Introduction
Breast cancer (BC) is the most prevalent cancer type
among females worldwide (1).
Advances in early systematic screening, effective treatments and
supportive care have significantly prolonged the survival time of
patients with BC (2). Due to the
high incidence and good prognosis of BC, the risk of developing a
second primary malignancy (SPM) thereafter may turn into a serious
health issue both for the patients and health care system. A large
study in the US found a higher risk of SPM associated with BC than
with other cancers in women (3).
An SPM is a second, unrelated cancer in a person who
has previously experienced another cancer at any time. The exact
incidence of SPMs is uncertain, though studies have provided
certain insight. One study evaluated over 2 million people who
developed the 10 most common types of cancer from 1992 to 2008, and
>10% of them developed an SPM (4). Previous population-based research has
examined the risk of developing SPMs among initial primary BC
survivors compared to the general population, but the results from
these studies were inconsistent in their risk estimation, finding a
wide risk range of 15-45% for any type of SPM (2). Therefore, accurately estimating the
SPM risk and profiling the characteristics of patients at risk
would be valuable.
An SPM may occur in the same tissue or organ as the
first cancer or in another region of the body (5). These second cancers may be related to
a genetic predisposition, common risk factors or treatments for the
original cancer, or they may simply occur sporadically, as cancer
commonly does (6,7). The link between the characteristics
of the primary cancer and the risk of SPM is controversial
(8,9). Little is known regarding the
simultaneous effect of intrinsic factors, such as age at diagnosis,
sex, marital status, ethnicity, hormone receptor (HR) status and
tumor characteristics, which lead to the development of a new
malignancy in a BC survivor.
The present study aimed to comprehensively profile
the characteristics of patients with BC harboring an SPM and to
further identify patients at high risk of developing SPMs using a
large, population-based cohort. First, the demographic, clinical
and histological differences between patients with BC with only one
primary malignancy (OOPM) and with SPMs were retrieved. The
influence of SPM on prognosis was then investigated. Finally, the
intrinsic factors associated with the development of SPM were
evaluated and a machine learning model was established to identify
BC survivors who were at high risk of developing SPMs.
Patients and methods
Data sources
Data were extracted from the Surveillance,
Epidemiology and End Results (SEER) research database. The SEER
program is the most authoritative and premier source of cancer
statistics in the US, collecting demographics, tumor
characteristics and survival data. The SEER data of the version
November 2019 (https://seer.cancer.gov/data-software/documentation/seerstat/nov2019/)
were downloaded. The downloaded data contained four compressed
files: file1Bvek1.rar, file9c7Clt.rar, fileb0bhOt.rar and
fileYhNIxM.rar. Each of the above files contained two files, titled
xxx.dic and xxx.txt. The dic file is the column id of the txt file
is the content. An in-house python script was used to read and
process these files. In brief, a function named extract_colname was
used to extract the column names from the dic file, which were
assigned to the corresponding txt file. The patients with BC were
then selected and the histology was rated according to criteria in
the 3rd edition of the International Classification of Diseases for
Oncology (10).
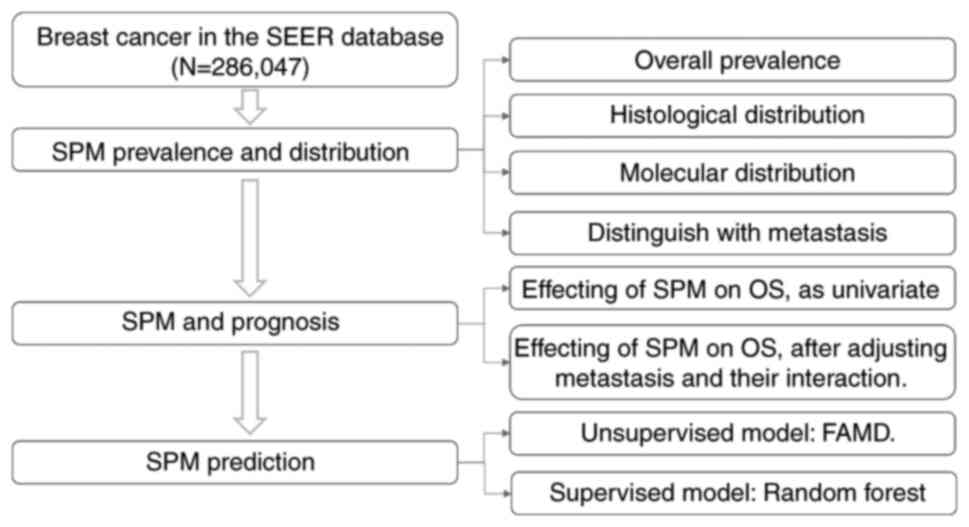
Study population
Patients with BC diagnosed after 2010 were included
because molecular subtypes were available from then on. Only cases
with complete data, without missing values on important covariates
(age, ethnicity, tumor site, grade, size) were eligible. Cases that
were reported from a death certificate or autopsy were excluded and
a 2-month latency exclusion was set to further distinguish SPMs
from simultaneous cancers. The identified patients with BC were
then categorized into two cohorts: The OOPM cohort and the SPM
cohort. The study design and workflow are presented in Fig. 1.
