Introduction
In patients with advanced cancer, distant metastasis
commonly occurs in bone. A recent study estimated the incidence
rate of bone metastasis within 10 years of an advanced cancer
diagnosis to be 8.4% (1).
Radiotherapy is a well-established treatment modality to relieve
pain from bone metastasis. Many guidelines for the management of
bone metastasis recommend 8 Gy of single-fraction radiotherapy as
palliative treatment as this regimen provides pain relief
comparable to fractionated moderate-dose palliative radiotherapy
(FMRT) (2,3). However, the need for multiple rounds
of treatment was higher for patients receiving single-fraction
radiotherapy than for those receiving FMRT (4). Therefore, the FMRT regimen of 10x3 Gy
(biologically effective dose (BED) calculated using an α/β of 10 Gy
(BED10), 39.0 Gy) remains the most widely used for bone metastasis
(5). Furthermore, some facilities
use stereotactic body radiotherapy (SBRT) for the local control
(LC) of bone metastasis in patients expected to have long-term
survival (6,7).
Recently, significant progress in systemic and
supportive therapies has improved the prognosis of patients with
various advanced cancers (8,9).
Patients with bone metastases are also expected to have prolonged
prognoses. Therefore, although many guidelines have recommended 8
Gy single-fraction radiotherapy as palliative treatment for bone
metastases for patients with expected short-term survival, FMRT may
be an appropriate treatment for bone metastases for some patients
expected to have comparatively good prognosis. Thus, in this study,
to examine the usefulness of FMRT for bone metastases in
intermediate-term survivors, LC of bone metastases treated with
FMRT in patients surviving for ≥1 year (intermediate-term
survivors) was investigated.
Materials and methods
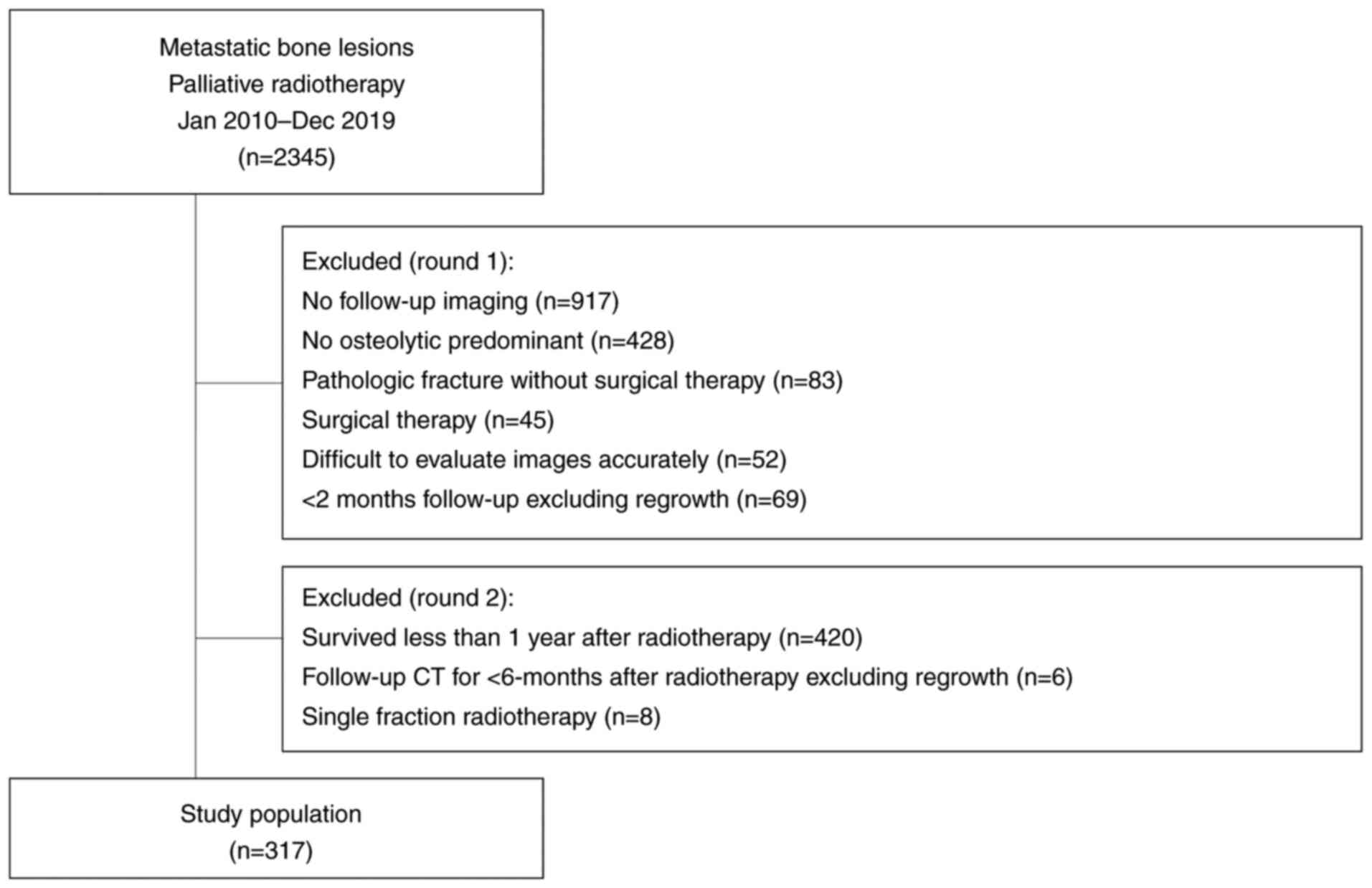
Study protocol and lesions
Majority of the eligibility criteria used in this
study have been reported previously (10). Briefly, from January 2010 to
December 2019, a total of 2,345 metastatic bone lesions in 1,750
patients were treated with palliative radiotherapy using
three-dimensional conformal radiotherapy in three institutions: a
cancer center (n=1514), a university hospital (n=594), and a
community hospital (n=237). The exclusion criteria were as follows:
absence of follow-up imaging data (n=917), predominantly not
