Introduction
Lymphoepithelial carcinoma (LEC), according to the
current World Health Organization Classification, is one of 21
subtypes of salivary malignant epithelial tumors and is
histologically an undifferentiated carcinoma with a syncytial
growth pattern accompanied by prominent non-neoplastic
lymphoplasmacytic infiltrates, mostly associated with Epstein-Barr
virus (EBV) infection (1,2). These histological features are
indistinguishable from those of undifferentiated nasopharyngeal
carcinoma (NPC), which is also associated with EBV infection. LEC
arising in the salivary glands is extremely rare, comprising only
0.4% of salivary carcinomas (3).
Thus, the information on this tumor is limited to case reports and
small case series, most of which described patients from Arctic
Inuit (Eskimo), Southern Chinese, and Japanese populations with
strong EBV involvement (1). Given
its rarity, neither the etiology nor clinical features of LEC of
the salivary gland have been fully elucidated. Here, we report a
rare case of EBV-associated LEC arising in the parotid gland, which
was treated with surgery followed by radiotherapy. Somatic mutation
test using next generation sequencing (NGS) was also performed and
showed no evidence of any mutations.
Case report
A 60-year-old Japanese woman with a 3-years history
of a slow-growing, painless mass in her left parotid gland was
admitted to our department. She had no medical or familial history
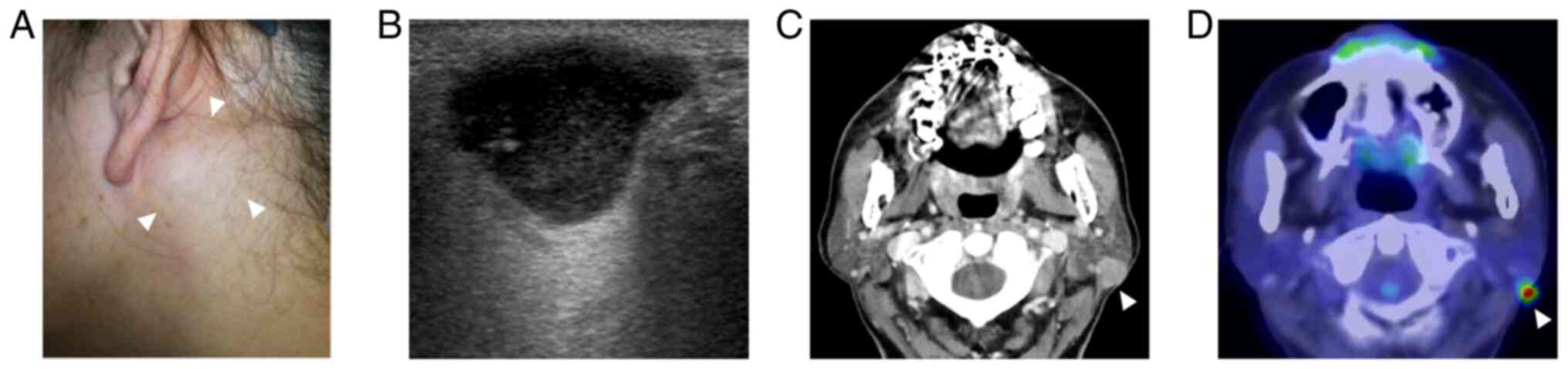
of note. A thumb-sized, hard mass was palpated on the left parotid
gland (Fig. 1A). No facial palsy
was observed. Endoscopic examination did not reveal any tumorous
lesions in the nasopharynx. Ultrasonography revealed a
well-circumscribed, lobulated, hypoechoic mass with posterior
enhancement measuring 19x12x10 mm in the left parotid gland
(Fig. 1B). Computed tomography
(CT) revealed a well-circumscribed, solid mass exhibiting
homogeneous enhancement in the posterior part of the left parotid
gland (Fig. 1C). No abnormal
enlargement of lymph nodes was identified.
Fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT
revealed uptake by the tumor, but not in other areas including the
nasopharynx (Fig. 1D). Fine-needle
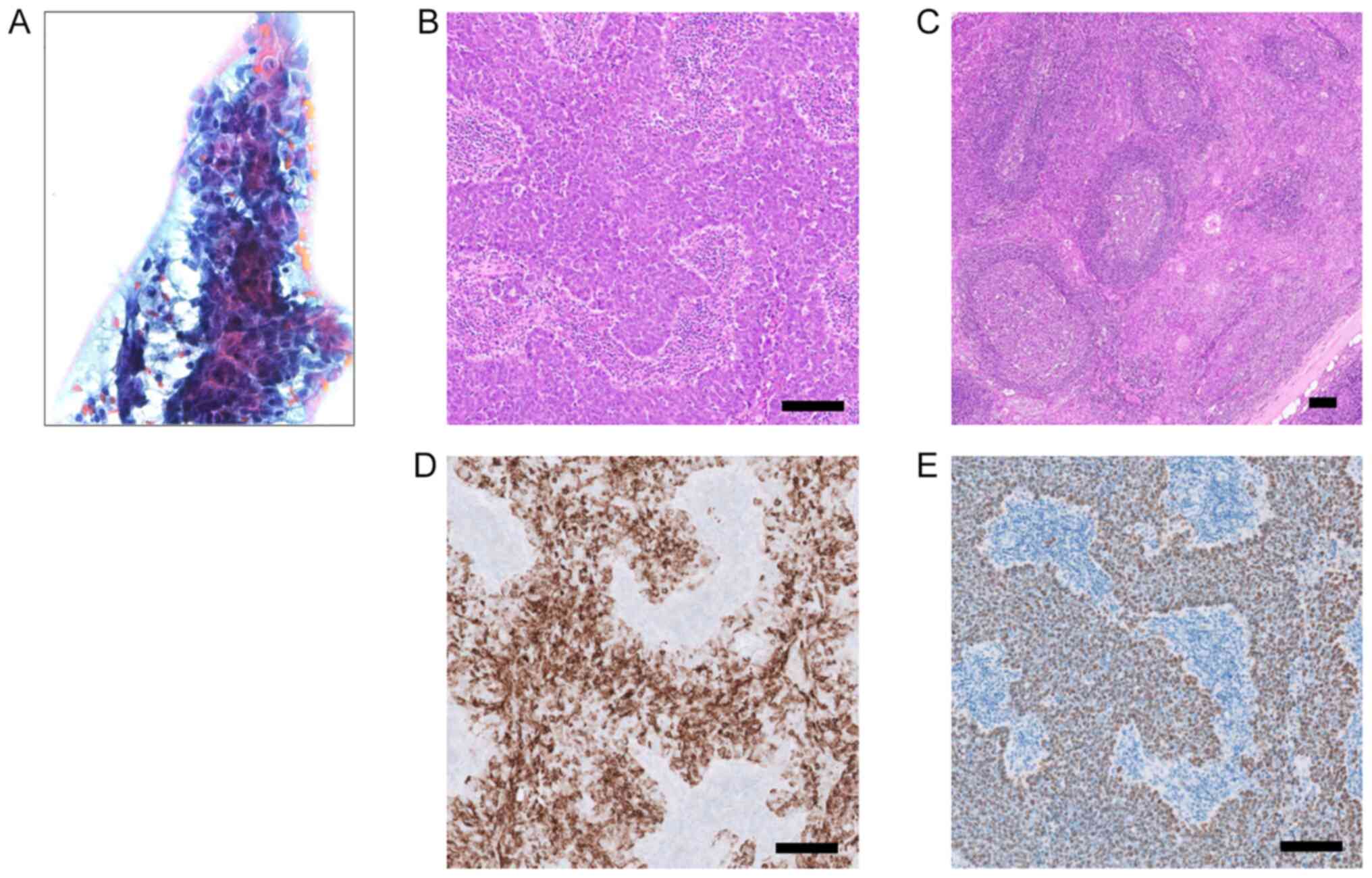
aspiration cytology (FNAC) demonstrated numerous irregularly shaped
trabecular clusters of relatively large cancer cells with vesicular
chromatin and prominent ‘cherry-red’ nucleoli (Fig. 2A). Lymphocytic infiltrate was not
conspicuous. Cytologically, poorly differentiated carcinoma was
suggested. The patient underwent left superficial parotidectomy
with adequate safety margins. After making an S-shaped incision,
the facial nerve was identified and preserved. The adjacent portion
of the sternocleidomastoid muscle was resected, and selective neck
dissection of Level II was added. Her clinical course proved
uneventful. Postoperatively, the patient received radiotherapy to
the parotid gland area at 50 Gy and then has been screened with
ultrasonography and CT scan at fixed intervals. No evidence of
recurrence has been observed as of 20 months postoperatively.
