Introduction
Breast cancer is the most common malignancy in women
and remains a major global challenge. Approximately 1.4 million
cases occur per year, worldwide (1,2).
Surgery, chemotherapy and targeted therapy are the standard
treatments for breast cancer. Numerous studies have reported that
changes in cell metabolism were associated with transformation and
tumor progression, which are associated with electron transport
(3,4). In cancer metabolism, electron acceptor
deficiency affects NAD+ regeneration from NADH, which indicates
that the chemical disposal of excess electrons to synthesize
nucleotides could limit proliferation (5). In addition, electron donor and
electron acceptor deficiencies lead to an increase in reactive
oxygen species (ROS) by affecting ROS detoxification and
mitochondrial electron transport chain function, which is
consistent with tumor growth (6).
Glyoxal (GO) is the smallest dialdehyde formed in
the oxidation-reduction reaction and is associated with electron
transfer and metabolism (7,8). A previous study has indicated that GO
levels increase in patients with diabetes and reacts with several
proteins to form advanced glycation end products via a
Maillard-like reaction (9);
however, few studies have focused on cancer. In addition, GO has
been shown to reduce keratinocyte migration, downregulation of
SNAI2 and inhibition of EGF-dependent proliferation (10). Recently, bis(thiosemicarbazones),
derived from 1,2-diones, such as GO, have been recognized as
potential therapeutic agents against cancer, by suppressing the
cell cycle in the human neuroblastoma cell line BE(2)-M17(11). Meanwhile, in a separate study, the
Cu(GTSC) complex, derived from GO,
GO-bis(4-methyl-4-phenyl-3-thiosemicarbazonato) copper(II),
inhibited tumor growth by 95.0±3.9% in the HCT116 xenograft mouse
model (12). Thus, GO could be an
important agent for disrupting tumor cell metabolism and a
potential anti-cancer target. However, similar to arsenic trioxide,
GO has been associated with strong intracellular, digestive and
respiratory toxicity, that lead to usage limitations (13,14).
Once the balance between the safety and the effects of GO has been
achieved, GO might become an effective agent against breast cancer
by disrupting cancer metabolism.
The present study aimed to investigate the
biofunctions of GO and investigate its molecular mechanisms in
breast cancer progression.
Materials and methods
Cell lines and culture
The MDA-MB-231, SUM149 and SUM159 cell lines were
used as representative triple-negative breast cancer cell models,
while the EMT6 and MCF-7 cell lines were used as estrogen
receptor-positive breast cancer cell models, and the MCF-10A cell
line was used to represent a normal breast cell line. All the cells
were purchased from the Shanghai Institutes for Biological Sciences
and cultured at 37˚C in a humidified incubator with 5%
CO2 in DMEM (HyClone; Cytiva) supplemented with 10% FBS
(Natocor) and antibiotics (Sigma-Aldrich; Merck KGaA) (15).
GO preparations
GO (Sinopharm Chemical Reagent Co., Ltd.) is an
organic compound with the chemical formula OCHCHO, and the Chemical
Abstract Service number, 107-22-2. GO was filtered using a 0.22 µm
filter (Millipore Sigma) and stored at 26˚C in the dark.
MTT assay
Cell viability was performed using the MTT assay
(Sigma-Aldrich; Merck KGaA). The MDA-MB-231, SUM149, SUM159, EMT6,
MCF-7 and MCF-10A cell lines (3x103 cells/well) were
seeded in 96-well plates and treated with different concentrations
of GO (0.129, 0.258, 0.516, 1.032, 2.065, 4.13 and 8.25 mmol/l for
MDA-MB-231, SUM149 and SUM159; and 1.032, 2.065 and 4.13 mmol/l for
EMT6, MCF-7 and MCF-10A). After 24 h, 20 µl 5 mg/ml MTT solution
was added to each well and the plates were further incubated at
37˚C for 4 h. Following which, the medium was aspirated, and 200 µl
dimethyl sulfoxide was added to each well. After the purple
formazan crystals had dissolved, the absorbance was determined at
492 nm using an INFINITE F50 microplate reader (Tecan Group, Ltd.).
According to the MTT data, IC50 values were computed by
GraphPad Prism (v8.0; GraphPad Prism Software, Inc.). Meanwhile,
the IC50 for GO was used for 24 h, and the morphology
was captured by Nikon TS100 microscope (Nikon Corporation). The
results were obtained from three independent experiments.
