown of Fyn mRNA expression after transfection of Fyn siRNA.
TaqMan chemistry was used to perform RT-qPCR according to the
manufacturer's protocol with TaqMan primer and probes for Fyn (cat.
no. Hs00941613_m1) and hypoxanthine phosphoribosyl-transferase 1
(cat. no. Hs02800695_ml, Thermo Fisher Scientific, Inc.) which was
used as an internal control (12).
First, total RNA was extracted from cells using a RNeasy Mini kit
(Qiagen GmbH). Next, total RNA (1 µg) was reverse transcribed into
cDNA using a High-Capacity cDNA Reverse Transcription kit (Thermo
Fisher Scientific, Inc.). Finally, qPCR was performed using TaqMan
Fast Universal PCR MasterMix (Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 20 sec; followed by 40 cycles at 95˚C for
3 sec and 60˚C for 30 sec. All procedures were performed in
accordance with the manufacturer's protocol. The relative changes
in gene expression were calculated using the 2-∆∆Cq
method (13).
Statistical analysis
Values are expressed as the mean ± standard
deviation. A Fisher's exact test was used for determining the
association between categorical variables in the two different
groups, and a Mann-Whitney U-test was used for comparing the median
and mean values between the two different groups. A Receiver
Operating Characteristic (ROC) curve was generated to determine the
cutoff value of the Fyn score to predict the effect of NAC
treatment. The Kaplan-Meier method and log-rank tests were used to
compare the survival between the two different groups. Three
replicants were performed in RT-qPCR, and ten replicates were