Definition of SPM
According to the SEER rules for classifying multiple
primary cancers, the definition was dependent on the cancer site of
origin, date of diagnosis, histology, tumor behavior (i.e., in
situ vs. invasive) and laterality of any paired organs. In
general, all SPMs occurring 2 or more months after the initial
diagnosis were considered separate primary cancers unless the
medical record stated that the tumor was recurrent or metastatic
(11,12). There were also two key variables
indicating multiple primary malignancies in SEER, the ‘total number
of in situ/malignant tumors for patient’ and the ‘sequence
number’ of the multiple primary malignancies. The former was used
to identify patients with an SPM and the latter to index the
sequence of multiple malignancies. A random variable that was named
‘indicator of SPM’ was defined to indicate whether the patient had
developed one.
Model creation using unsupervised and
supervised methods
An unsupervised machine learning method called
factor analysis of mixed data (FAMD), which is generally used to
analyze datasets containing both quantitative and qualitative
variables, was used to transform data. In brief, FAMD may be
regarded as a mix between principal component analysis (PCA) and
multiple correspondence analysis. It acts as PCA for quantitative
variables and as multiple correspondence analysis for qualitative
variables. This was achieved by an R package FactoMineR (13).
The total dataset was randomly split 75/25% into the
training set and testing set, stratified by the existence of SPMs.
A popular supervised machine learning method called random forest
was applied to the training set to predict the likelihood of
developing SPMs. The performance of the random forest classifier
was evaluated in the testing set with 50 repetitions to reduce the
influence of randomization. The outcome was visualized by the
receiver operating characteristic (ROC) curve. Feature importance
was calculated by their contribution to the prediction ability of
the model. The above steps were implemented in python using the
sci-kit-learn package.
Statistical analysis
To compare distributions between variables, the
χ2 test was generally applied for discrete variables,
Student's t-test for continuous variables satisfying a normal
distribution and the Mann-Whitney U-test for continuous variables
otherwise. For survival analysis, both the non-parametric
Kaplan-Meier model and the semi-parametric Cox proportional hazard
model were used to evaluate the influence of variables on overall
survival (OS); when both methods produced a significant P-value,
the result was regarded to be significant. P<0.05 was considered
to indicate statistical significance.
Results
Clinical, histological and molecular
characteristics of patients with BC with SPMs
A total of 286,047 patients with BC were identified
from the SEER database. Of them, 26,657 (9.32%) developed SPMs
within a maximum follow-up of ~7 years. The characteristics of the
patients with OOPM and SPM are compared in Table I. In general, ~99% of patients with
BC were females and ~50% of BCs were in the left breast.
Specifically, the SPM cohort had significantly more patients with
well (23.91%) or moderately (44.22%) differentiated pathology and
with more widowed patients (14.87%), while the OOPM cohort had a
significantly higher percentage of patients poorly differentiated