Crystal violet assay
The MDA-MB-231, SUM149, SUM159, EMT6, MCF-7 and
MCF-10A cell lines were seeded in 24-well plates, at a density of
1x103 cells/well and incubated for 24 h. The cells were
then treated with different concentrations of GO (0.516, 1.032,
2.065, 4.13 and 8.25 mmol/l) continuously for 5 days. After
fixation with 4% paraformaldehyde for 30 min at 26˚C, the cells
were stained with crystal violet solution for 2 h at 26˚C. The
results were obtained from three independent experiments.
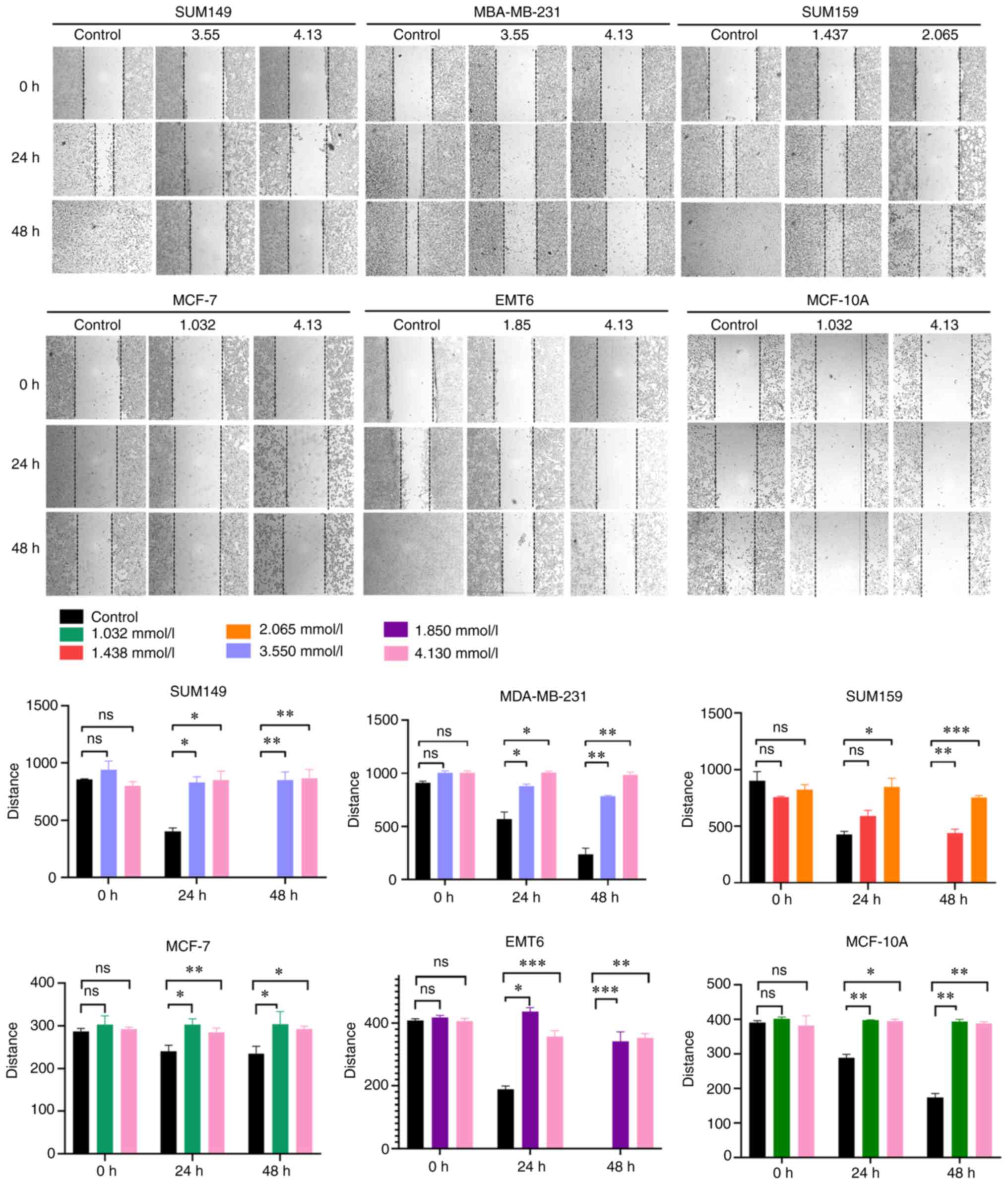
Cell migration assay
Cell migration ability was performed using a
wound-healing assay. Approximately 2x104 cells were
seeded into each well of a 6-well plate without serum and a light
microscope was used the following day to confirm that each well was
coated with cells at ~90% confluence. A 1.0 ml pipette tip was used
to remove the cells in the wound-healing region and the plates were
washed with PBS three times to remove the displaced cells. The
cells were treated with different concentrations of GO (3.550/4.130
mmol/l for MDA-MB-231 and SUM149; 1.437/2.065 mmol/l for SUM159;
1.85/4.13 mmol/l for EMT6; and 1.032/4.13 mmol/l for MCF-7 and
MCF-10A). The control cells were incubated with serum-free medium
at 37˚C with 5% CO2. At 0, 24 and 48 h, images were
captured using a Nikon TS100 microscope (Nikon Corporation), at x40
magnification and the wound was measured using ImageJ software
(v1.52a; National Institutes of Health). The experiment was
repeated three times.