performed in cell survival assays. P<0.05 was considered to
indicate a statistically significant difference. GraphPad Prism
Version 8 (GraphPad Software, Inc.) was used for all statistical
analyses.
Results
Patient characteristics and overall
survival
There were 23 patients in the NAC success group and
21 patients in the NAC failure group. Comparison of the patients'
characteristics between the two groups showed no significant
differences in age, BMI, serum SCC value, and tumor size before NAC
(Table I). Tumor size after NAC
was significantly larger in the NAC failure group than in the NAC
successful group (P<0.001; Table
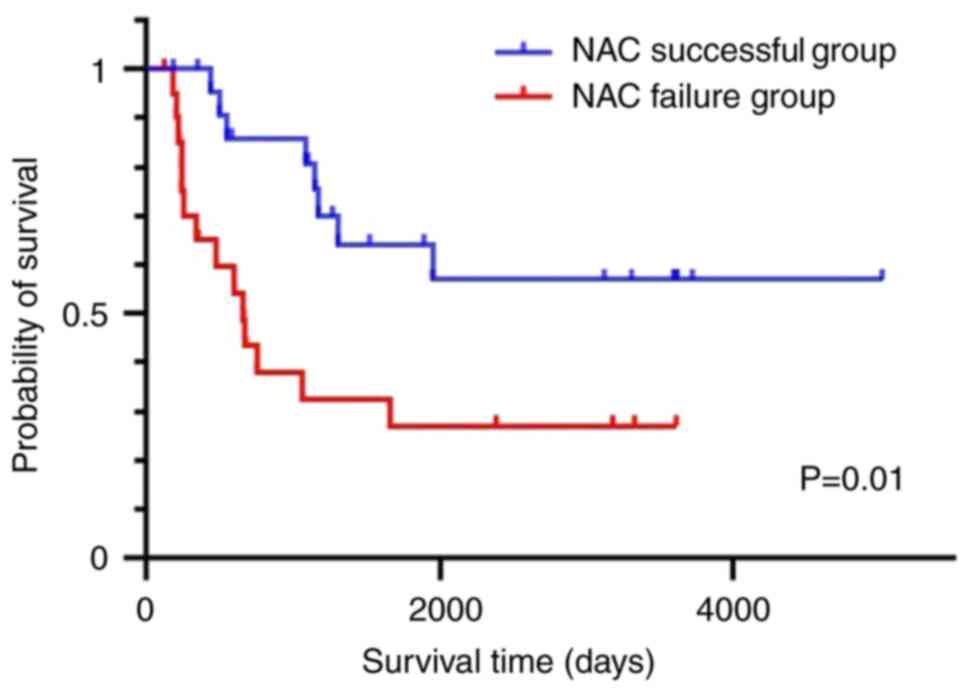
I). Regarding the overall survival, the NAC success group had
significantly better overall survival than the NAC failure group
(P=0.01; Fig. 1).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | NAC successful
group | NAC failure
group | P-value |
|---|
| n | 23 | 21 | |
| Age,
yearsa | 47 (24-59) | 55 (37-68) |
0.241b |
| BMIa | 21.7
(14.6-29.6) | 21.3
(12.7-27.1) | 0.891b |
| SCC antigen,
ng/mla | 8.45
(0.7-187.0) | 11.1
(1.6-49.3) | 0.539b |
| Tumor size before
NAC, mma | 43.5 (25-80) | 48.0 (35-78) | 0.257b |
| Tumor size after
NAC, mma | 0 (0-45) | 35.5 (5-51) |
<0.001b |
Fyn expression and Fyn cutoff values
for predicting NAC efficacy
The expression of Fyn was compared between the two
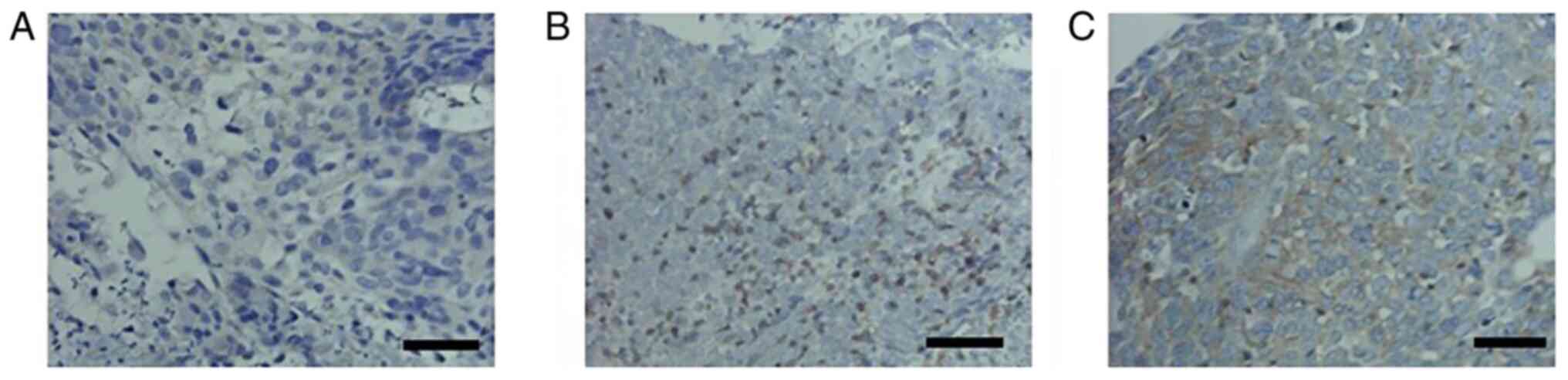
groups using IHC. Fyn protein expression was observed primarily at
the cell membrane and in the cytoplasm (Fig. 2). The difference in expression was