Histologically, a well-circumscribed, encapsuled
nodule was composed of sheets and cords of relatively large cancer
cells with vesicular nuclei, prominent nucleoli, and indistinct
cell borders (Fig. 2B). Scattered
mitotic figures were observed. The sheets and cords of tumor were
intermingled with abundant lymphoplasmacytic infiltrate with
secondary lymphoid follicles (Fig.
2C). No neck lymph node metastasis was identified. Surgical
margins were negative for malignancy. Immunoperoxidase staining of
formalin-fixed, paraffin-embedded (FFPE) tissue sections with
anti-keratin/cytokeratin monoclonal antibody (clone AE1/AE3;
Nichirei Biosciences) was performed using a Ventana OptiView DAB
IHC detection kit (Roche Diagnostics). Tumor cells were positive
for AE1/AE3 (Fig. 2D) and negative
for human epidermal growth factor receptor-2, androgen receptor,
S-100 protein, α-smooth muscle actin, synaptophysin, and
chromogranin (data not shown). In situ hybridization for
EBV-encoded RNA (EBER) on FFPE tissue sections was assessed by
using a fluorescein isothiocyanate-conjugated EBER peptide nucleic
acid (PNA)-probe (DaKoCytomation, Glostrup, Denmark) and PNA in
situ hybridization detection kit (DaKoCytomation) according to
the manufacturer's instructions. Diffuse nuclear staining was
observed in the tumor cells (Fig.
2E). These histologic features, immunohistochemical profile,
and in situ hybridization for EBER were those of an
EBV-associated LEC, pT1N0M0, pStage I on the 8th edition of the
AJCC/TNM staging system (4).
Blood examination showed high levels of serum
anti-viral capsid antigen (VCA) immunoglobulin (Ig)G (1:10.6) and
anti-EBV-nuclear antigen (EBNA) IgG (1:4) antibodies, and negative
results for anti-VCA IgA, anti-VCA IgM, and anti-early antigen (EA)
IgG antibodies. Targeted-NGS was performed as previously described
(5). Briefly, total DNA was
extracted from FFPE tissue sections using a Maxwell 16 FFPE Plus
LEV DNA purification kit (Promega). Amplicon sequencing of targeted
regions of 160 cancer-related genes (Table SI) was carried out using the
GeneRead DNAseq Targeted Panels V2 Human Clinically Relevant Tumor
Panel (NGHS-101X; Qiagen). Libraries were sequenced using an
Illumina MiSeq platform (Illumina). Raw read data obtained from the
amplicon sequencing were processed using online analytical
resources from the GeneRead DNAseq Variant Calling Service for
analysis of mutations. No mutations, including known significant
mutations reported in NPC (6),
were identified.
Discussion
Clinical characteristics of 27 patients with
EBV-associated LEC arising in the parotid gland that have been
described in the literature with detailed information since 2005,
including the present case, are shown in Table I (7-18).
These tumors occurred across a wide age range (21-67 years; median,
46 years), and a female predominance was evident (17 females, 10
males). Swelling in the parotid area as clinical presentation was
seen in all 27 patients. In addition, three patients (11%) showed
facial nerve palsy and only one (4%) showed a painful mass. The
time intervals from first symptom to treatment ranged from 3 to 72
months (median, 11 months). Maximum lesion diameters ranged from 15
to 93 mm.
 | Table IClinical characteristics of patients
with EB virus-positive lymphoepithelial carcinoma arising in the
parotid gland since 2005. |
Table I
Clinical characteristics of patients
with EB virus-positive lymphoepithelial carcinoma arising in the
parotid gland since 2005.