osteolytic cancer (n=428), pathologic fracture without surgical
therapy (n=83), surgical therapy (n=45), lack of accurate
evaluation of images (n=52), and follow-up time of <2 months
excluding regrowth (n=69). Thus, 751 predominantly osteolytic
metastatic bone lesions in 536 patients were followed-up using
computed tomography (CT).
In addition, patients who survived for <1 year
after radiotherapy (n=420), had follow-up CT for <6 months after
radiotherapy excluding regrowth (n=6), and had single fraction
radiotherapy of 8 Gy (n=8) were excluded. Finally, we
retrospectively evaluated LC of 317 lesions in 240 patients with
bone metastasis treated with FMRT (Fig. 1). This retrospective study was
approved by the appropriate institutional review boards of Ehime
University Hospital (1912010), Shikoku Cancer Center (RIN2019-79),
and Saiseikai Imabari Hospital (I2-1-2), and we applied the opt-out
method to obtain consent for this study.
Effectiveness assessment
For the purpose of the present study, local failure
was defined as enlargement of lytic change or extraosseous mass at
the FMRT sites of bone metastases based on the size of osteolytic
change before FMRT as a reference. Two observers (a radiologist and
a radiation oncologist) were blinded to the follow-up information
and outcomes during the evaluation of the images.
Radiotherapy
The doses of FMRT were determined at the discretion
of each physician and institution. The most common dose
administered was 30 Gy in 10 fractions. To compare the various
fractionated schedules, BED was calculated. The BED10 (BED
calculated using an α/β of 10 Gy) was calculated using the
equation: n x d (1 + d/(α/β)), where d is the fraction dose, n is
the number of fractions, and α/β is 10 Gy.
Statistical analysis
The time of survival and LC of the FMRT sites was
calculated from the beginning of FMRT. The Kaplan-Meier method was
used to generate the LC curves. Cox proportional hazards models
were used to determine hazard ratios (HRs), including 95%
confidence intervals (CIs), and P-values. Univariate and
multivariate analyses were used to assess the predictive factors
associated with LC rates of FMRT sites. Statistical significance
was defined as a two-sided P-value <0.05. Statistical analyses
were performed using JMP software (JMP version 14.3.0; SAS
Institute, Cary, NC, USA).
