Introduction
The small bowel is the longest portion of the
gastrointestinal tract; however, the incidence of small bowel
cancer is far less than that of colorectal cancer; e.g., in 2022,
11,000 vs. 150,000 new cases, respectively, are predicted to be
diagnosed in the US (1). Overall,
small bowel cancers account for 3-5% of all gastrointestinal
tumors, and the most common histological subtypes are
adenocarcinoma, neuroendocrine tumors, gastrointestinal stromal
tumors and lymphoma. Small bowel adenocarcinoma (SBA) accounts for
30-40% of all primary small bowel cancers (2-5).
The vast majority of SBAs originate from the shortest portion of
the small intestine, the duodenum (52-82%), followed by the jejunum
(11-25%) (6-9).
The mean age group for diagnosis is the fifth and sixth decade of
life (10). The risk factors
include Peutz-Jeghers syndrome, inflammatory bowel disease,
familial adenomatous polyposis, Lynch syndrome, celiac disease,
cystic fibrosis and peptic ulcer disease, in addition to
environmental and dietary factors (10,11).
Surgical resection and lymph node dissection are the
mainstays of localized disease treatment. However, the clinical
diagnosis of SBA is challenging and symptoms usually do not occur
in localized disease (11).
Therefore, a significant number of cases are diagnosed in the
advanced stage due to delays in diagnosis, and despite advances in
diagnostic tools, the time required for diagnosis has remained
unchanged over time (6,9).
The factors associated with short overall survival
(OS) are advanced stage, lack of surgery, older age, primary
duodenal site and high baseline neutrophil-lymphocyte ratio (NLR)
(11-13).
Overall, there are limited studies regarding the disease
characteristics and outcomes of SBA, particularly from the Arab
regions, due to the rarity of the disease. In the present study,
the patient characteristics and clinical outcomes for patients with
SBA treated at our tertiary hospital were described.
Materials and methods
Patients and methods
A retrospective review of consecutive patients
diagnosed with SBA between January 2007 and December 2020 at King
Faisal Specialist Hospital & Research Center (Riyadh, Saudi
Arabia) was performed. Study data were collected and managed using
REDCap electronic data capture tools hosted at King Faisal
Specialist Hospital & Research Center (Riyadh, Saudi Arabia)
(14,15). Ethical approval was obtained from
the Research Ethics Committee at King Faisal Specialist Hospital
& Research Center (Riyadh, Saudi Arabia) and the requirement
for informed consent from the patients was waived. The data
obtained included age at diagnosis, sex, Eastern Cooperative
Oncology Group Performance Status (ECOG PS), past medical and
surgical history, family history, baseline laboratory test results,
TNM staging, management and outcomes, including best responses to
chemotherapy, time-to-progression and status at the last follow-up.
Performance status was evaluated using the Eastern Cooperative
Oncology Group Performance Status (ECOG PS) assessment tool
(16). The patients were staged
according to the American Joint Committee on Cancer Union for
International Cancer Control staging system (17). The disease response was evaluated
using the Response Evaluation Criteria in Solid Tumors (version
1.1) (18). Disease-free survival
(DFS) was defined as the time from surgery until either disease
recurrence or death. Progression-free survival (PFS) was defined as
the time from the beginning of management (chemotherapy, surgery,
radiation therapy or best supportive care) until either disease
progression or death, and OS was defined as the time from the
beginning of management until death from any cause.
Statistical analysis
Categorical variables are described as frequencies
and continuous variables are described as the median and
interquartile range. The association of categorical variables with
metastasis at diagnosis was examined by χ2 tests and
that of continuous variables by using the Mann-Whitney U-test.
Factors tested for associations with the metastatic stage at
diagnosis were presenting symptoms, age, sex, history, baseline
serum albumin level, pretreatment NLR, platelet-lymphocyte ratio
(PLR), tumor markers (CEA and CA19-9 levels) and baseline
hemoglobin (Hb) levels. The tumor markers were defined as positive
if either CEA (>4.3 µg/l) or Ca 19-9 (>27 U/ml) was present.