| First author,
year | Age/Sex | Symptom duration,
months | Size, mm | FNAC | Stage | Neck node | Treatment | Prognosis
(months) | (Refs.) |
|---|
| Saqui-Salces,
2006 | 56/F | 72 | 25 | Biphasic
neoplasm | III | + | PD + ND, RT | ANED (108) | (7) |
| Saqui-Salces,
2006 | 28/F | 36 | 15 | Poorly diff. ca. | IVa | + | CT | DOD (18) | (7) |
| Manganaris, 2007 | 67/F | 12 | 55x42x24 | Pleomorphic
adenoma | III | - | PD, RT | ANED (12) | (8) |
| Gupta, 2012 | 40/F | 12 | 25x22x17 | Reactive lymphoid
cells | II | - | PD | | (9) |
| Spencer, 2012 | 27/F | 12 | | Lymphoma | | - | PD | | (10) |
| Tang, 2012 | 29/F | 10 | 41x29x37 | Poorly diff. ca. | IVa | + | PD + ND, RT | | (11) |
| Kim, 2017 | 44/M | 12 | 19x17x19 | Squamous cell
ca. | I | - | PD | ANED (60) | (12) |
| Kim, 2017 | 35/F | 36 | 17x15x18 | | I | - | PD | ANED (60) | (12) |
| Topal, 2017 | 66/F | 36 | 93x66x45 | Ca. | IVb | - | PD + ND | DOD (48) | (13) |
| Maeda, 2018 | 21/M | 4 | 20x20 | Negative finding | IVa | + | CRT | ANED (60) | (14) |
| Halder, 2018 | 41/F | 5 | | Poorly diff.
ca. | | + | PD + ND | DOD (3) | (15) |
| Halder, 2018 | 47/M | | | | | + | PD + ND, RT | | (15) |
| Whaley, 2020 | 52/F | 4 | 33 | | IVa | + | PD | DOD (50) | (16) |
| Whaley, 2020 | 60/F | 3 | 30 | | IVa | + | PD, CRT | ANED (112) | (16) |
| Whaley, 2020 | 50/F | 12 | 15 | | I | - | PD, RT | ANED (19) | (16) |
| Whaley, 2020 | 53/F | 11 | 16 | | I | - | PD | DOD (73) | (16) |
| Whaley, 2020 | 54/M | 3 | 45 | | IVb | + | PD, RT | ANED (6) | (16) |
| Whaley, 2020 | 59/M | 7 | 42 | | IVb | + | PD, CRT | AWM (21) | (16) |
| Lv, 2021 | 27/M | 24 | | | IVb | + | CRT | ANED (6) | (17) |
| Lv, 2021 | 33/M | 6 | | | IVb | + | CRT | ANED (50) | (17) |
| Chou, 2022 | 48/M | | 16x12 | | IVa | + | PD + ND, CRT | ANED (163) | (18) |
| Chou, 2022 | 51/F | | 20x12 | | II | - | PD, RT | ANED (120) | (18) |
| Chou, 2022 | 44/M | | 30x18 | | II | - | PD, RT | ANED (54) | (18) |
| Chou, 2022 | 47/F | | 20x15 | | IVa | + | PD + ND, CRT | DOD (40) | (18) |
| Chou, 2022 | 31M | | 24x17 | | IVa | + | PD + ND, CRT | ANED (8) | (18) |
| Chou, 2022 | 27F | | 25x18 | Cytologic
atypia | II | - | PD + ND, RT | ANED (4) | (18) |
| Present case | 60/F | 36 | 19x12x10 | Poorly diff.
ca. | I | - | PD + ND, RT | ANED (20) | - |
Ultrasonographically, LEC lesions have been
described as a hypoechoic solid mass with posterior enhancement and
increased vascularity on color Doppler images (12). On CT scan, the lesions have been
depicted as exophytic, solid masses with good contrast enhancement
in the parotid gland (12).
Fifteen of 27 patients (56%) with EBV-associated LEC revealed neck
lymph node metastasis (Table I).
Identification of enlarged lymph nodes by imaging studies should
merit consideration of neck lymph node dissection.