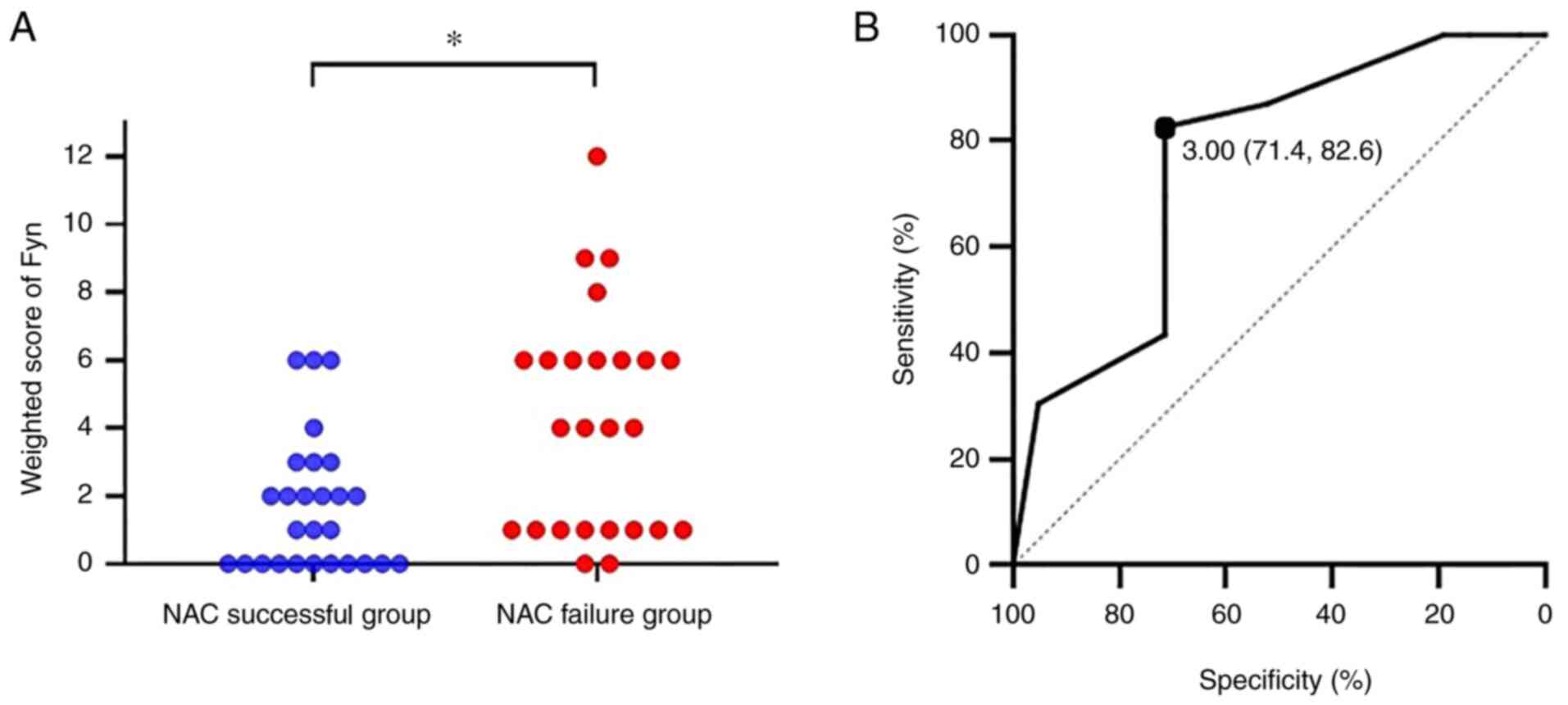
evaluated using weighted scores, and the results showed that the
expression in the NAC failure group was significantly higher than
in the NAC success group (P=0.003; Fig. 3A). Next, we evaluated the cutoff
value of the weighted score to predict the efficacy of NAC using a
ROC curve. The ROC curve showed that a cutoff value of 3 predicted
the NAC efficacy with a sensitivity of 82.6% and specificity of
71.4%, with an area under the curve of 0.759 and a 95% confidence
interval of 0.613-0.905 (Fig.
3B).
Contribution of Fyn expression to NAC
efficacy
Based on the cut off value of 3, patients were
divided into two groups, a low expression group (n=25) in which the
weighted score was ≤3 and a high expression group (n=19) in which
the weighted score ≥4. The rate of NAC success between the two
groups was compared. In the low expression group, the success rate
was 76% and the failure rate was 24%, in the high expression group,
the success rate was 21.1% and the failure rate was 78.9% (Table II). The success rate in the low
expression group was significantly higher than that in the high
expression group (P<0.001), which was evaluated with the
Fisher's exact test determining the association of categories in
two group variables (NAC successful group and NAC failure group).
This indicated that high expression of Fyn contributed to reduced
sensitivity to NAC in local advanced uterine cervical cancer
patients.
 | Table IIAssociation between Fyn expression
and NAC efficacy. |
Table II
Association between Fyn expression
and NAC efficacy.