Hypoalbuminemia was defined as an albumin level <34 g/l. The
best NLR and PLR cutoff was obtained using a receiver operating
characteristic (ROC) curve (19,20).
Uni- and multivariate logistic regression analysis was used to
estimate the association of these variables with the metastatic
stage at diagnosis. The Kaplan-Meier method was used to estimate
DFS and OS, and a log-rank test was used to determine factors
associated with survival outcomes. Statistical analyses were
performed using SPSS v.28 (IBM Corporation). P<0.05 was
considered to indicate statistical significance.
Results
Patient characteristics
Of 137 small bowel primary tumors diagnosed during
the study period, 43 cases of SBA were identified and included in
the analysis. The median age at diagnosis was 53 years (range,
44-66 years) and the majority of patients (76.7%) were males. The
detailed patient and disease characteristics are presented in
Table I. The most common primary
site was the duodenum (60.5%), followed by the jejunum (27.9%) and
ileum (6.9%). The most common diagnostic modalities were EGD
(60.5%) and CT scan (23.3%). The diagnosis was established
intraoperatively in eight patients. The tumor markers were elevated
in 21 patients (48.8%): CEA was elevated in 10 patients (23.3%) and
CA19-9 was elevated in 17 patients (39.5%). Furthermore, 18
patients (41.9%) presented with synchronous metastasis and the most
common sites for metastases were the liver (n=10 patients),
followed by peritoneum (n=8), lung (n=8), lymph nodes (n=5) and
bone (n=2) (data not shown).
 | Table IPatients and disease characteristics
(n=43). |
Table I
Patients and disease characteristics
(n=43).
| Characteristic | Value |
|---|
| Median age at
diagnosis, years | 53 (44-66) |
| Male sex | 33 (76.7) |
| PMH | |
|
Celiac
disease | 3 (6.9) |
|
Lynch
syndrome | 1 (2.3) |
|
Familial
adenomatous polyposis | 1 (2.3) |
|
Multiple
colonic polyps (non-APC) | 1(2.3) |
| PSH | |
|
Cholecystectomy | 7 (16.2) |
|
Hemicolectomy | 6 (13.9) |
| Presentation | |
|
Abdominal
pain | 24 (55.8) |
|
Vomiting | 17 (39.5) |
|
Bowel
obstruction | 13 (30.2) |
|
Anemia | 12 (27.9) |
|
Overt
gastrointestinal tract bleeding | 5 (11.6) |
|
Weight
loss | 8 (18.6) |
|
Jaundice | 5 (11.6) |
| Baseline laboratory
parameters, and normal values | |
|
Hb, g/dl
(NR, 11.6-16.6) | 10.5
(7.8-12.2) |
|
CEA, µg/l
(NR, 0-4.3) | 2.15 (1.6-4.2) |
|
CA19-9, U/ml
(NR, 0-27) | 30 (12-77) |
|
Albumin, g/l
(NR, 34-54) | 34 (30.9-38.7) |
|
Bilirubin,
mg/dl (NR, 0.1-1.2) | 6 (4.0-9.5) |
|
NLR | 1.46
(0.75-3.7) |
|
PLR | 133.3
(103-267) |
| ECOG PS | |
|
0/I | 22 (51.2) |
|
II | 7 (16.3) |
|
III | 11 (25.5) |
|
NA | 3 (6.9) |
| Site of primary
tumor | |
|
Duodenum | 26 (60.5) |
|
Jejunum | 12 (27.9) |
|
Ileum | 3 (6.9) |
|
Unspecified | 2 (4.7) |
| Tumor grade | |
|
G1 | 3 (6.9) |
|
G2 | 33 (76.7) |
|
G3 | 5 (11.6) |
|
NA | 2 (4.7) |
| Stage | |
|
I | 3 (6.9) |
|
II | 12 (27.9) |
|
III | 10 (23.3) |
|
IV | 18 (41.9) |
Factors associated with metastatic
stage at diagnosis
The continuous values of baseline albumin (P=0.01),
NLR (P<0.001) and PLR (P=0.01) were associated with the
metastatic stage at diagnosis. There was no association of
presenting symptoms, age, sex, history of cholecystectomy, CEA
level, CA19-9 level or baseline Hb with metastasis at diagnosis
(data not shown). The best cutoff for the NLR was >0.85 and that
for PLR was >125 (Fig. S1).