Results
Clinical characteristics
Data from 240 patients (male/female=113/127; median
age [range]: 66 (34-90) years) with 317 lesions were included in
the analysis. The median follow-up and radiographic follow-up times
were 24 (range, 12-123) and 20 (range, 1-119) months, respectively.
The characteristics of the lesions are presented in Table I. The median FMRT dose was
BED10=39.0 Gy (30 Gy in 10 fractions). The other fraction
schedules, in sequential order, were as follows: 28.0 Gy (5x4 Gy),
31.2 Gy (10x2.5 Gy), 46.9-50.0 Gy (15-16x2.5 Gy), 46.8-58.5 Gy
(12-15x3 Gy), 60.0 Gy (25x2 Gy), 39.7 Gy (5x4 Gy + 3x3 Gy), and
71.7 Gy (3x3 Gy + 25x2 Gy).
 | Table ICharacteristics of lesions. |
Table I
Characteristics of lesions.
| Characteristic | No. of lesions
(%) |
|---|
| Age, years | |
|
<70 | 213 (67.2) |
|
≥70 | 104 (32.8) |
| Sex | |
|
Male | 147 (46.4) |
|
Female | 170 (53.6) |
| Primary tumor
sites | |
|
Lung | 93 (29.3) |
|
Breast | 103 (32.5) |
|
Head and
neck | 27 (8.5) |
|
Esophagus | 3 (0.1) |
|
Hepatobiliary/pancreatic | 19 (6.0) |
|
Kidney/ureter | 30 (9.5) |
|
Colorectal | 10 (3.2) |
|
Gynecological | 9 (2.8) |
|
Sarcoma/melanoma/mesothelioma | 8 (2.5) |
|
Others | 15 (4.7) |
| FMRT sites | |
|
Vertebral | 201 (63.4) |
|
Pelvis | 72 (22.7) |
|
Rib | 24 (7.6) |
|
Others | 20 (6.3) |
| Bone cortex
destruction | |
|
Yes | 225 (71.0) |
|
No | 92 (29.0) |
| FMRT dose
(BED10) | |
|
<39.0 | 18 (5.7) |
|
39 | 176 (55.5) |
|
>39.0 | 123 (38.8) |
| Post-FMRT BMAs | |
|
Yes | 223 (70.3) |
|
No | 94 (29.7) |
| Pre-FMRT ATs | |
|
Yes | 180 (56.8) |
|
No | 137 (43.2) |
| Post-FMRT ATs | |
|
Yes | 252 (79.5) |
|
No | 65 (20.5) |
The 2- and 3-year LC rates of the FMRT sites were 88
and 84%, respectively (Fig. 2A).
Local recurrence was observed in 12.9% (41 of 317 lesions) of the
lesions, and the median time to recurrence was 10 (range, 1-106)
months.
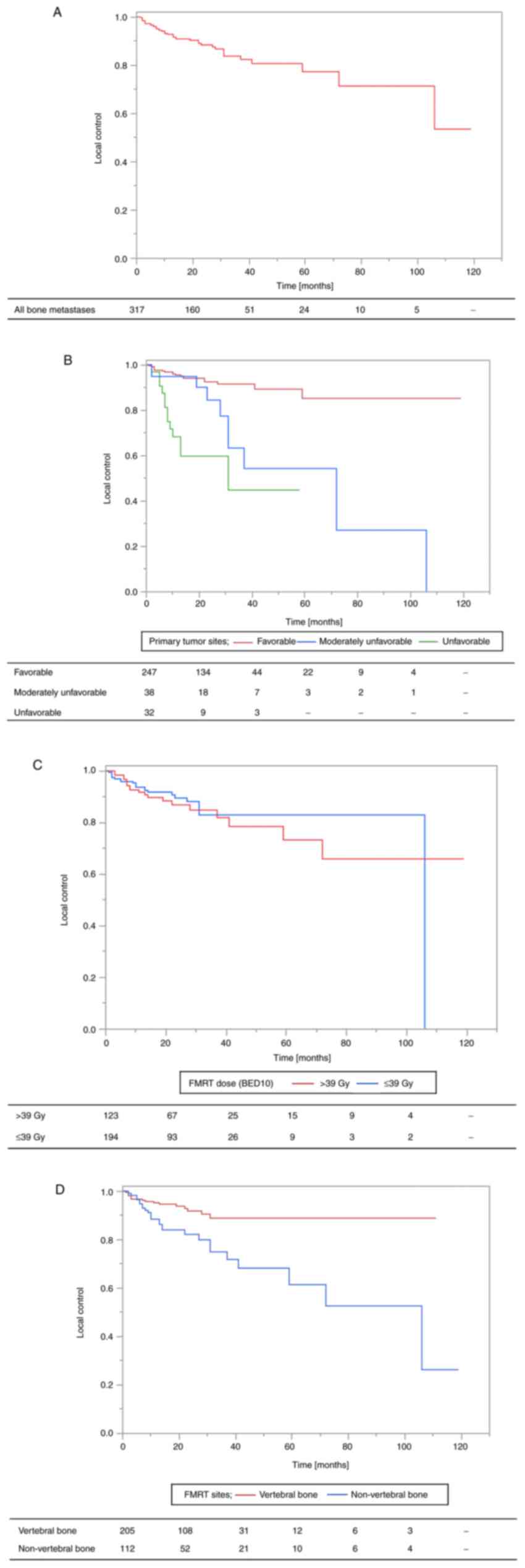 | Figure 2Local control of bone metastases. (A)
Local control of all bone metastatic lesions. (B) Primary tumor
sites (favorable group vs. moderate group vs. unfavorable group;
favorable group, head and neck, lung/mediastinal, breast, gastric,