| Fyn expression | NAC successful
group | NAC failure
group | P-value |
|---|
| Low expression, n
(%) | 19(76) | 6(24) |
<0.001a |
| High expression, n
(%) | 4 (21.1) | 15 (78.9) | |
Contribution of Fyn knockdown to the
cisplatin sensitivity of cervical cancer cells
Next, the effect of Fyn knockdown on cisplatin
sensitivity was evaluated in vitro using human cervical
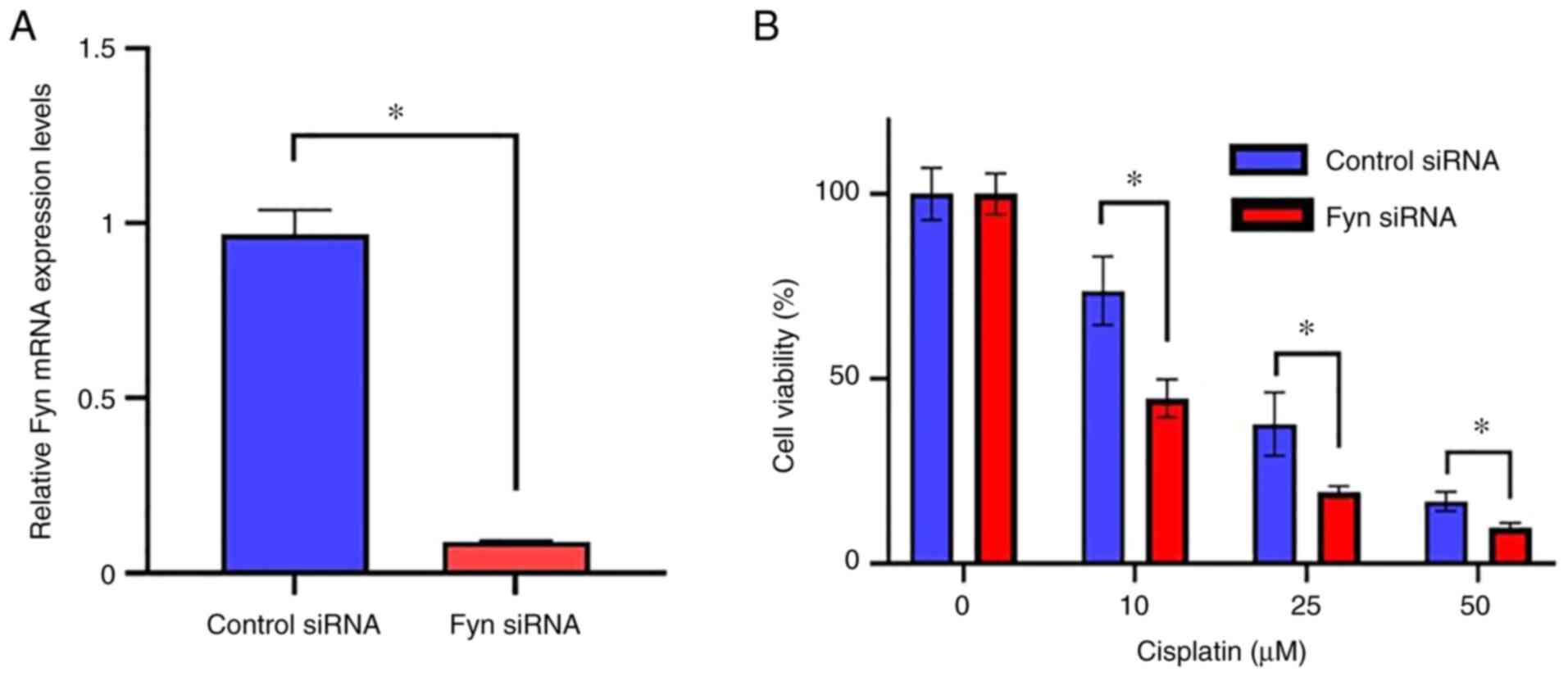
cancer cells. Fyn expression was knocked down by transfection of
si-Fyn. The downregulation of Fyn mRNA expression was confirmed by
RT-qPCR. As shown in Fig. 4A, Fyn
mRNA expression was significantly suppressed in the treated cells
compared with the control cells transfected with the control siRNA
(P<0.05). After confirmation of knockdown of Fyn, the effect of
Fyn knockdown on the sensitivity to cisplatin in the uterine
cervical cancer cells was determined. The cell viability of cells
in which Fyn was knocked down was significantly lower than in the
cells transfected with the control siRNA when treated with 10, 25,
or 50 µM cisplatin (Fig. 4B).
These results indicate that Fyn knockdown contributed to the
enhancement of cisplatin sensitivity on uterine cervical cancer
cells.
Discussion
NAC followed by surgery is considered the standard
treatment option for patients with locally advanced uterine
cervical cancer, and there are numerous studies revealing the
effectiveness of NAC for patients with locally advanced uterine
cervical cancer. Nguyen et al (5) performed a meta-analysis that showed
that dose-intense cisplatin-based NAC followed by surgery increases
survival in stage IB2-IVA uterine cervical cancer patients. Mori
et al (14) reported that
NAC using paclitaxel and carboplatin followed by surgery was a
promising mode of therapy for stage IB2-IIIB uterine cervical
cancer patients with a good probability of improving the prognosis.