Univariate logistic regression was significant for hypoalbuminemia
[odds ratio (OR): 3.75, 95% CI: 1.01-13.7; P=0.04] and high NLR
(OR: 20.2, 95% CI: 2.2-182.4; P<0.01). There was no significant
association between primary disease site (OR: 2.1, 95% CI:
0.77-6.11; P=0.1), tumor grade (OR: 1.1, 95% CI: 0.26-4.5; P=0.9),
PLR (OR: 4.3, 95% CI: 0.95-19.5; P=0.06) and tumor markers (OR:
1.2, 95% CI: 0.33-4.6; P=0.7) with metastasis at diagnosis.
Multivariate analysis indicated that in comparison to patients with
a low NLR (<0.85), patients with a high NLR were more likely to
be in the metastatic stage, with an OR of 17.6 (95% CI: 1.7-178;
P=0.01). Furthermore, patients with hypoalbuminemia were more
likely to be in the metastatic stage at diagnosis (OR: 5.5, 95% CI:
0.9-31.5); however, the P-value was insignificant (P=0.06) (data
not shown).
Characteristics of management
A total of 23 (92%) out of 25 patients received
treatment for localized disease. Furthermore, 17 patients (68%)
underwent surgery (microscopically margin-negative resection, R0
achieved in 13 patients), and 11 patients received chemotherapy:
Adjuvant, 5 patients; and upfront, 6 patients (XELOX, 6 patients;
and FOLFOX, 5 patients). The median duration of chemotherapy was
3.75 months (range, 0.5-6.0 months). A total of 9 patients
developed recurrence, 4 received second-line chemotherapy and 1
underwent cytoreductive surgery with hyperthermic intraperitoneal
chemotherapy. None of the patients received chemotherapy beyond the
second line. In the metastatic group, 12 out of 18 patients
received treatment. A total of 10 patients (55%) received
chemotherapy (FOLFOX, 6 patients; XELOX, 3 patients; and nivolumab,
1 patient), the median duration of chemotherapy was 3.5 months
(range, 1.0-6.0 months) and the best response was partial response
(n=1), stable disease (n=1), progressive disease (n=5) and unknown
in 3 patients. A total of 6 patients underwent surgery (R0, 2
patients) and 2 received radiation therapy. Furthermore, 3 patients
received second-line chemotherapy (data not shown).