tumors (31.86%) and of married patients (55.04%). The SPM cohort
was significantly older than the OOPM cohort (median age, 63 vs. 60
years; P<0.001). Of note, the SPM frequency was significantly
(P=0.003) higher in stage M0 (25,316/245,406, 10.32%) than in stage
M1 (1,089/11,590, 9.39%; Table
I).
 | Table ICharacteristics of patients with BC
with OOPM and SPM. |
Table I
Characteristics of patients with BC
with OOPM and SPM.
| Parameter | OOPM (n=259,390) | SPM (n=26,657) |
|---|
| Sex | | |
|
Female | 257,517 (99.28) | 26,432 (99.16) |
|
Male | 1,873 (0.72) | 225 (0.84) |
| Age at diagnosis,
years [median (range)] | 60.0 (2.0-117.0) | 63.0
(21.0-103.0) |
| Marital status | | |
|
Married | 142,757 (55.04) | 14,109 (52.93) |
|
Single | 39,001 (15.04) | 3,937 (14.77) |
|
Widowed | 32,868 (12.67) | 3,964 (14.87) |
|
Divorced | 27,488 (10.60) | 2,910 (10.92) |
|
Separated | 2,822 (1.09) | 257 (0.96) |
|
Unmarried or
domestic partner | 753 (0.29) | 93 (0.35) |
| Laterality | | |
|
Left | 131,723
(50.78) | 13,197 (49.51) |
|
Right | 127,288
(49.07) | 13,444 (50.43) |
|
Left or
righta | 49 (0.02) | 2 (0.01) |
|
Bilateral | 42 (0.02) | 0 (0.00) |
| Ethnicity | | |
|
White | 202,991
(78.26) | 21,556 (80.86) |
|
Black | 29,453 (11.35) | 2,765 (10.37) |
|
Asian or
Pacific Islander | 23,403 (9.02) | 2,063 (7.74) |
|
American
Indian/Alaska Native | 1,570 (0.61) | 159 (0.60) |
| Grade | | |
|
I: Well
differentiated | 55,797 (21.51) | 6,373 (23.91) |
|
II:
Moderately differentiated | 108,397
(41.79) | 11,788 (44.22) |
|
III: Poorly
differentiated | 82,637 (31.86) | 7,227 (27.11) |
|
IV:
Undifferentiated | 900 (0.35) | 74 (0.28) |
| BC subtype | | |
|
HER2+/HR+ | 26,825 (10.34) | 2,199 (8.25) |
|
HER2+/HR- | 11,292 (4.35) | 876 (3.29) |
|
HER2-/HR+ | 177,409
(68.39) | 19,487 (73.10) |
|
Triple
negative | 28,180 (10.86) | 2,402 (9.01) |
| T stage | | |
|
T1 | 146,231
(56.37) | 14,865 (55.76) |
|
T2 | 76,558 (29.51) | 7,865 (29.50) |
|
T3 | 14,686 (5.66) | 1,721 (6.46) |
|
T4 | 6,170 (2.38) | 697 (2.61) |
| N stage | | |
|
N0 | 170,761
(65.83) | 17,547 (65.83) |
|
N1 | 60,316 (23.25) | 6,092 (22.85) |
|
N2 | 13,472 (5.19) | 1,408 (5.28) |
|
N3 | 10,488 (4.04) | 1,177 (4.42) |
| M stage | | |
|
M0 | 245,406
(94.61) | 25,316 (94.97) |
|
M1 | 11,590 (4.47) | 1,089 (4.09) |
| Stage | | |
|
I | 120,561
(46.48) | 12,247 (45.94) |
|
II | 94,164 (36.30) | 9,664 (36.25) |
|
III | 29,340 (11.31) | 3,268 (12.26) |
|
IV | 11,590 (4.47) | 1,089 (4.09) |
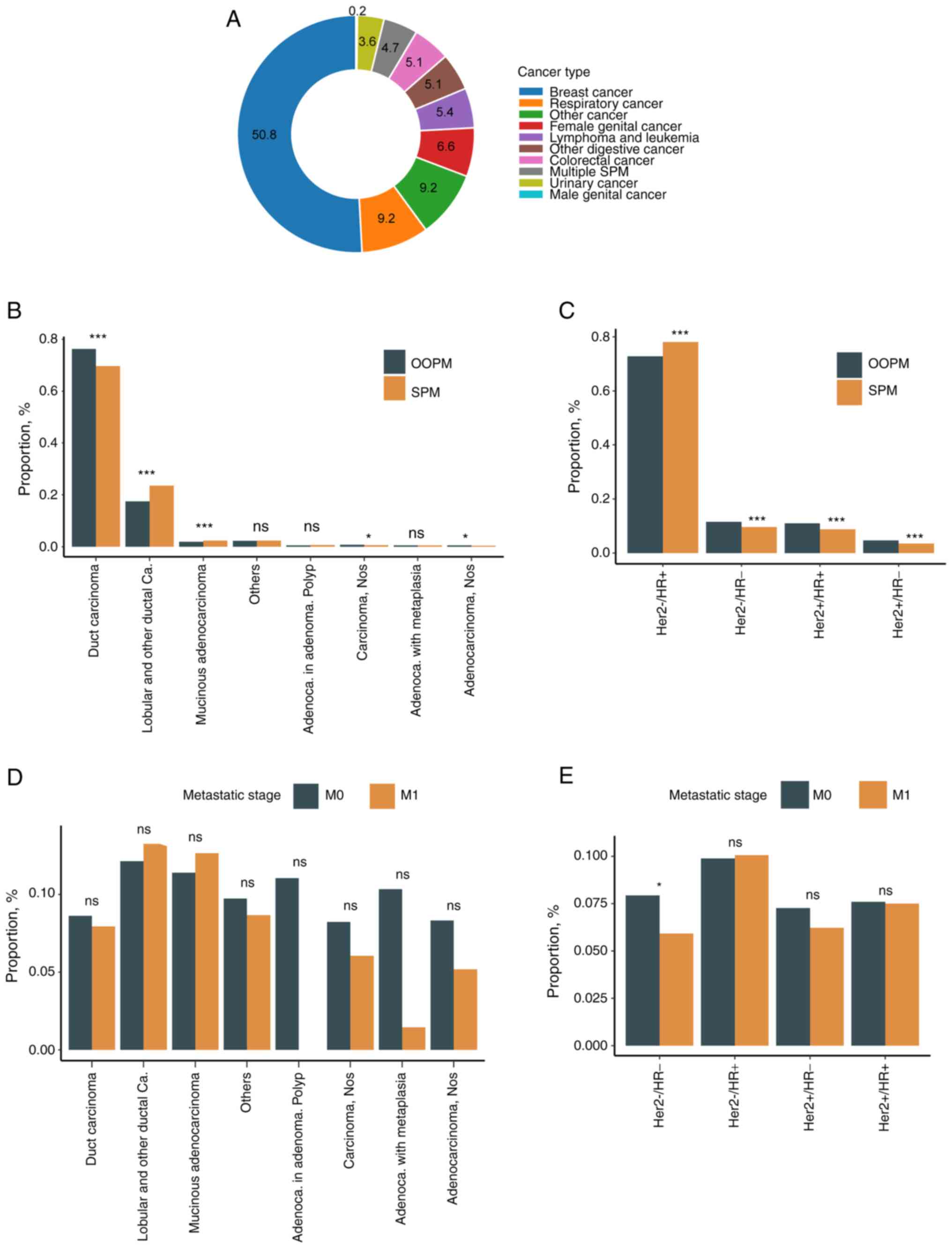
The cancer types of the SPMs are profiled in
Fig. 2A. Half of the SPMs occurred
in the breast and half were found evenly in the other systems,
including the respiratory, female germline, lymphatic/leukocytic,
colorectal, other digestive and urinary systems. of note, 0.2% of
SPMs were detected in the male germline system.
The histological type distribution in the SPM cohort
was compared with that in the OOPM cohort (Fig. 2B). Most SPMs were ductal carcinoma
in both cohorts, but the frequency of ductal carcinoma was
significantly lower in the SPM cohort (69.1%) than in the OOPM
cohort (75.2%, Fisher's exact P<0.001). On the other hand, the
frequency of lobular carcinoma, which was the second most common
carcinoma in both cohorts, was significantly more common in the SPM
cohort (23.3%) than in the OOPM cohort (17.1%, Fisher's exact
P<0.001).