Shoji et al (15) reported
that NAC using cisplatin and irinotecan followed by surgery was a
useful therapeutic strategy for management of stage IB2-IIIB
uterine cervical cancer with a good probability of improving the
prognosis. Finally, Sala et al (6) demonstrated that NAC followed by
surgery improved survival outcomes for stage IB2-IVA uterine
cervical cancer patients, and suggested that NAC followed by
surgery was an effective alternative treatment option to CCRT
standard treatment strategy.
Currently, for locally advanced uterine cervical
cancer, especially for patients with stage IIIB-IVA cancer, CCRT is
the standard treatment strategy (2); however, the prognostic outcomes of
these patients have remained poor (3). Therefore, there is an urgent need to
establish an effective treatment strategy for these patients. One
candidate strategy to improve the prognosis is NAC followed by
surgery. However, if NAC is not effective, surgery cannot be
performed and the only treatment option available is radiotherapy,
which may result in unfavorable treatment outcomes due to the delay
before the initiation of the core treatment. Therefore, if the
efficacy of NAC can be predicted in advance, the patients who will
benefit from NAC effectively can be selected before the initiation
of treatment. To this end, it is crucial to identify biomarkers
that can be used in predicting NAC efficacy.
Tyrosine kinases can be divided into two groups,
receptor tyrosine kinases, and non-receptor tyrosine kinases.
Receptor tyrosine kinases include vascular endothelial growth
factor receptor (VEGFR), epidermal growth factor receptor (EGFR),
and mesenchymal-epithelial transition factor, and they receive
signals via soluble ligands. Non-receptor tyrosine kinases include
families such as Src, Abl, focal adhesion kinase, and the Janus
kinase (16). Dysregulation of the
activation of these tyrosine kinases causes cancer by altering
cellular growth, function, and shape which are hallmarks of
malignancy (17). The SFKs
consists of the following members: c-Src, Fyn, Lck, Yes, Lyn, Fgr,
Blk, and Hck. Among these, Fyn, c-Src, and Yes are expressed
ubiquitously, whereas the others exhibit restricted tissue
expression (7,18,19).
Fyn is a non-receptor tyrosine kinase that is localized to the
inner side of the cell membrane; however, when activated, it is
translocated to other cellular components such as the nucleus
(20). Fyn has been reported to
exhibit a wide variety of biological functions, such as signal
transduction in the nerves thus contributing to the regulation of
brain function, signal transduction through T cell receptors, and
adhesion-mediated signal transduction under physiological
conditions. Additionally, Fyn has been reported to contribute to
the development and progression of several malignancies through
regulation of cell growth, cell death, cell motility, morphogenic
transformation, and migration, and is considered an essential
factor in the development, progression, and metastasis of cancer
(8,21). Fyn has been reported to be involved
in a wide variety of malignancies such as breast cancer, prostate
cancer, glioma, melanoma, squamous cell carcinoma of the head and
neck, chronic myeloid leukemia, cholangiocarcinoma, gastric cancer,
thyroid cancer, and esophageal squamous cell carcinoma (8,21-27).
Fyn has been reported to be involved in the receptor
tyrosine kinase (EGFR, VEGF, platelet-derived growth factor
receptor, fibroblast growth factor) pathway in cancer. Fyn
transmits signals through Ras-independent pathways (via PIK3/Akt,
STAT3, FAK, β-catenin, VAV1, paxillin, and/or SHC) and
Ras-dependent pathways (via Ras/MEK/ERK) (28). Through involvement in these
pathways, Fyn mediates the growth factor-induced anti-apoptotic
effects of Akt/PKB (8). As a
result, there are also several reports showing the correlation
between Fyn expression and sensitivity to anti-cancer agents due to
the regulation of apoptosis by Fyn. Knockdown of Fyn facilitates
doxorubicin-induced apoptosis and increases the sensitivity to
doxorubicin in doxorubicin-resistant cells (29). Additionally, the levels of Fyn
expression markedly influence the efficacy of PP2, which is an SFK
inhibitor, by inducing apoptosis (9). Furthermore, there are several reports
on the correlation between Fyn expression and sensitivity to
anti-cancer agents. Fyn was reported to be upregulated in
tamoxifen-resistant estrogen receptor-positive breast cancer cell
lines, and when Fyn expression was knocked down the estrogen
receptor-positive breast cancer cell lines became sensitive to
tamoxifen (30). Upregulated
expression of Fyn has been reported to be involved in resistance to
imatinib in chronic myeloid leukemia (31). Fyn upregulation has also been
reported to exert negative effects on chemosensitivity to
gemcitabine in pancreatic ductal adenocarcinoma via regulation of
miR-125a-3p (32). Fyn is
considered an important molecule that can confer resistance to
anti-cancer agents, and it exerts its effect predominantly by
inducing apoptosis (9,29).