FNAC diagnosis of parotid LEC is challenging given
its rarity. In the previous reports, FNAC could not make the
definitive diagnosis of LEC in any cases and the interpretation
included carcinomas in 7 patients, lymphoma in 1 patient, and
benign lesions in 3 patients (Table
I). Cytologic features of LEC described are large, single and
clustered, polygonal and spindle-shaped tumor cells with a high
nucleus-to-cytoplasm ratio in syncytial sheets (3,19).
The nuclei are vesicular with prominent nucleoli. Most display a
prominent mixed lymphoid population that may mask the epithelial
elements, resulting in misdiagnosis of lymphoid entities such as
lymphoma. In the present case, FNAC revealed findings of a poorly
differentiated carcinoma but could not suggest LEC due to its
scarcity of lymphocytes. With cell block material from the tumor
available, differential diagnosis could have been narrowed down
based on positivity for EBER in situ hybridization (19). Stage of the disease was Stage I in
5 (21%), Stage II in 4 (17%), Stage III in 2 (8%), Stage IVa in 7
(30%), and Stage IVb in 6 (25%) of the 24 patients.
Histologically, LEC consists of infiltrative sheets,
islands and cords of cancer cells that are separated by dense
lymphoid stroma. The tumor cells are characterized by indistinct
cell borders (i.e., syncytial), lightly eosinophilic cytoplasm,
oval moderately pleomorphic vesicular nuclei with conspicuous
nucleoli (1). Immunohistochemical
examination is of great help in the diagnosis of LEC. Epithelial
markers such as AE1/AE3 highlight cancer cells that might be
markedly obscured by densely infiltrating lymphocytes and plasma
cells. The main differential includes metastasis from either NPC or
lymphoepithelial-like carcinoma arising in other organs (e.g.,
stomach) to not only parotid parenchyma but also to intraglandular
lymph node. In the present case, endoscopic examination of the
nasopharynx and FDG-PET/CT excluded metastasis.
Identification of EBV infection is not essential for
the diagnosis of LEC in the parotid glands; however, if found to be
associated with EBV infection, such parotid carcinoma is highly
suggestive of LEC especially when there is no known carcinoma
elsewhere in the body. Parotid LEC in the endemic areas was nearly
100% associated with EBV (3),
suggesting a strong relationship between EBV infection and its
tumorigenesis. Progression of LEC may involve malignant
transformation of glandular or ductal inclusions in intraparotid
gland lymph nodes or transformation of benign lymphoepithelial
lesions (3). EBV-associated NPC is
characterized by the lymphoepithelial features, suggesting that
lymphoid infiltrate may represent a host immune reaction to the
EBV-associated antigens expressed on the tumor cells (20). Our case also demonstrated dense
lymphoplasmacytic infiltrate not only in the stroma but within the
sheets/nests of tumor cells. In the present case, blood examination
showed high levels of serum anti-VCA IgG and anti-EBNA IgG
antibodies, and negative results for anti-VCA IgA and anti-EA IgG
antibodies, suggesting a latent viral infection of EBV. This is
different from the pattern of NPC, which shows positive results for
serum anti-VCA-IgA and anti-EA-IgG antibodies.
Somatic mutations have been reported at a low
frequency but have been widely distributed among cancer-related
genes such as TP53 in 9.5%, SYNE1 in 7.9%,
MLL2 in 5.5%, PIK3CA in 4.7%, ARID1A in 3.9%,
FGFR2 in 3.9%, NOTCH3 in 3.9% in NPC (6). Little is known about somatic mutation
for LEC arising in the parotid gland. Negative results were
obtained in the present case, with no mutations identified using a
targeted amplicon-based NGS panel of the major cancer-related
genes. Somatic mutations in genes other than we analyzed by the
DNA-based platform or gene arrangements could possibly be related
to the tumorigenesis in LEC.
The mainstay of treatment of parotid LEC is surgical
resection with adequate safety margins for patients with resectable
tumor (3). Twenty-three of 27
patients (85%) reported were treated with excision as described by
a variety of terminologies for the parotid surgery (Table I). Neck dissection was added in 9
of 23 patients (39%). Owing to its high radiosensitivity,
postoperative radiotherapy may offer significantly improved
survival compared to surgery alone (12,21,22),
although no standard of therapy has not yet been proposed.