Molecular status had been determined by a
combination of immunohistochemistry, fluorescence in situ
hybridization, chromogenic in situ hybridization and other
methods by the SEER group (https://seer.cancer.gov/seerstat/databases/ssf/her2-derived.html).
The HER2-/HR+ subtype was significantly
enriched in the SPM cohort compared to the OOPM cohort (78.1% vs.
72.8%, P<0.001; Fig. 2C). Of
note, HER2-/HR+ was the most common subtype
in both the OOPM cohort and SPM cohort, and the
HER2-/HR+ subtype was more likely to have an
SPM (10%) than other subtypes. The proportion of
HER2+/HR+, HER2+/HR-
and triple-negative subtypes was generally lower in the SPM cohort
than in the OOPM cohort.
Since it is at times difficult to distinguish
metastasis and SPM, the SPM frequency was compared between stage M0
and stage M1, stratified by histological and molecular subtype.
Although the SPM frequency was slightly higher in stage M0 than in
stage M1 across numerous histological types, no significant
difference in SPM frequency was detected in any histological type
(Fig. 2D). As for molecular
subtypes, the SPM frequency was significantly higher in stage M0 in
the HER2-/HR- subtype (Fig. 2E).
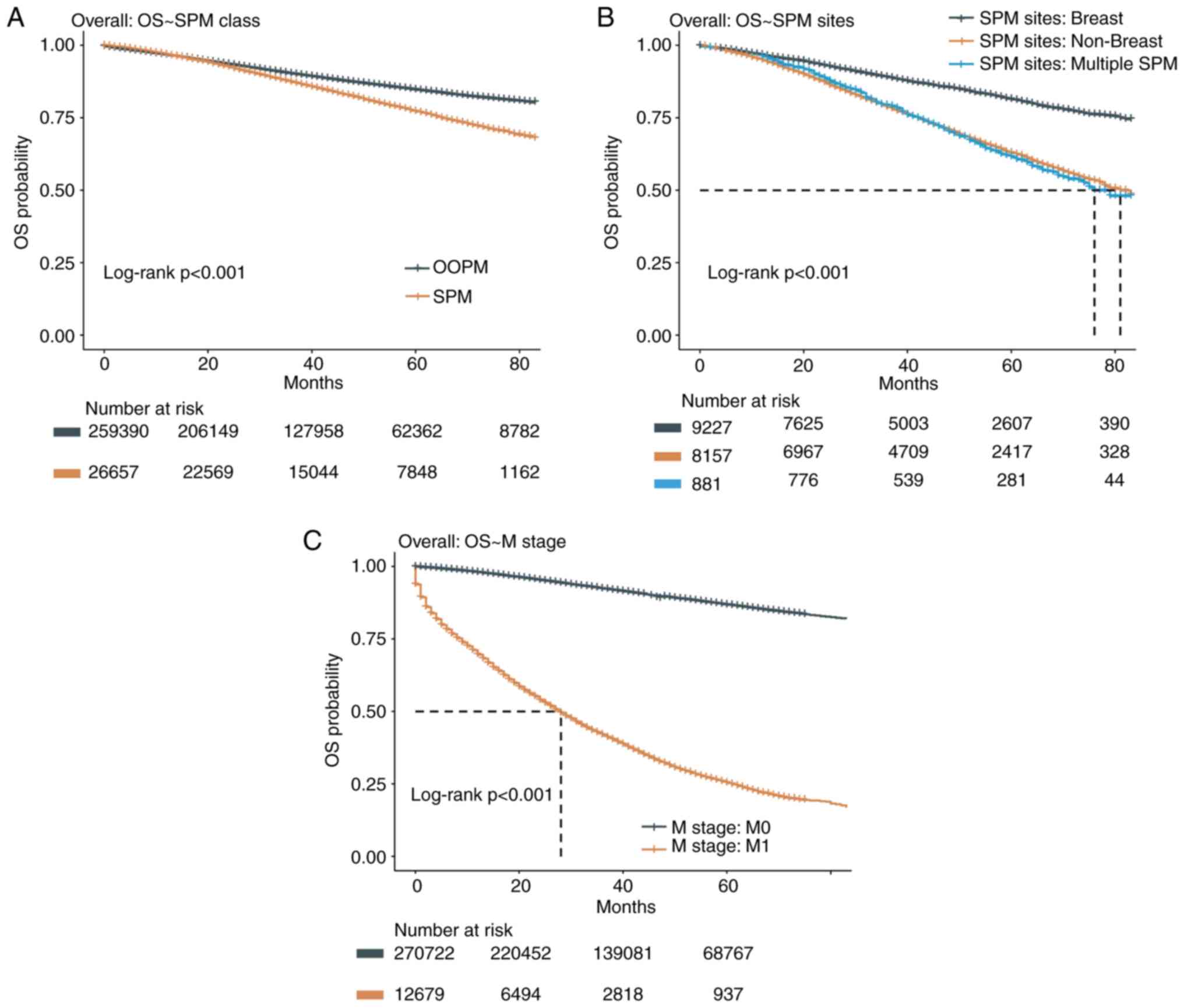
OS of patients with BC with SPMs
The OS of the SPM cohort was significantly worse
than that in the OOPM cohort (hazard ratio: 1.49; 95% CI:
1.44-1.53; log-rank P<0.001), indicating the role of SPMs in
accelerating patient death (Fig.