The antitumor effects of platinum-based anti-tumor
drugs including cisplatin are achieved by covalently binding to the
DNA of cancer cells (33). There
are several mechanisms that contribute to the resistance of
platinum-based anti-tumor drugs such as inactivation of apoptotic
signaling pathways (34,35), enhanced DNA damage repair capacity
(36,37), increased cisplatin detoxification
(38), decreased cellular uptake
of cisplatin (39,40), and other epigenetic modifications
that occur at the molecular and cellular levels (41,42).
Thus far, several biomarkers that exhibit potential
for predicting the efficacy of NAC to uterine cervical cancer
patients, such as uncoupling protein 2, protein arginine
methyltransferase, and T-box 2, have been identified (43-45).
In the current study, high Fyn expression was shown to be
negatively associated with the effectiveness of NAC using cisplatin
for locally advanced uterine cervical cancer, and knockdown of Fyn
increased the sensitivity to cisplatin in uterine cervical cancer
cells in vitro. It is hypothesized that the accumulation of
these findings will allow for a more accurate prediction of the
effects of NAC treatment. If the efficacy of NAC can be predicted
using biopsy specimens prior to the initiation of treatment, the
optimal treatment strategy can be predicted for patients. Taking
pathological samples from the uterine cervix via punch biopsy is a
routine procedure in the clinic, thus there is no need for further
invasive procedures for the patients.
To the best of our knowledge, this study is the
first to show the association between Fyn expression and the
efficacy of NAC for patients with locally advanced uterine cervical
squamous cell carcinoma. In the present study, Fyn was shown to
contribute to the prognosis of patients; however, as this study is
a retrospective study with a relatively small number of cases from
a single institute, further larger prospective studies with
patients from multiple institutes, ideally from several different
countries are required to confirm the results presented here.
In conclusion, this study showed that Fyn expression
may be a potentially useful predictive biomarker of the response to
NAC for patients with locally advanced uterine cervical squamous
cell carcinoma that is easy to evaluate using biopsy specimens. The
results also suggest that Fyn may be a promising molecular target
for the management of uterine cancer.
Acknowledgements
The authors would like to thank Dr Yukimi Kira
(Research Support Platform of Osaka Metropolitan University
Graduate School of Medicine; Osaka Japan) for their technical
support.
Funding
Funding: The present study was funded by The Osaka Medical
Research Foundation for Intractable Diseases (grant no.
27-2-4).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SN, TF and TS designed the study. SN, TN, EU, YA, KI
and MY performed the experiments and collected the data. SN, TF, TY
and TS analyzed the data. SN and TF wrote the manuscript. SN and TF
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
Institutional Review Board of Osaka City University Hospital
(approval no. 2019-01; Osaka Japan). Written informed consent was
obtained from all patients prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gennigens C, De Cuypere M, Hermesse J,
Kridelka F and Jerusalem G: Optimal treatment in locally advanced
cervical cancer. Expert Rev Anticancer Ther. 21:657–671.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Kawamura N, Ogita S, Kamino T, Nakamura K and Yamada R:
Balloon-occluded arterial infusion chemotherapy, simple total
hysterectomy, and radiotherapy as a useful combination-therapy for
advanced cancer of the uterine cervix. Oncol Rep. 7:141–144.
2000.PubMed/NCBI
|
|
5
|
Nguyen VT, Winterman S, Playe M, Benbara
A, Zelek L, Pamoukdjian F and Bousquet G: Dose-intense
cisplatin-based neoadjuvant chemotherapy increases survival in
advanced cervical cancer: An up-to-date meta-analysis. Cancers
(Basel). 14(842)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sala P, Bogliolo S, Barra F, Fazio A,
Maramai M, Cassani C, Gardella B, Babilonti L, Giannelli F,
Mammoliti S, et al: Neoadjuvant chemotherapy followed by radical
surgery versus concurrent chemo-radiotherapy in the treatment of
locally advanced cervical cancer: A multicenter retrospective
analysis. J Invest Surg. 35:308–314. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yeatman TJ: A renaissance for SRC. Nat Rev
Cancer. 4:470–480. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Elias D and Ditzel HJ: Fyn is an important
molecule in cancer pathogenesis and drug resistance. Pharmacol Res.