gynecologic, prostate, bladder and skin cancer; moderate group,
kidney/ureter and non-epithelial cancer; unfavorable group,
esophageal, colorectal and hepatobiliary/pancreatic cancer). (C)
FMRT dose (BED10) (≤39.0 vs. >39.0). (D) FMRT sites (vertebral
bone vs. non-vertebral bone). BED, biological effective dose; FMRT,
fractionated moderate-dose palliative radiotherapy. |
LC according to primary tumor
sites
The primary tumor sites were classified into three
groups based on reported radiosensitivity and the results of the 1-
and 3-year LC rates (unfavorable group, 1-year LC of <50%;
moderately unfavorable group, 3-year LC of <50%; favorable
group, 3-year LC of ≥50%) in our previous study (10,11).
Esophageal, colorectal, and hepatobiliary/pancreatic cancers were
classified in the unfavorable group (n=32), kidney/ureter cancer
and non-epithelial cancers were classified in the moderately
unfavorable group (n=38), and the remaining cancers (i.e., lung
cancer, breast cancer, head and neck cancer, gastric cancer,
genitourinary cancer, and skin cancers other than melanoma) were
classified in the favorable group (n=247).
The number of recurrent bone metastatic sites was
18/247 (7.3%) in the favorable group, 10/38 (26.3%) in the
moderately unfavorable group, and 13/32 (40.5%) in the unfavorable
group. The 2- and 3-year LC rates were 60 and 45% for the
unfavorable group, 84 and 63% for the moderately unfavorable group,
and 93 and 92% for the favorable group, respectively (Fig. 2B). In univariate analysis, LC rates
were significantly lower in the unfavorable group than in the
moderately unfavorable group (HR 2.47, 95% CI 1.06-5.76, P=0.036)
and significantly higher in the favorable group than in the
moderately unfavorable group (HR 0.11, 95% CI 0.05-0.23,
P<0.001, Table II).
 | Table IILC rates after FMRT and results of
univariate and multivariate analyses. |
Table II
LC rates after FMRT and results of
univariate and multivariate analyses.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | 2-year LC, % | 3-year LC, % | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<70 years
vs. ≥70 years) | 92 vs. 80 | 87 vs. 77 | 2.45
(1.31-4.58) | 0.006 | 3.53
(1.62-7.67) | 0.002 |
| Sex (female vs.