3A). As more than half of SPMs occurred in the breast again,
while the rest seemed to occur in other organs at random, their OS
rates were compared to explore the influence of SPM location on
survival. The OS of breast SPMs was significantly better than that
of other single-organ SPMs (hazard ratio: 0.46; 95% CI: 0.45-0.49;
log-rank P<0.001) and that of multiple-organ SPMs (hazard ratio:
0.44; 95% CI: 0.39-0.50; log-rank P<0.001; Fig. 3B), while there was no significant
OS difference between the latter two groups (hazard ratio: 0.98;
95% CI: 0.87-1.10; log-rank P=0.69). Median OS was not achieved
(i.e. the survival rate remained >50%) in patients with SPMs of
the breast, while it was 81 and 76 months in patients with SPMs of
other organs and in patients with multiple SPMs, respectively.
Since distant metastasis is a key factor influencing
OS, this was validated in the present dataset (Fig. 3C). To better understand the role of
distant metastasis and SPM on OS, multivariate Cox survival
analysis was performed for distant metastasis, SPM and their
interacting effect as covariates. The results indicated that both
distant metastasis and SPM were significantly associated with OS
after adjusting for each other (for SPM, hazard ratio: 1.71; 95%
CI: 1.70-1.82; for M1, hazard ratio: 12.64, 95% CI: 12.38-13.05;
Table II). The significant
interaction P-value implies that the influence of SPM on OS was
more significant in patients with stage M0.
 | Table IIUnivariate and multivariate Cox
regression analysis of SPM group and metastasis status for overall
survival. |
Table II
Univariate and multivariate Cox
regression analysis of SPM group and metastasis status for overall
survival.
| | Univariate | Multivariate |
|---|
| Factor | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| SPM (vs. OOPM) | 1.49
(1.44-1.53) | <0.001 | 1.71
(1.70-1.82) | <0.001 |
| Metastasis status
(M1 vs. M0) | 11.37
(11.09-11.67) | <0.001 | 12.64
(12.38-13.05) | <0.001 |
| Interaction
terma | | | 0.40
(0.36-0.43) | <0.001 |
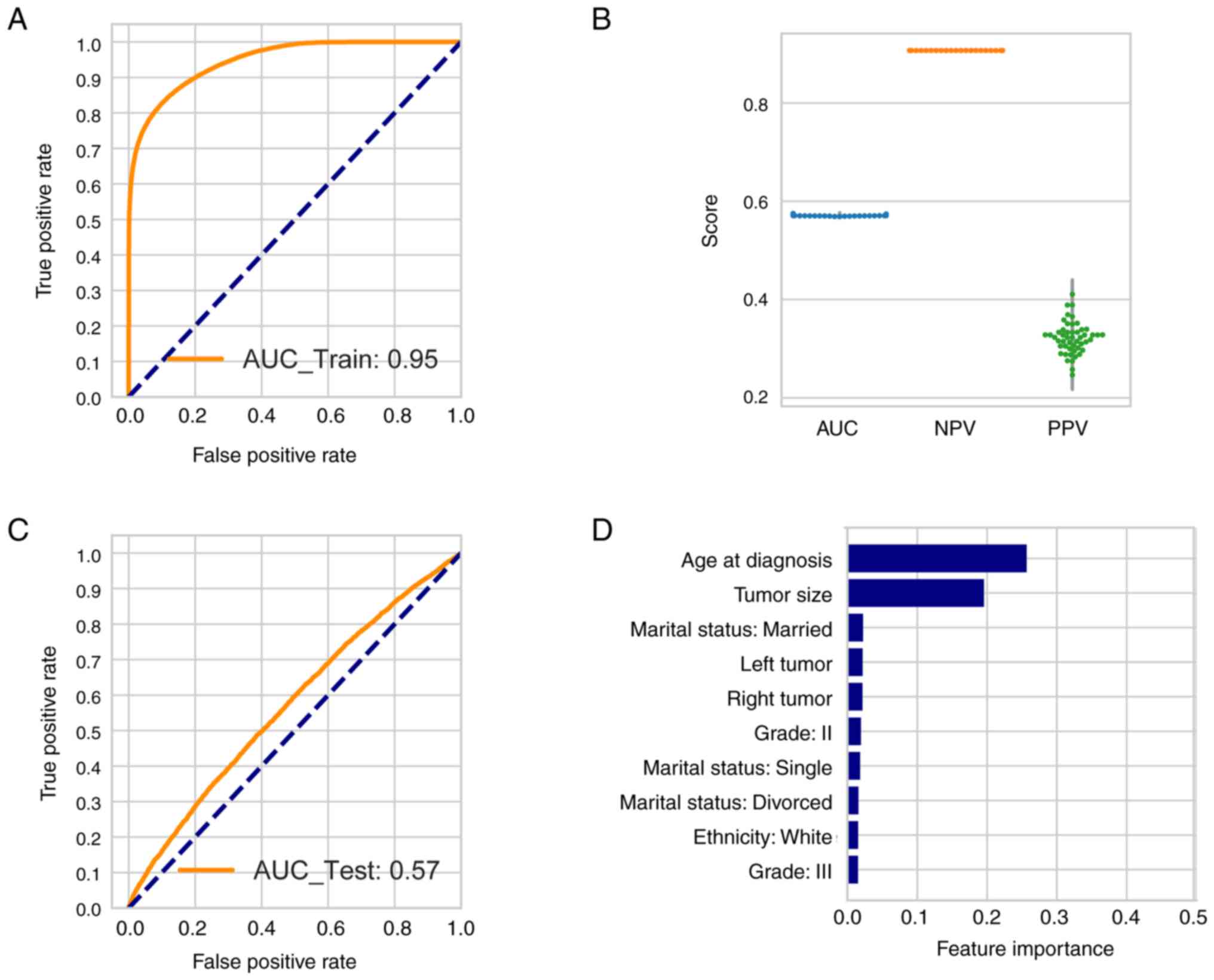
Predicting the occurrence of SPMs in
patients with BC
To detect whether the SPM and OOPM cohorts may be
distinguished by certain features, unsupervised transformation was
performed using FAMD, which was an extension of PCA. The general
purpose of PCA is to find transformed features that may cluster the
patients into two or more clusters and the transformed features are
a combination of the original variables. In the present study,
there were 23 original variables. After transformation, the top 5
features were extracted. Only slightly >10% of the variance of
the data was able to be explained by the top five transformed
features (Fig. S1A), while the
top two transformed features were only able to explain 6.5% of the
variance. The tumor stage contributed the most to the variance
detected by the top two transformed features (Fig. S1B). Patients with SPMs were not
able to be clustered together using the top two transformed
features (Fig. S1C). Therefore,
using supervised learning, a random forest model was created to
predict the probability of SPM in patients with BC.