Survival outcomes
The median duration of follow-up was 12 months
(range, 2-47 months). The median DFS for patients who achieved
complete resection (R0 vs. R1) was 49 vs. 5 months (P=0.02). The
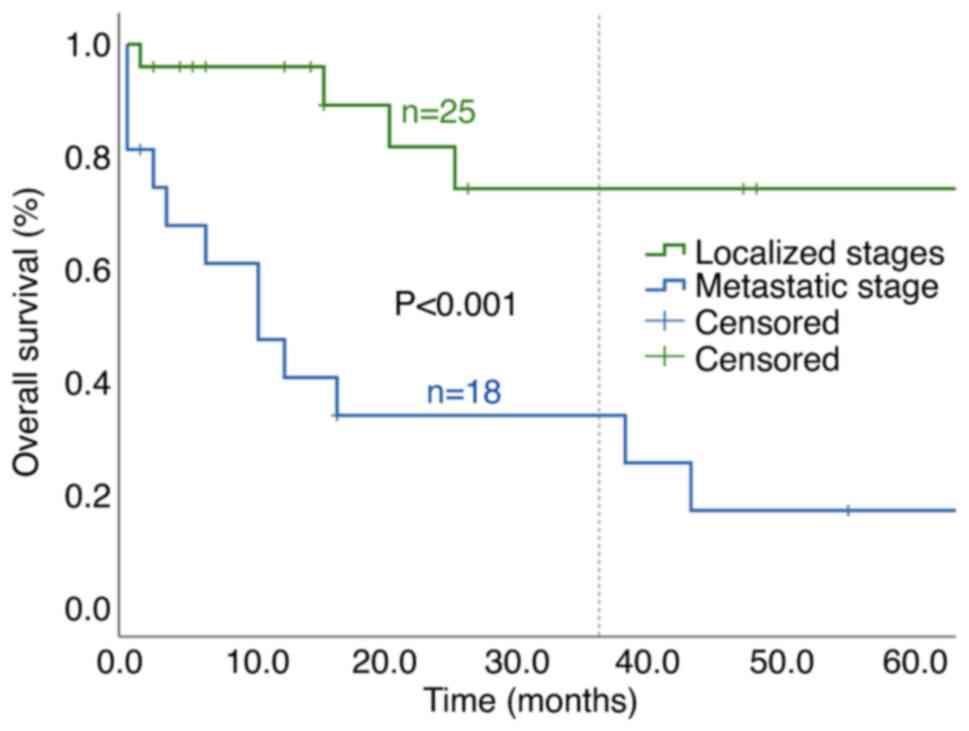
median OS for localized disease vs. metastatic stage was not
reached vs. 10 months and the 3-year OS was 74.3 vs. 33.9%,
respectively (P<0.001; Fig. 1).
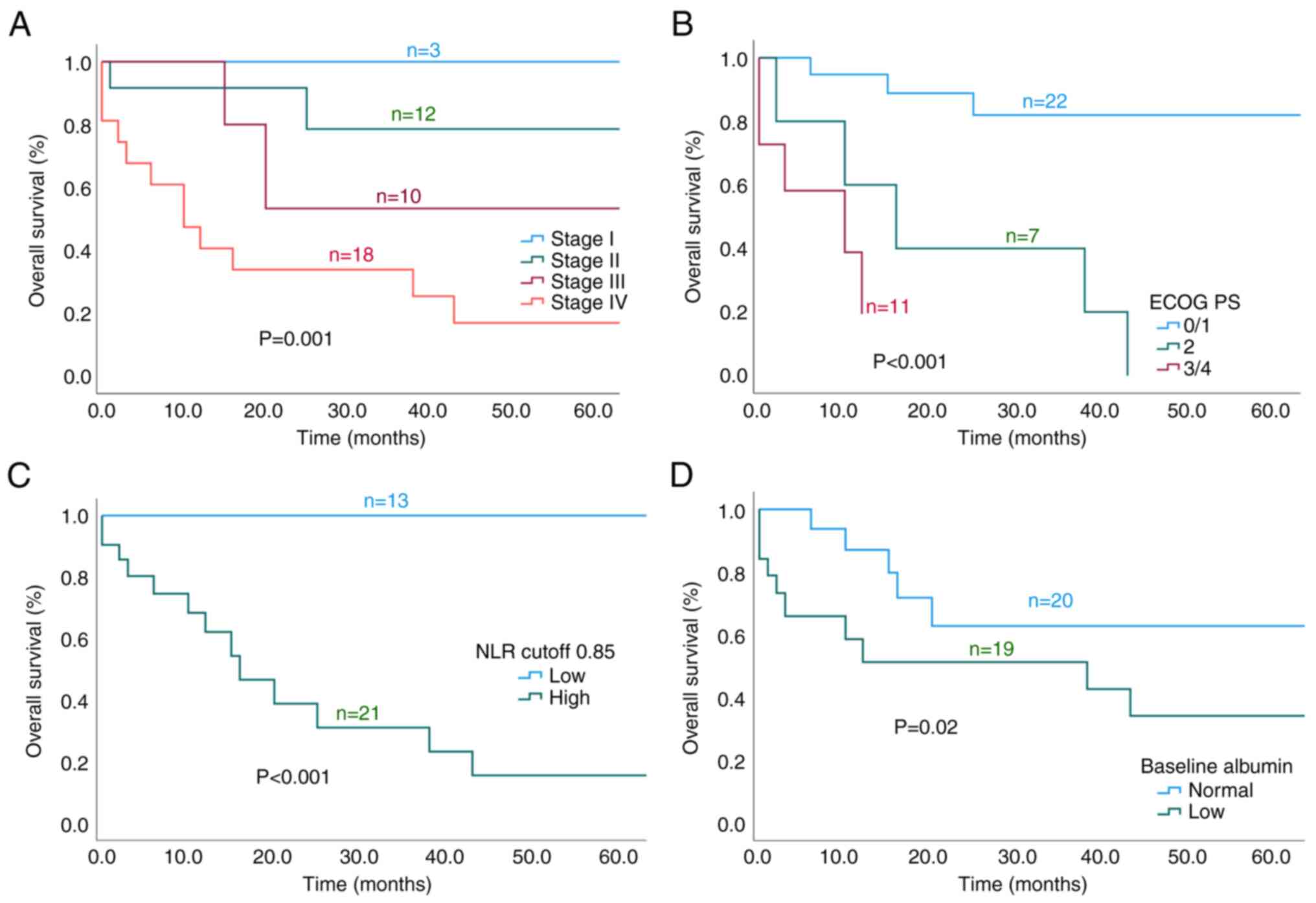
The 3-year OS rates based on disease stage were 100% (I), 85% (II),
53% (III) and 33.9% (IV) (P=0.001). Furthermore, a lower ECOG PS
(P<0.001), low baseline NLR (P<0.001) and no hypoalbuminemia
(P=0.02) were associated with better OS (Fig. 2A-D). Chemotherapy administration
for metastatic disease was associated with better PFS and OS; the
median PFS was 6 vs. 1 month (P=0.03) and the median OS was 38 vs.
3 months (P=0.02). There was a trend of better survival with low
CA19-9 and low PLR, but it did not reach statistical significance
(P=0.051 and P=0.33, respectively). There was no association
between grade and OS (P=0.92). Furthermore, there was no
significant difference in OS by primary site (duodenum, jejunum or
ileum). The 1-year OS rate was 77, 73 and 66.7%, respectively
(P=0.60) (data not shown).
Discussion
The findings of the current study are consistent
with previous reports and support recent findings related to the
association of the baseline NLR with OS. Furthermore, the results
indicated that a high baseline NLR was independently associated
with a more advanced stage at diagnosis. Early-stage disease,
better performance status, low NLR, normal albumin level and
chemotherapy in the advanced stage were associated with better
OS.
Older age at diagnosis in the patients of the
present study did not correlate with survival outcomes. However,
the median age in the present cohort was 53 years, which is
relatively younger than the worldwide median age at diagnosis for
SBA, perhaps due to the younger age distribution in the local
population. In contrast to the young age at diagnosis in the
present cohort (50% were younger than 55 years), other studies have
found SBA to primarily be a disease of the elderly (3,12,13,21).
SBA tends to occur more frequently in males (6,7,9,10,22),
consistent with the present cohort. However, certain studies
reported a relatively equal distribution by sex (12,13,21).
There was no association between sex and survival outcomes in the
present cohort; however, male sex was previously reported to be
associated with worse survival outcomes in SBA (3,23).
A total of 30.2% of the patients of the current
study presented with bowel obstruction or overt bleeding (11.6%),
perhaps due to late presentation. These rates are similar to those
of previous studies (6,24-26).