male) | 91 vs. 85 | 86 vs. 80 | 1.88
(1.01-3.51) | 0.047 | 1.39
(0.70-2.77) | 0.344 |
| Primary tumor
sites | | | | | | |
|
Moderately
unfavorable vs. favorable | 84 vs. 93 | 63 vs. 92 | 0.11
(0.05-0.23) | <0.001 | 0.23
(0.10-0.54) | 0.001 |
|
Moderately
unfavorable vs. unfavorable | 84 vs. 60 | 63 vs. 45 | 2.47
(1.06-5.76) | 0.036 | 2.70
(1.08-6.74) | 0.033 |
| FMRT sites
(vertebral bone vs. non-vertebral bone) | 92 vs. 82 | 89 vs. 75 | 3.14
(1.66-5.94) | <0.001 | 2.25
(1.17-4.31) | 0.015 |
| FMRT dose (BED10)
(≤39.0 Gy vs. >39.0 Gy) | 90 vs. 87 | 83 vs. 85 | 1.19
(0.64-2.22) | 0.580 | - | - |
| Post-FMRT BMAs (yes
vs. no) | 95 vs. 72 | 90 vs. 68 | 4.69
(2.47-8.89) | <0.001 | 3.54
(1.85-6.78) | <0.001 |
| Post-FMRT ATs (yes
vs. no) | 91 vs. 78 | 85 vs. 78 | 2.63
(1.36-5.07) | 0.004 | 1.04
(0.49-2.19) | 0.924 |
| Bone cortex
destruction (yes vs. no) | 85 vs. 90 | 78 vs. 86 | 0.54
(0.29-1.02) | 0.057 | 0.59
(0.30-1.16) | 0.128 |
| Pre-FMRT ATs (yes
vs. no) | 89 vs. 87 | 81 vs. 87 | 0.99
(0.53-1.84) | 0.972 | - | - |
LC according to FMRT dose (BED10)
The 2- and 3-year LC rates were 90 and 83% for bone
metastasis with a FMRT dose (BED10) of ≤39.0 Gy and 87 and 85% for
a dose >39.0 Gy, respectively (Fig.
2C). In univariate analysis, LC rates were not significantly
lower in the FMRT dose (BED10) of ≤39.0 Gy than in a dose >39.0
Gy (HR 1.19, 95% CI 0.64-2.22, P=0.580, Table II).
In the favorable group, the 2-year LC rates of
patients treated with a FMRT dose (BED10) ≤39.0 Gy (n=159) and
>39.0 Gy (n=88) were 95 and 91%, respectively (P=0.507,
log-rank). In the unfavorable and moderately unfavorable groups,
the 2-year LC rates of patients treated with a FMRT dose (BED10)
≤39.0 Gy (n=35) and >39.0 Gy (n=35) were 67 and 73%,
respectively (P=0.990, log-rank).
LC according to FMRT sites
The 2-year and 3-year LC rates were 92 and 89% for
vertebral bones, and 82 and 75% for non-vertebral bones (pelvic
bone, 87 and 77%; other bone, 75 and 75%), respectively (Fig. 2D). In univariate analysis, LC rates
were significantly lower in non-vertebral bones than in vertebral
bones (HR 3.14, 95% CI 1.66-5.94, P<0.001, Table II).
LC according to other factors
Older age (≥70 years) and non-administration of
post-FMRT bone-modifying agents (BMAs) and/or antineoplastic agents
(ATs) were statistically significant unfavorable factors for LC of
bone metastasis in the univariate analysis (Table II). Furthermore, bone cortex
destruction and sex were significantly associated with LC (Table II). In addition, LC rates were not
significantly low in non-administration of pre-FMRT ATs than in
administration of pre-FMRT ATs (Table
II).
Multivariate Cox regression
analysis
On multivariate analysis, older age (≥70 years),
non-vertebral bone metastasis, bone metastasis from unfavorable and
moderately unfavorable primary tumor sites, and no administration
of post-FMRT BMAs were significantly unfavorable factors for LC of
bone metastasis in long-term survivors (Table II).
Discussion
This study showed that age, primary tumor sites,
FMRT sites, and administration of post-FMRT BMAs were significant
factors for LC of bone metastasis after FMRT in intermediate-term
survivors (surviving for ≥1 year). However, higher FMRT doses
(BED10 >39.0 Gy [10x3 Gy]) and the administration of pre- and
post-FMRT ATs were not significant factors for LC of bone
metastasis.