The patient population was randomly split into a
training set (75%) and a testing set (25%), each stratified by the
presence of SPMs. Parameters including maximum depth and class
weight were learned from the training set and the parameters that
generated the highest positive predictive value (PPV) in the
out-of-bag mode were adopted to create the random forest model. The
model generated an overall area under the curve (AUC) of 0.95 in
the training set (Fig. 4A). To
reduce the influence of randomization, the model was tested 50
times using different random seeds. The mean consistency of the
testing set was 0.91, but in the imbalance dataset, the PPV and
negative predictive value (NPV) were more important features. The
mean AUC, NPV and PPV in the testing set were 0.57, 0.91 and 0.32,
respectively (Fig. 4B). The median
value of the above parameters was the same as their mean value.
A representative ROC curve with its AUC in the
testing set is illustrated in Fig.
4C. The top 10 features contributing to the estimation in the
testing set were displayed in Fig.
4D. Age at diagnosis and tumor size had the highest weight, at
~25 and 20%, respectively.
Discussion
BC has the highest incidence among all cancers in
the world in females, since its incidence has surpassed that of
lung cancer (14). Most studies
have indicated that the clinical factors influencing the survival
of patients with BC include tumor stage, estrogen receptor status,
progesterone receptor status and HER2 status (15). However, with the aging of the
population and the continuous extension of the survival time of
patients with BC, the incidence rate of multiple primary
malignancies has gradually increased in recent years (16). The OS of patients with BC is
related to not only the primary malignancy but also the nature of
SPMs and the organs bearing the SPMs. Determining the risk of SPMs
in patients with BC, predicting patients at high risk for SPMs, and
closely monitoring these patients have a vital role in improving
the OS and guiding clinical practice.
Carcinogenesis is a multistep, long-term process. As
life expectancy increases, the likelihood of being diagnosed with
cancer also increases. Therefore, the risk of developing SPMs
gradually increases with age (17,18).
In addition, compared with the general population, cancer patients
have a much higher risk of developing SPMs (16). The results of the present study
indicated that 9.32% of patients with BC developed SPMs within 7
years after the diagnosis of the primary malignancy and that this
proportion would keep increasing if the follow-up were to be
continued. This finding is similar to the results of Xiao et
al (16).
The present study suggested that approximately half
of SPMs in patients with BC occurred in the breast, while the rest
appeared to occur randomly in other organs. This finding suggests
that the primary malignancy of BC may change the mammary gland
microenvironment and contribute to the occurrence of SPMs (19), or it may be interpreted through the
notion of hereditary cancer syndromes reported in previous studies.
Hereditary BC and ovarian cancer syndrome are hereditary
malignancies that may be confirmed by detecting germline mutations
in the BRCA1 or BRCA2 genes (20). Compared with the general
population, females with BRCA1 or BRCA2 gene
mutations have a significantly higher risk of BC and ovarian cancer
(21). In the present study, SPM
occurred most frequently in the breast, which reflects the
susceptibility of the breast to the invasion of primary BC, in line
with the studies mentioned above, which suggested that the
implementation of preventive mastectomy may obtain a survival
benefit for patients with BC. Numerous studies on the association
between BC and colon cancer have reported the coexistence of common
extrinsic and genetic predisposition factors (18). A prospective cohort study of a
female BC population suggested that the standardized incidence
ratio of secondary primary colorectal cancer in BC survivors was
1.59(22). BRCA mutations
may increase the risks of colorectal cancer (23), ovarian cancer, pancreatic cancer
and prostate cancer (21). The
present study indicated that nearly half of the SPMs occurred
randomly in organs other than the breast (such as the digestive,
respiratory, blood and reproductive systems). The random occurrence
of SPMs may reflect the genetic tendency of these patients
(24), which may also correspond
to the more common sites mentioned in certain studies (18,25-27).
Although a small number of studies have looked into
whether menopausal women are more likely to develop SPMs, the
present study found that patients with SPMs were mostly
HER2-/HR+ menopausal patients with a median
age of 63 years, consistent with the finding of Xiao et al
(16) that >70% of the patients
had reached menopause prior to the diagnosis of the SPMs.