Of note, 16.2% of the patients of the present study had a history
of cholecystectomy; in two-thirds of them, the duodenum was the
primary site and it was the jejunum in one-third. The Swedish
registry included a quarter million patients who underwent
cholecystectomy and reported a significant increase in small
intestine cancers after surgery that correlated with the distance
from the common bile duct (4,27).
The findings of the present study were similar to
those of previous studies that reported the benefit of R0 resection
in terms of prolonged survival outcomes in metastatic settings
(10,11). Patients with advanced stage and
poor ECOG PS had worse OS, consistent with other reported series
(2,8,21,22,25,28).
Nearly half of the patients of the present study had
increased tumor markers, which were not associated with survival
outcomes. Of note, high CA19-9 was associated with a trend of
longer survival that was more pronounced in advanced settings, but
it was not statistically significant (P=0.06). However, high CA19-9
was associated with shorter OS, particularly in the advanced stage
(12,29). Hypoalbuminemia in the present
cohort exhibited an association with a more advanced stage at
diagnosis and a significant association with worse OS, consistent
with the report by Sakae et al (28). Furthermore, a previous report also
indicated that high lactate dehydrogenase is a prognostic factor
for poor OS (28).
The NLR reflects the underlying inflammatory and
immunity processes, two essential parts of the hallmarks of cancer
(30). The NLR has been proven to
have prognostic survival value in a variety of solid tumors,
including gastrointestinal malignancies (31,32).
Recently, two studies indicated that a high NLR is associated with
poor survival outcomes in patients with SBA (13,33).
Yanko et al (13) used 4.5
as the optimal cutoff for the NLR. They selected 4.5 based on the
high median NLR in their cohort and the optimal NLR cutoff (median
3.5-4.5) of a previous study (34). However, that study included
metastatic diseases and did not consider cancer site specificity
(34). In the cohort of the
present study, the median NLR was 1.46 and it was prespecified that
the optimal NLR would be obtained from the ROC curve (19,20).
However, in the present cohort, patients with NLR >4.5 had worse
survival, with a 3-year OS of 16.7 vs. 63% (P<0.001). Of note, a
high NLR in this cohort demonstrated an association with the
metastatic stage at diagnosis, reflecting the aggressiveness of the
disease. Despite the small sample size, the present results support
the value of the NLR as an available biomarker that may be
incorporated into the management of SBA. Further research is
required to investigate the value of NLR in this setting and with
immunotherapy (35). A low PLR was
associated with a trend toward better OS, but it was not
statistically significant. The median OS for low vs. high PLR was
65 vs. 38 months (P=0.33).
It should be acknowledged that the small sample size
and retrospective nature of the present study are significant
limitations. However, to the best of our knowledge, the present
study was the first to explore the clinical characteristics and
outcomes for SBA in a population from any Arab country, in this
case Saudi Arabia.
In conclusion, the NLR is associated with a more
advanced stage at the time of diagnosis of SBA. In addition to the
ECOG PS, the stage at diagnosis, hypoalbuminemia and NLR are
promising prognostic factors for survival.
Supplementary Material
ROC curves showing the association of
(A) the baseline NLR and (B) PLR with the metastatic stage at
diagnosis of small bowel adenocarcinoma. ROC, receiver operating
characteristic; AUC, area under the ROC curve; NLR,
neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
Acknowledgements
Early results were presented as an abstract at the
American Society of Clinical Oncology Conference (ASCO) 2021 and
this abstract was published in the Journal of Clinical Oncology
(abstract no. e16277; available at https://ascopubs.org/doi/10.1200/JCO.2021.39.15_suppl.e16277).
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BA, AB, AS, MAE and AHA conceived the study and
wrote the proposal. BA, MA, AS, MAE and SB collected the data. BA,
MA, AB, SB and AHA analyzed the data. BA, MA and AHA confirm the
authenticity of all of the raw data. BA wrote the first draft of
the manuscript. All authors critically revised the manuscript for
important intellectual content and have read and approved the final
version.