100:250–254. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Noronha G, Barrett K, Boccia A, Brodhag T,
Cao J, Chow CP, Dneprovskaia E, Doukas J, Fine R, Gong X, et al:
Discovery of [7-(2,6-dichlorophenyl)-5-methylbenzo
[1,2,4]triazin-3-yl]-[4-(2-pyrrolidin-1-ylethoxy)phenyl]amine-a
potent, orally active Src kinase inhibitor with anti-tumor activity
in preclinical assays. Bioorg Med Chem Lett. 17:602–608.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fresno Vara JA, Cáceres MA, Silva A and
Martín-Pérez J: Src family kinases are required for prolactin
induction of cell proliferation. Mol Biol Cell. 12:2171–2183.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
12
|
Valadan R, Hedayatizadeh-Omran A,
Alhosseini-Abyazani MN, Amjadi O, Rafiei A, Tehrani M and
Alizadeh-Navaei R: Data supporting the design and evaluation of a
universal primer pair for pseudogene-free amplification of HPRT1 in
real-time PCR. Data Brief. 4:384–389. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mori T, Hosokawa K, Sawada M, Kuroboshi H,
Tatsumi H, Koshiba H, Okubo T and Kitawaki J: Neoadjuvant weekly
carboplatin and paclitaxel followed by radical hysterectomy for
locally advanced cervical cancer: Long-term results. Int J Gynecol
Cancer. 20:611–616. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shoji T, Takatori E, Furutake Y, Takada A,
Nagasawa T, Omi H, Kagabu M, Honda T, Miura F, Takeuchi S, et al:
Phase II clinical study of neoadjuvant chemotherapy with
CDDP/CPT-11 regimen in combination with radical hysterectomy for
cervical cancer with a bulky mass. Int J Clin Oncol. 21:1120–1127.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Boggon TJ and Eck MJ: Structure and
regulation of Src family kinases. Oncogene. 23:7918–7927.
2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vlahovic G and Crawford J: Activation of
tyrosine kinases in cancer. Oncologist. 8:531–538. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Frame MC: Src in cancer: Deregulation and
consequences for cell behaviour. Biochim Biophys Acta.
1602:114–130. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Thomas SM and Brugge JS: Cellular
functions regulated by Src family kinases. Annu Rev Cell Dev Biol.
13:513–609. 1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Campbell EJ, McDuff E, Tatarov O, Tovey S,
Brunton V, Cooke TG and Edwards J: Phosphorylated c-Src in the
nucleus is associated with improved patient outcome in ER-positive
breast cancer. Br J Cancer. 99:1769–1774. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Saito YD, Jensen AR, Salgia R and Posadas
EM: Fyn: A novel molecular target in cancer. Cancer. 116:1629–1637.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang C, Zhou J, Nie Y, Guo G, Wang A and
Zhu X: A new finding in the key prognosis-related proto-oncogene
FYN in hepatocellular carcinoma based on the WGCNA hub-gene
screening trategy. BMC Cancer. 22(380)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jiang P, Li Z, Tian F, Li X and Yang J:
Fyn/heterogeneous nuclear ribonucleoprotein E1 signaling regulates
pancreatic cancer metastasis by affecting the alternative splicing
of integrin β1. Int J Oncol. 51:169–183. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lyu SC, Han DD, Li XL, Ma J, Wu Q, Dong
HM, Bai C and He Q: Fyn knockdown inhibits migration and invasion
in cholangiocarcinoma through the activated AMPK/mTOR signaling
pathway. Oncol Lett. 15:2085–2090. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang X, Huang Z, Guo Y, Xiao T, Tang L,
Zhao S, Wu L, Su J, Zeng W, Huang H, et al: The phosphorylation of
CD147 by Fyn plays a critical role for melanoma cells growth and
metastasis. Oncogene. 39:4183–4197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu J, Zhou Z, Wei Z, Wu J, OuYang J, Huang
W, He Y and Zhang C: FYN promotes gastric cancer metastasis by
activating STAT3-mediated epithelial-mesenchymal transition. Transl
Oncol. 13(100841)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu D, Gao M, Wu K, Zhu D, Yang Y and Zhao
S: LINC00152 facilitates tumorigenesis in esophageal squamous cell
carcinoma via miR-153-3p/FYN axis. Biomed Pharmacother.