Therefore, it may be reasonable to suggest that SPM monitoring
should begin after the end of the BC regimen. Postmenopausal
elderly patients with a HER2-/HR+ molecular
subtype should be more watchful for SPMs. In particular, for
patients who deny a family history of BC at the first diagnosis but
a hereditary tumor-related syndrome is detected during the
follow-up, as well as in patients with a known family history of
BC, ovarian cancer, pancreatic cancer, prostate or gastrointestinal
cancer, not only routine reexaminations should be performed after
BC operation to exclude recurrence and metastasis, but also the
family tumor history of patients should be reviewed at each
reexamination, so as to avoid missing a diagnosis of SPM due to
ignoring hereditary tumor syndrome. Misdiagnosis or missed
diagnosis should be avoided and early detection, early diagnosis
and early treatment should be aimed for.
The OS of the SPM cohort was significantly lower
than that of the OOPM cohort, indicating that the occurrence of SPM
had a certain role in accelerating disease progression and
deterioration. Compared with the patients with SPMs in non-breast
organs, the patients with SPMs in the breast had significantly
better OS. Compared with patients with SPMs in non-breast organs
and patients with multiple SPMs, the patients with SPMs in the
breast had 54 and 56% lower risks of death, respectively. In
addition, OS was not significantly different between patients with
SPMs in non-breast organs and patients with multiple SPMs, which
indicates that the organs bearing SPMs had a significantly greater
impact on prognosis than other factors, such as the number of
SPMs.
To predict the occurrence of SPM at the time when
the primary BC was diagnosed, a supervised machine learning model
was created based on clinical characteristics and features of the
primary tumor, such as age at diagnosis, marital status and tumor
location. The model of the present study had a PPV of 32% and NPV
of 91%. This performance is not very good, but this was the best
result that was achieved when using the above features after
comparing various models. Compared to the unsupervised machine
learning model, which was not able to clearly distinguish SPMs from
OOPMs, the model of the present study achieved an acceptable PPV
and a high NPV. Of all the features used to create the model, age
at diagnosis and tumor size were the two most important features
predicting SPM, which is reasonable and consistent with previous
reports (4,28). It may be possible to further
improve the performance of the model by adding more features, such
as genetic variation.
The present study has several limitations. First, it
is retrospective and the data originated from different centers;
therefore, it has limitations inherent to such studies such as
heterogeneity regarding data recording and patient management etc.
Furthermore, the differential diagnosis between SPMs and metastatic
lesions is still difficult, so diagnostic confusion between the two
is inevitable. Finally, there is a lack of information regarding
the treatment given after surgery or diagnosis, which is an
important prognostic variable. However, considering the large
population base, the present study made valuable contributions.
In conclusion, the present study describes the
clinical, histological and molecular characteristics of patients
with BC with SPMs based on the SEER dataset. The results suggested
that the OS of the SPM cohort was significantly worse than that of
the OOPM cohort, and the OS of the patients with SPMs in the breast
was significantly better than that of the patients with SPMs in
other organs. Furthermore, the negative effect of SPM on OS was
independent of the metastasis status. A supervised machine learning
model was created that had a 32% PPV and 91% NPV using certain
clinical characteristics and characteristics of the primary
malignancy. In addition, postmenopausal elderly patients with a
HER2-/HR+ molecular subtype should be more
watchful for SPMs. The present results suggest that SPMs in the
breast should be considered a prognostic factor; the association
between BC and SPMs should not be ignored only because of
metastasis. In addition, adequate diagnosis and long-term regular
follow-up are of great significance to patients with malignancies.
Therefore, attention should be paid to SPM monitoring to avoid
misdiagnoses or missed diagnoses and to achieve early detection,
early diagnosis and early treatment in these patients.
Supplementary Material
Unsupervised classification of all
patients with BC. 23 variables, including sex, age and tumor size,
were inputted into the model and the model attempted to find the
eigenvectors and eigenvalues in the 23-dimension space. These
eigenvectors are a linear combination of the original 23 variables
and are also referred to as transformed features. The corresponding
eigenvalues stand for their contribution to the data variance. In
general, features with high eigenvalues may be used to distinguish
data sub-classes. (A) The top 5 transformed features and the
corresponding variance they indicate. (B) Weights of the clinical
characteristics that were the top two transformed features; each
datapoint stands for an original feature, with tumor stage at the
top right. (C) Unsupervised classification of overall BC using the
top two transformed features. Each datapoint stands for one
patient; the red triangles indicate patients with SPM and the blue
circles patients with OOPM. Patients of the SPM and OOPM cohorts
were not able to be distinguished from the figure. SPM, second
primary malignancy; OOPM, only one primary malignancy; BC, breast
cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data used in this study are available from the
SEER research database.
Authors' contributions
Conception and design: QL and HL. Collection and
collation of data: QL and FZ. Data analysis and interpretation: FZ.
Manuscript writing: All authors. All authors have read and approved
the final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T,
Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et
al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2016:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 4:1553–1568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Molina-Montes E, Requena M,
Sanchez-Cantalejo E, Fernandez MF, Arroyo-Morales M, Espin J,
Arrebola JP and Sánchez MJ: Risk of second cancers cancer after a
first primary breast cancer: A systematic review and meta-analysis.
Gynecol Oncol. 136:158–171. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Song F, Qureshi AA, Giovannucci EL, Fuchs
CS, Chen WY, Stampfer MJ and Han J: Risk of a second primary cancer
after non-melanoma skin cancer in white men and women: A
prospective cohort study. PLoS Med. 10(e1001433)2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Donin N, Filson C, Drakaki A, Tan HJ,
Castillo A, Kwan L, Litwin M and Chamie K: Risk of second primary
malignancies among cancer survivors in the United States, 1992
through 2008. Cancer. 122:3075–3086. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heard A, Roder D and Luke C: Multiple
primary cancers of separate organ sites: Implications for research
and cancer control (Australia). Cancer Causes Control. 16:475–481.