Ethics approval and consent to
participate
All methods followed the relevant guidelines and
regulations. The study was approved and the requirement for
informed patient consent was waived by the Research Advisory
Council at King Faisal Specialist Hospital and Research Centre
(Riyadh, Saudi Arabia; no. 2221168).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lepage C, Bouvier AM, Manfredi S, Dancourt
V and Faivre J: Incidence and management of primary malignant small
bowel cancers: A well-defined French population study. Am J
Gastroenterol. 101:2826–2832. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY,
Bennett CL and Talamonti MS: Small bowel cancer in the United
States: Changes in epidemiology, treatment, and survival over the
last 20 years. Ann Surg. 249:63–71. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schottenfeld D, Beebe-Dimmer JL and
Vigneau FD: The epidemiology and pathogenesis of neoplasia in the
small intestine. Ann Epidemiol. 19:58–69. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Barsouk A, Rawla P, Barsouk A and Thandra
KC: Epidemiology of cancers of the small intestine: Trends, risk
factors, and prevention. Med Sci (Basel). 7(46)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dabaja BS, Suki D, Pro B, Bonnen M and
Ajani J: Adenocarcinoma of the small bowel: Presentation,
prognostic factors, and outcome of 217 patients. Cancer.
101:518–526. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moon YW, Rha SY, Shin SJ, Chang H, Shim HS
and Roh JK: Adenocarcinoma of the small bowel at a single Korean
institute: Management and prognosticators. J Cancer Res Clin Oncol.
136:387–394. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aparicio T, Zaanan A, Svrcek M,
Laurent-Puig P, Carrere N, Manfredi S, Locher C and Afchain P:
Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis
and treatment. Dig Liver Dis. 46:97–104. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Colina A, Hwang H, Wang H, Katz MHG, Sun
R, Lee JE, Thomas J, Tzeng CW, Wolff RA, Raghav K and Overman MJ:
Natural history and prognostic factors for localised small bowel
adenocarcinoma. ESMO Open. 5(e000960)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lech G, Korcz W, Kowalczyk E, Słotwiński R
and Słodkowski M: Primary small bowel adenocarcinoma: Current view
on clinical features, risk and prognostic factors, treatment and
outcome. Scand J Gastroenterol. 52:1194–1202. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Overman MJ: Recent advances in the
management of adenocarcinoma of the small intestine. Gastrointest
Cancer Res. 3:90–96. 2009.PubMed/NCBI
|
|
12
|
Hong SH, Koh YH, Rho SY, Byun JH, Oh ST,
Im KW, Kim EK and Chang SK: Primary adenocarcinoma of the small
intestine: Presentation, prognostic factors and clinical outcome.
Jpn J Clin Oncol. 39:54–61. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yanko E, Le D, Mahmood S, Ginther DN,
Chalchal HI, Kanthan R, Haider K, Zaidi A, Dueck DA, Ahmed O, et
al: Outcomes of patients with small intestine adenocarcinoma in a
canadian province: A retrospective multi-center population-based
cohort study. Cancers (Basel). 14(2581)2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Harris PA, Taylor R, Minor BL, Elliott V,
Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J,
et al: The REDCap consortium: Building an international community
of software platform partners. J Biomed Inform.
95(103208)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Harris PA, Taylor R, Thielke R, Payne J,
Gonzalez N and Conde JG: Research electronic data capture
(REDCap)-a metadata-driven methodology and workflow process for
providing translational research informatics support. J Biomed
Inform. 42:377–381. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Conill C, Verger E and Salamero M:
Performance status assessment in cancer patients. Cancer.