In our present study, LC did not differ according to
the FMRT dose in intermediate-term survivors, in contrast to the
results of our previous study (10). The selection bias of including only
patients who survived for >1 year in this study could have
resulted in the relatively high number of patients with indolent
tumors and a good response to systemic therapy. Although the
palliative radiotherapy for bone metastases does not contribute to
prolonged prognosis (12), SBRT
was associated with an improvement in overall survival in patients
with oligometastatic paradigm (13). In the cases of non-oligometastatic
bone metastases, FMRT was an acceptable option in terms of the LC
of FMRT sites even when intermediate-term survival was
expected.
Although all patients in the unfavorable group
survived for >1 year, only 68% of them experienced LC in 1-year
(data not shown). Moreover, LC of bone metastasis from unfavorable
primary tumor sites is insufficient even when SBRT, a more
aggressive treatment than FMRT, is used (14,15).
However, although moderate-dose escalation did not improve LC of
bone metastasis, an extremely high radiation dose may have the
potential to control bone metastasis from these tumors (16,17).
When bone metastases from unfavorable primary tumor sites are
treated using radiotherapy and intermediate- and long-term survival
is desired, extremely high radiation doses using
intensity-modulated radiotherapy or heavy ion radiotherapy may be
warranted. On the contrary, some studies have shown that SBRT
resulted in good LC of bone metastases from moderately unfavorable
primary tumor sites (18-20).
Thus, SBRT, rather than FMRT, should be aggressively utilized for
bone metastases from moderately unfavorable primary tumor sites for
patients expected to have intermediate- and long-term survival.
In addition, the sites of the bone metastases seemed
to influence the LC. Non-vertebral bone metastases had unfavorable
LC in our study. Although the reasons were unclear, one of the
possible explanations is that vertebral bone metastases often occur
via Batson's vertebral venous plexus (21); these may have occurred early as
compared to the non-vertebral bone metastases. Therefore,
non-vertebral bone metastases may represent a more aggressive tumor
compared to vertebral bone metastases.
In contrast to the results of our previous study,
the administration of post-FMRT ATs did not improve the LC of FMRT
sites (10), perhaps due to the
indolent nature of the tumors in intermediate-term survivors
without post-FMRT ATs. Furthermore, Ahmed et al (22) showed that the radiation sensitivity
of each metastatic site was different according to the anatomical
location of metastases, even if each metastasis occurred from the
same primary tumor site. In addition, the development and
progression of bone metastases are influenced by osteoclasts and
osteoblasts, which are different from the microenvironment of other
metastases (23,24). Even when a comparatively low FMRT
dose (≤39.0 Gy) and no post-FMRT ATs are used to treat bone
metastasis, local regrowth may be restricted by the damage to
osteoclasts and osteoblasts.
This study had some limitations due to its
retrospective nature. First, overall survival could not be
evaluated because only patients surviving ≥1 year were included in
this study. Second, although some studies examined the prognostic
factors in patients with bone metastases (25,26),
our study could not investigate the relationship between prognosis
and LC of FMRT sites because the large number of missing values
(general condition, the severity of spinal cord palsy, the number
of bone metastases, and metastases to the major internal organs)
limited the detailed evaluation. Third, in this study, although the
tumor aggressiveness is relatively homogeneous because only cases
that survived >1 year were evaluated, the influence of tumor
aggressiveness could not be completely excluded. Therefore, careful
interpretation of the results is necessary. Furthermore, tumor
aggressiveness may depend on the unfavorable group (primary tumor
sites) and systemic control. However, because some patients in the
unfavorable group survived for ≥1 year and had local failure within
a few months, increased intensity treatment may be necessary for
these cases. Finally, this study was substantially affected by the
small number of recurrences that occurred compared with our
previous study (10); thus, future
studies are required to verify these results. In addition, this
study aimed to investigate LC in patients surviving for ≥1 year, in
contrast to our previous study (10). In patients with a short prognosis,
pain relief with a single-fraction radiotherapy is optimal. In
cases with a long prognosis (especially oligometastatic bone
lesion), SBRT may be preferable. However, in cases with
intermediate prognosis, it is often difficult to select an
appropriate irradiation dose in clinical practice. Based on the
results of the previous study alone, it was difficult to consider
cases in which FMRT is truly preferable. Therefore, this study
focusing on LC of bone metastases with palliative radiotherapy
could help in determining the appropriate radiation dose in cases
with intermediate prognosis.