2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hemminki K and Boffetta P: Multiple
primary cancers as clues to environmental and heritable causes of
cancer and mechanisms of carcinogenesis. IARC Sci Publ.
2004:289–297. 2004.PubMed/NCBI
|
|
7
|
Travis LB, Rabkin CS, Brown LM, Allan JM,
Alter BP, Ambrosone CB, Begg CB, Caporaso N, Chanock S, DeMichele
A, et al: Cancer survivorship-genetic susceptibility and second
primary cancers: Research strategies and recommendations. J Natl
Cancer Inst. 98:15–25. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bergfeldt K, Einhorn S, Rosendahl I and
Hall P: Increased risk of second primary malignancies in patients
with gynecological cancer. A Swedish record-linkage study. Acta
Oncol. 34:771–777. 1995.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee KD, Chen CY, Huang HJ, Wang TY, Teng
D, Huang SH, Lai CH and Chen MC: Increased risk of second primary
malignancies following uterine cancer: A population-based study in
Taiwan over a 30-year period. BMC Cancer. 15(393)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fritz April, Percy Constance, Jack Andrew,
Shanmugaratnam Kanagaratnam, Sobin Leslie H, et al: International
classification of diseases for oncology, 3rd edition. World Health
Organization, Geneva, 2020. https://apps.who.int/iris/handle/10665/42344.
|
|
11
|
Copur MS and Manapuram S: Multiple primary
tumors over a lifetime. Oncology (Williston Park).
33(629384)2019.PubMed/NCBI
|
|
12
|
Shaitelman SF, Grills IS, Kestin LL, Ye H,
Nandalur S, Huang J and Vicini FA: Rates of second malignancies
after definitive local treatment for ductal carcinoma in situ of
the breast. Int J Radiat Oncol Biol Phys. 81:1244–1251.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Goodman ZD: Neoplasms of the liver. Mod
Pathol. 20 (Suppl 1):S49–S60. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miller KD, Nogueira L, Devasia T, Mariotto
AB, Yabroff KR, Jemal A, Kramer J and Siegel RL: Cancer treatment
and survivorship statistics, 2022. CA Cancer J Clin. 72:409–436.
2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiao L, Cao T, Ou J and Liang W: Clinical
characteristics and prognostic analysis of multiple primary
malignant neoplasms in female patients with breast cancer or
genitalia malignancies. PeerJ. 10(e13528)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Luciani A, Ascione G, Marussi D, Oldani S,
Caldiera S, Bozzoni S, Codecà C, Zonato S, Ferrari D and Foa P:
Clinical analysis of multiple primary malignancies in the elderly.
Med Oncol. 26:27–31. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tripodi D, Cannistra' C, Gagliardi F,
Casella G, Lauro A, De Luca A, Amabile MI, Palumbo P, Pironi D,
Mascagni D, et al: Coincidental or Causal? concurrence of
colorectal carcinoma with primary breast cancer. Dig Dis Sci.
67:437–444. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Y and Cao X: Characteristics and
significance of the Pre-metastatic Niche. Cancer Cell. 30:668–681.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Noh SK, Yoon JY, Ryoo UN, Choi CH, Sung
CO, Kim TJ, Bae DS and Kim BG: A case report of quadruple cancer in
a single patient including the breast, rectum, ovary, and
endometrium. J Gynecol Oncol. 19:265–269. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
National Comprehensive Cancer Network
(NCCN): Breast cancer (version 2.2022). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
|
|
22
|
Lu Y, Segelman J, Nordgren A, Lindström L,
Frisell J and Martling A: Increased risk of colorectal cancer in
patients diagnosed with breast cancer in women. Cancer Epidemiol.
41:57–62. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Phelan CM, Iqbal J, Lynch HT, Lubinski J,
Gronwald J, Moller P, Ghadirian P, Foulkes WD, Armel S, Eisen A, et
al: Incidence of colorectal cancer in BRCA1 and BRCA2 mutation
carriers: Results from a follow-up study. Br J Cancer. 110:530–534.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yousefi M, Nosrati R, Salmaninejad A,
Dehghani S, Shahryari A and Saberi A: Organ-specific metastasis of
breast cancer: Molecular and cellular mechanisms underlying lung
metastasis. Cell Oncol (Dordr). 41:123–140. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Babacan NA, Aksoy S, Cetin B, Ozdemir NY,
Benekli M, Uyeturk U, Ali Kaplan M, Kos T, Karaca H, Oksuzoglu B,
et al: Multiple primary malignant neoplasms: Multi-center results
from Turkey. J BUON. 17:770–775. 2012.PubMed/NCBI
|
|
26
|
Lv M, Zhang X, Shen Y, Wang F and Yang J,
Wang B, Chen Z, Li P, Zhang X, Li S and Yang J: Clinical analysis
and prognosis of synchronous and metachronous multiple primary
malignant tumors. Medicine (Baltimore). 96(e6799)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhai C, Cai Y, Lou F, Liu Z, Xie J, Zhou
X, Wang Z, Fang Y, Pan H and Han W: Multiple primary malignant
tumors-A clinical analysis of 15,321 patients with malignancies at
a single center in China. J Cancer. 9:2795–2801. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Contrera KJ, Yong V, Reddy CA, Berber E
and Lorenz RR: Second primary tumors in patients with a head and
neck paraganglioma. Head Neck. 41:3356–3361. 2019.PubMed/NCBI View Article : Google Scholar
|