Postoperative radiotherapy was performed in 17 of 23 patients
(74%). Three of 27 patients (11%) had unresectable LEC and were
treated with chemoradiotherapy. Platinum-based chemotherapy was
administered for the patients with advanced-stage disease (14,17).
In terms of prognosis, a 5-year survival rate of 75-86% has been
reported in patients treated by combined surgery (including neck
dissection) followed by radiotherapy (1,21).
Six of 23 patients (26%) were died of LEC, mainly due to metastasis
to the lungs, bone, and liver. EBV-associated NPC showed abundant
lymphocytic infiltration to the tumor and high expression of
programmed death-ligand 1 (PD-L1) (23), suggesting the utility of programmed
death-1 (PD-1)/PD-L1 inhibitors in patients with advanced stage of
NPC. There may be a possible role of immune checkpoint inhibitors
in advanced LEC as well.
In conclusion, we report a rare case of LEC arising
in the parotid gland. No recurrence was observed as of 20 months
after superficial parotidectomy and postoperative radiotherapy. No
mutations of major cancer-related genes were identified using NGS.
One limitation of this report was that type of latency of EBV could
not be analyzed for the LEC. EBV-associated NPC is categorized into
type II latency infection in which EBV-encoded nuclear antigen
(EBNA)-1 and latent membrane protein (LMP)-1 are expressed, and
EBNA-2 is absent (24). Further
analysis would be needed to confirm the type of latency by testing
the expression of EBV-related antigens for EBV-associated
tumors.
Supplementary Material
Cancer-related genes in the Human
Comprehensive Cancer Panel (NGHS-501X)
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to privacy reasons but
are available from the corresponding author on reasonable
request.
Authors' contributions
AK, NB and TG conducted the surgery and provided
bedside care. AK, NB and HT drafted the manuscript. KIM performed
radiation therapy. TY and HT performed cytologic diagnosis. YK
performed mutational analyses. HN performed pathologic
investigations. AK and NB confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed on patient tumor samples in
this study were conducted in accordance with the ethical standards
of the 1964 Declaration of Helsinki and its later amendments or
comparable ethical standards. The present study was approved by the
Ethics Committee of Hokuto Hospital (approval no. 1078; Obihiro,
Japan).
Patient consent for publication
Written informed consent for publication of clinical
details and images was obtained from the patient and her
family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seethala R, Thompson LDR, Wenig M, Nagao T
and Whaley RD: Lymphoepithelial Carcinoma. In: WHO Classification
of Head and Neck Tumours. Skalova A, Hyrcza MD and Mehrotra R
(eds). 5th edition. IARC Press, Lyon, 2022.
|
|
2
|
Zhang C, Gu T, Tian Z, Wang L, Han J, Hu
Y, Xia R and Li J: Lymphoepithelial carcinoma of the parotid gland:
Clinicopathological analysis of 146 cases from a single institute.
Head Neck. 44:2055–2062. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thompson LDR and Whaley RD:
Lymphoepithelial carcinoma of salivary glands. Surg Pathol Clin.
14:75–96. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Salivary Gland Cancer Stages: American
Joint Committee on Cancer (AJCC) Staging Manual. 8th edition.
Springer, New York, NY, pp95, 2017. Available from: https://www.cancer.org/cancer/salivary-gland-cancer/detection-diagnosis-staging/staging.html.
|
|
5
|
Kono M, Bandoh N, Matsuoka R, Goto T,
Akahane T, Kato Y, Nakano H, Yamaguchi T, Harabuchi Y and Nishihara
H: Glomangiopericytoma of the nasal cavity with CTNNB1 p.S37C
mutation: A case report and literature review. Head Neck Pathol.
13:298–303. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin DC, Meng X, Hazawa M, Nagata Y, Varela
AM, Xu L, Sato Y, Liu LZ, Ding LW, Sharma A, et al: The genomic
landscape of nasopharyngeal carcinoma. Nat Genet. 46:866–871.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Saqui-Salces M, Martinez-Benitez B and
Gamboa-Dominguez A: EBV+ lymphoepithelial carcinoma of the parotid
gland in Mexican Mestizo patients with chronic autoimmune diseases.