In conclusion, the sites of bone metastasis and
primary tumors, as well as the administration of post-FMRT BMAs
were significant factors associated with LC of bone metastasis in
intermediate-term survivors. However, an FMRT dose (BED10) >39.0
Gy and the administration of post-FMRT ATs were not significantly
useful for the LC of bone metastasis. Although further study of LC
of bone metastasis from various cancer sites is warranted, these
results should be considered for the individualized radiotherapy
for bone metastasis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM and YH designed the study concepts and confirm
the authenticity of all the raw data. KM and YH prepared the
manuscript and MK and HK edited the manuscript. KM, YH, HK, MK, SY,
KN, HI, NT, ST, KU and TK collected patient data, were involved in
the data analysis, drafted the article and collaborated in the
discussion. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were performed in accordance with the
ethical standards of the institutional research committee and with
the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. The present study was approved by
Ethics Committee of Ehime University Hospital (Toon, Ehime, Japan;
approval reference no. 1912010), Ethics Committee of Shikoku Cancer
Center (Matsuyama, Ehime, Japan; approval reference no. RIN2019-79)
and Ethics Committee of Saiseikai Imabari Hospital (Imabari, Ehime,
Japan; approval reference no. I2-1-2). Patients consented in
writing to the use of their anonymous data for research at the time
of treatment and consented to this research via the opt-out
method.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hernandez RK, Wade SW, Reich A, Pirolli M,
Liede A and Lyman GH: Incidence of bone metastases in patients with
solid tumors: Analysis of oncology electronic medical records in
the United States. BMC Cancer. 18(44)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lutz S, Balboni T, Jones J, Lo S, Petit J,
Rich SE, Wong R and Hahn C: Palliative radiation therapy for bone
metastases: Update of an ASTRO Evidence-Based Guideline. Pract
Radiat Oncol. 7:4–12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Coleman R, Hadji P, Body JJ, Santini D,
Chow E, Terpos E, Oudard S, Bruland Ø, Flamen P, Kurth A, et al:
Bone health in cancer: ESMO Clinical Practice Guidelines. Ann
Oncol. 31:1650–1663. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rich SE, Chow R, Raman S, Liang Zeng K,
Lutz S, Lam H, Silva MF and Chow E: Update of the systematic review
of palliative radiation therapy fractionation for bone metastases.
Radiother Oncol. 126:547–557. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wegner RE, Matani H, Colonias A, Price F,
Fuhrer R and Abel S: Trends in radiation fractionation for bone
metastases: A contemporary nationwide analysis. Pract Radiat Oncol.
10:402–408. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bedard G, McDonald R, Poon I, Erler D,
Soliman H, Cheung P, Chung H, Chu W, Loblaw A, Chow E and Sahgal A:
Stereotactic body radiation therapy for non-spine bone metastases-a
review of the literature. Ann Palliat Med. 5:58–66. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kowalchuk RO, Waters MR, Richardson KM,
Spencer K, Larner JM, McAllister WH, Sheehan JP and Kersh CR:
Stereotactic body radiation therapy for spinal metastases: A novel
local control stratification by spinal region. J Neurosurg Spine.
34:1–10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Li KH, de Wit M, et
al: Overall survival with durvalumab after chemoradiotherapy in
stage III NSCLC. N Engl J Med. 379:2342–2350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Makita K, Hamamoto Y, Kanzaki H, Kataoka
M, Yamamoto S, Nagasaki K, Ishikawa H, Takata N, Tsuruoka S, Uwatsu
K and Kido T: Local control of bone metastases treated with
external beam radiotherapy in recent years: A multicenter
retrospective study. Radiat Oncol. 16(225)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gerszten PC, Mendel E and Yamada Y:
Radiotherapy and radiosurgery for metastatic spine disease: What
are the options, indications, and outcomes? Spine (Phila Pa 1976).