112(108654)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Comba A, Dunn PJ, Argento AE, Kadiyala P,
Ventosa M, Patel P, Zamler DB, Núñez FJ, Zhao L, Castro MG and
Lowenstein PR: Fyn tyrosine kinase, a downstream target of receptor
tyrosine kinases, modulates antiglioma immune responses. Neuro
Oncol. 22:806–818. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mi H, Wang X, Wang F, Li L, Zhu M, Wang N,
Xiong Y and Gu Y: miR-381 induces sensitivity of breast cancer
cells to doxorubicin by inactivation of MAPK signaling via FYN. Eur
J Pharmacol. 839:66–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Elias D, Vever H, Lænkholm AV, Gjerstorff
MF, Yde CW, Lykkesfeldt AE and Ditzel HJ: Gene expression profiling
identifies FYN as an important molecule in tamoxifen resistance and
a predictor of early recurrence in patients treated with endocrine
therapy. Oncogene. 34:1919–1927. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Grosso S, Puissant A, Dufies M, Colosetti
P, Jacquel A, Lebrigand K, Barbry P, Deckert M, Cassuto JP, Mari B
and Auberger P: Gene expression profiling of imatinib and
PD166326-resistant CML cell lines identifies Fyn as a gene
associated with resistance to BCR-ABL inhibitors. Mol Cancer Ther.
8:1924–1933. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu G, Ji L, Ke M, Ou Z, Tang N and Li Y:
miR-125a-3p is responsible for chemosensitivity in PDAC by
inhibiting epithelial-mesenchymal transition via Fyn. Biomed
Pharmacother. 106:523–531. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bose RN: Biomolecular targets for platinum
antitumor drugs. Mini Rev Med Chem. 2:103–111. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Q, Shi S, He W, Padilla MT, Zhang L,
Wang X, Zhang B and Lin Y: Retaining MKP1 expression and
attenuating JNK-mediated apoptosis by RIP1 for cisplatin resistance
through miR-940 inhibition. Oncotarget. 5:1304–1314.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: The role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu RY, Dong Z, Liu J, Yin JY, Zhou L, Wu
X, Yang Y, Mo W, Huang W, Khoo SK, et al: Role of eIF3a in
regulating cisplatin sensitivity and in translational control of
nucleotide excision repair of nasopharyngeal carcinoma. Oncogene.
30:4814–4823. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Surowiak P, Materna V, Kaplenko I,
Spaczyński M, Dietel M, Lage H and Zabel M: Augmented expression of
metallothionein and glutathione S-transferase pi as unfavourable
prognostic factors in cisplatin-treated ovarian cancer patients.
Virchows Arch. 447:626–633. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Morimoto A, Serada S, Enomoto T, Kim A,
Matsuzaki S, Takahashi T, Ueda Y, Yoshino K, Fujita M, Fujimoto M,
et al: Annexin A4 induces platinum resistance in a chloride-and
calcium-dependent manner. Oncotarget. 5:7776–7787. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu RY, Dong Z, Liu J, Zhou L, Huang W,
Khoo SK, Zhang Z, Petillo D, The BT, Qian CN and Zhang JT:
Overexpression of asparagine synthetase and matrix
metalloproteinase 19 confers cisplatin sensitivity in
nasopharyngeal carcinoma cells. Mol Cancer Ther. 12:2157–2166.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Imai K, Fukuda T, Wada T, Kawanishi M,
Tasaka R, Yasui T and Sumi T: UCP2 expression may represent a
predictive marker of neoadjuvant chemotherapy effectiveness for
locally advanced uterine cervical cancer. Oncol Lett. 14:951–957.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shimomura M, Fukuda T, Awazu Y, Nanno S,
Inoue Y, Matsubara H, Yamauchi M, Yasui T and Sumi T: PRMT1
expression predicts response to neoadjuvant chemotherapy for
locally advanced uterine cervical cancer. Oncol Lett.
21(150)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Inoue Y, Fukuda T, Nanno S, Awazu Y,
Shimomura M, Matsubara H, Yamauchi M, Yasui T and Sumi T: T-box 2
expression is a useful indicator of the response to neoadjuvant
chemotherapy for patients with locally advanced uterine cervical
squamous cell carcinoma. Oncol Lett. 22(755)2021.PubMed/NCBI View Article : Google Scholar
|