65:1864–1866. 1990.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schwartz LH, Litière S, De Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Badora-Rybicka A, Nowara E and
Starzyczny-Słota D: Neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio before chemotherapy as potential
prognostic factors in patients with newly diagnosed epithelial
ovarian cancer. ESMO Open. 1(e000039)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Noh H, Eomm M and Han A: Usefulness of
pretreatment neutrophil to lymphocyte ratio in predicting
disease-specific survival in breast cancer patients. J Breast
Cancer. 16:55–59. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang D, Li C, Li Y, Liu W, Zhao L, Güngör
C, Tan F and Zhou Y: Specific survival nomograms based on SEER
database for small intestine adenocarcinoma. Ann Palliat Med.
10:7440–7457. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tsushima T, Taguri M, Honma Y, Takahashi
H, Ueda S, Nishina T, Kawai H, Kato S, Suenaga M, Tamura F, et al:
Multicenter retrospective study of 132 patients with unresectable
small bowel adenocarcinoma treated with chemotherapy. Oncologist.
17:1163–1170. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gu Y, Deng H, Wang D and Li Y: Metastasis
pattern and survival analysis in primary small bowel
adenocarcinoma: A SEER-based study. Front Surg.
8(759162)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Negoi I, Paun S, Hostiuc S, Stoica B,
Tanase I, Negoi RI and Beuran M: Most small bowel cancers are
revealed by a complication. Einstein (Sao Paulo). 13:500–505.
2015.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
25
|
Chaiyasate K, Jain AK, Cheung LY, Jacobs
MJ and Mittal VK: Prognostic factors in primary adenocarcinoma of
the small intestine: 13-Year single institution experience. World J
Surg Oncol. 6(12)2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Farhat MH, Shamseddine AI and Barada KA:
Small bowel tumors: Clinical presentation, prognosis, and outcome
in 33 patients in a tertiary care center. J Oncol.
2008(212067)2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lagergren J, Ye W and Ekbom A: Intestinal
cancer after cholecystectomy: Is bile involved in carcinogenesis?
Gastroenterology. 121:542–547. 2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sakae H, Kanzaki H, Nasu J, Akimoto Y,
Matsueda K, Yoshioka M, Nakagawa M, Hori S, Inoue M, Inaba T, et
al: The characteristics and outcomes of small bowel adenocarcinoma:
A multicentre retrospective observational study. Br J Cancer.
117:1607–1613. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zaanan A, Costes L, Gauthier M, Malka D,
Locher C, Mitry E, Tougeron D, Lecomte T, Gornet JM, Sobhani I, et
al: Chemotherapy of advanced small-bowel adenocarcinoma: A
multicenter AGEO study. Ann Oncol. 21:1786–1793. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Roxburgh CSD and McMillan DC: Role of
systemic inflammatory response in predicting survival in patients
with primary operable cancer. Future Oncol. 6:149–163.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Howard R, Kanetsky PA and Egan KM:
Exploring the prognostic value of the neutrophil-to-lymphocyte
ratio in cancer. Sci Rep. 9(19673)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Huffman BM, Patel S, Yadav S, Jin Z and
Mahipal A: Lymphocyte-to-monocyte ratio and
neutrophil-to-lymphocyte ratio independently predict survival in
resected small bowel adenocarcinoma. J Clin Oncol. 37 (Suppl
4)(S426)2019.
|
|
34
|
Vano YA, Oudard S, By MA, Têtu P, Thibault
C, Aboudagga H, Scotté F and Elaidi R: Optimal cut-off for
neutrophil-to-lymphocyte ratio: Fact or Fantasy? A prospective
cohort study in metastatic cancer patients. PLoS One.
13(e0195042)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Valero C, Lee M, Hoen D, Weiss K, Kelly
DW, Adusumilli PS, Paik PK, Plitas G, Ladanyi M, Postow MA, et al:
Pretreatment neutrophil-to-lymphocyte ratio and mutational burden
as biomarkers of tumor response to immune checkpoint inhibitors.
Nat Commun. 12(729)2021.PubMed/NCBI View Article : Google Scholar
|