34 (Suppl 22):S78–S92. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van der Linden YM, Steenland E, van
Houwelingen HC, Post WJ, Oei B, Marijnen CA and Leer JW: Dutch Bone
Metastasis Study Group. Patients with a favorable prognosis are
equally palliated with single and multiple fraction radiotherapy:
Results on survival in the Dutch bone metastasis study. Radiother
Oncol. 78:245–253. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Palma DA, Olson R, Harrow S, Gaede S,
Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP,
et al: Stereotactic ablative radiotherapy versus standard of care
palliative treatment in patients with oligometastatic cancers
(SABR-COMET): A randomised, phase 2, open-label trial. Lancet.
393:2051–2058. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ito K, Ogawa H, Shimizuguchi T, Nihei K,
Furuya T, Tanaka H and Karasawa K: Stereotactic body radiotherapy
for spinal metastases: Clinical experience in 134 cases from a
single Japanese institution. Technol Cancer Res Treat.
17(1533033818806472)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ito K, Yamaguchi T, Ogawa H, Nakajima Y
and Karasawa K: Stereotactic body radiotherapy for bone metastases
in patients with colorectal cancer. Jpn J Clin Oncol. 50:1442–1446.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McGee HM, Carpenter TJ, Ozbek U, Kirkwood
KA, Tseng TC, Blacksburg S, Germano IM, Green S and Buckstein M:
Analysis of local control and pain control after spine stereotactic
radiosurgery reveals inferior outcomes for hepatocellular carcinoma
compared with other radioresistant histologies. Pract Radiat Oncol.
9:89–97. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jung IH, Yoon SM, Kwak J, Park JH, Song
SY, Lee SW, Ahn SD, Choi EK and Kim JH: High-dose radiotherapy is
associated with better local control of bone metastasis from
hepatocellular carcinoma. Oncotarget. 8:15182–15192.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Smith BW, Joseph JR, Saadeh YS, La Marca
F, Szerlip NJ, Schermerhorn TC, Spratt DE, Younge KC and Park P:
Radiosurgery for treatment of renal cell metastases to spine: A
systematic review of the literature. World Neurosurg. 109:e502–9.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Leeman JE, Bilsky M, Laufer I, Folkert MR,
Taunk NK, Osborne JR, Arevalo-Parez J, Zatcky J, Alektiar KM,
Yamada Y, et al: Stereotactic body radiotherapy for metastatic
spinal sarcoma: A detailed patterns-of-failure study. J Neurosurg
Spine. 25:52–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stinauer MA, Kavanagh BD, Schefter TE,
Gonzalez R, Flaig T, Lewis K, Robinson W, Chidel M, Glode M and
Raben D: Stereotactic body radiation therapy for melanoma and renal
cell carcinoma: Impact of single fraction equivalent dose on local
control. Radiat Oncol. 6(34)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Batson OV: The vertebral vein system as
mechanism for spread of metastases. Am J Roentgenol Radium Ther
Nucl Med. 48:715–718. 1942.https://pubmed.ncbi.nlm.nih.gov/13444513/.
|
|
22
|
Ahmed KA, Fulp WJ, Berglund AE, Hoffe SE,
Dilling TJ, Eschrich SA, Sridhar R and Torres-Roca JF: Differences
between colon cancer primaries and metastases using a molecular
assay for tumor radiation sensitivity suggest implications for
potential oligometastatic SBRT patient selection. Int J Radiat
Oncol Biol Phys. 92:837–842. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Morony S, Capparelli C, Sarosi I, Lacey
DL, Dunstan CR and Kostenuik PJ: Osteoprotegerin inhibits
osteolysis and decreases skeletal tumor burden in syngeneic and
nude mouse models of experimental bone metastasis. Cancer Res.
61:4432–4436. 2001.PubMed/NCBI
|
|
24
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tokuhashi Y, Matsuzaki H, Toriyama S,
Kawano H and Ohsaka S: Scoring system for the preoperative
evaluation of metastatic spine tumor prognosis. Spine (Phila Pa
1976). 15:1110–1113. 1990.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tokuhashi Y, Matsuzaki H, Oda H, Oshima M
and Ryu J: A revised scoring system for preoperative evaluation of
metastatic spine tumor prognosis. Spine (Phila Pa 1976).
30:2186–2191. 2005.PubMed/NCBI View Article : Google Scholar
|