Pathol Oncol Res. 12:41–45. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Manganaris A, Patakiouta F, Xirou P and
Manganaris T: Lymphoepithelial carcinoma of the parotid gland: Is
an association with Epstein-Barr virus possible in non-endemic
areas? Int J Oral Maxillofac Surg. 36:556–559. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gupta S, Loh KS and Petersson F:
Lymphoepithelial carcinoma of the parotid gland arising in an
intraglandular lymph node: Report of a rare case mimicking
metastasis. Ann Diagn Pathol. 16:416–421. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Spencer CR, Skilbeck CJ, Thway K and
Nutting CM: Lymphoepithelial carcinoma of the parotid gland: A rare
neck lump. JRSM Short Rep. 3(28)2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang CG, Schmidtknecht TM, Tang GY,
Schloegel LJ and Rasgon B: Lymphoepithelial carcinoma: A case of a
rare parotid gland tumor. Perm J. 16:60–62. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim YJ, Hong HS, Jeong SH, Lee EH and Jung
MJ: Lymphoepithelial carcinoma of the salivary glands. Medicine
(Baltimore). 96(e6115)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Topal O and Erinanc H: Coexistence of
lymphoepithelial carcinoma of the parotid gland and submandibular
gland pleomorphic adenoma. J Craniofac Surg. 28:e453–e454.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Maeda H, Yamashiro T, Yamashita Y,
Hirakawa H, Agena S, Uehara T, Matayoshi S and Suzuki M:
Lymphoepithelial carcinoma in parotid gland related to EBV
infection: A case report. Auris Nasus Larynx. 45:170–174.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Halder A, Sommerville J and Gandhi M:
Primary lymphoepthelial carcinoma of the parotid gland, pictorial
review of a rare entity. J Med Imaging Radiat Oncol. 62:355–360.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Whaley RD, Carlos R, Bishop JA, Rooper L
and Thompson LDR: Lymphoepithelial carcinoma of salivary gland
EBV-association in endemic versus non-endemic patients: A report of
16 cases. Head Neck Pathol. 14:1001–1012. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lv S, Xie D, Wu Z, Wang L and Su Y: Is
surgery an inevitable treatment for advanced salivary
lymphoepithelial carcinoma? Three case reports. Ear Nose Throat J.
100:NP402–NP406. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chou CT, Ou CY, Lee WT and Hsu HJ:
Clinical features in salivary gland lymphoepithelial carcinoma in
10 patients: Case series and literature review. Laryngoscope
Investig Otolaryngol. 7:779–784. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Hipp JA, Jing X, Zarka MA, Schmitt AC,
Siddiqui MT, Wakely P Jr, Bishop J and Ali SZ: Cytomorphologic
characteristics and differential diagnoses of lymphoepithelial
carcinoma of the parotid. J Am Soc Cytopathol. 5:93–99.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bauer M, Jasinski-Bergner S, Mandelboim O,
Wickenhauser C and Seliger B: Epstein-Barr virus-associated
malignancies and immune escape: The role of the tumor
microenvironment and tumor cell evasion strategies. Cancers
(Basel). 13(5189)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhan KY, Nicolli EA, Khaja SF and Day TA:
Lymphoepithelial carcinoma of the major salivary glands: Predictors
of survival in a non-endemic region. Oral Oncol. 52:24–29.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Deng DF, Zhou Q, Ye ZM, Xu Z and Shen L:
Clinical analysis of 12 patients with primary lymphoepithelial
carcinoma of the parotid gland. Eur Arch Otorhinolaryngol.
279:2003–2008. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ,
Jin YT and Chang Y: Increase of programmed death-1-expressing
intratumoral CD8 T cells predicts a poor prognosis for
nasopharyngeal carcinoma. Mod Pathol. 23:1393–1403. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fåhraeus R, Fu HL, Ernberg I, Finke J,
Rowe M, Klein G, Falk K, Nilsson E, Yadav M, Busson P, et al:
Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal
carcinoma. Int J Cancer. 42:329–338. 1988.PubMed/NCBI View Article : Google Scholar
|