3D laser scanning confocal microscope
and transmission electron microscope (TEM)
The MDA-MB-231, SUM149, SUM159 and EMT6 cell lines
were treated with different concentrations of GO (3.550, 3.550,
1.437 and 2.22 mmol/l, respectively) for 24 h. For TEM, a total of
1x107 cells were pelleted by centrifugation at 2,683 x g
for 5 min at 26˚C, then washed three times with PBS. The cells were
then fixed in 2.5% glutaraldehyde at 4˚C for 24 h. Next, the cells
were washed with PBS three times and post-fixed in 1% osmium
tetroxide for 60 min at 4˚C, encapsulated in 1% agar and stained
with uranyl acetate and phosphotungstic acid for 60 min at 4˚C. The
cells were then dehydrated in a graded ethanol series and
subsequently incubated in propylene oxide for 35 min at 26˚C. The
TEM images were captured using a Hitachi TEM system (Hitachi
High-Technologies Corporation).
For 3D micro-morphology, the volume and height of
the SUM149, MDA-MB-231, SUM159 and EMT6 cell lines were measured
using a VK-V150 laser microscopy system (Keyence Corporation).
Phase-contrast observations of the cells were performed using an
Olympus IX71 microscope (Olympus Corporation). The results were
obtained from three independent experiments.
Western blot analysis
The protein expression level of ERK, phosphorylated
(p)-ERK, MEK, p-MEK, AKT, p-AKT-Ser473, p-AKT-Thr308, mTOR and
p70-S6k was measured using western blot analysis in the MDA-MB-231,
SUM149 and SUM159 cell lines, which were each treated with the
IC50 of GO. All cells were lysed in RIPA lysis buffer
(cat. no. P0013B; Beyotime Institute of Biotechnology) and then
centrifuged at 15,702 x g for 15 min at 4˚C. Protein concentrations
were determined using a BCA kit (Beyotime Institute of
Biotechnology). A total of 20 µg protein was separated on 6-10%
gels using SDS-PAGE and transferred to PVDF membranes
(MilliporeSigma). The membranes were blocked for 1 h at 26˚C with
5% bovine serum albumin containing 0.1% Tween-20. Immunoblotting
was performed using the following primary antibodies: ERK (cat. no.
13-6200, 1:1,000), p-ERK (cat. no. 44-680G; 1:500), MEK (cat. no.
PA5-116802; 1:500), p-MEK (cat. no. 44-452, 1:1,000), AKT (cat. no.
MA191204; 1:1,000), mTOR (cat. no. A301-144A-T; 1:1,000), p70-S6k
(cat. no. MA5-36267; 1:1,000) (all Invitrogen; Thermo Fisher
Scientific, Inc.) and Tubulin (cat. no. AF1216; 1:1,000; Beyotime
Institute of Biotechnology) overnight at 4˚C. The membranes were
then washed with 1% TBS-Tween-20 three times and incubated with the
corresponding secondary antibodies (cat. no. A0208; goat
anti-rabbit; 1:5,000; Beyotime Institute of Biotechnology) at 37˚C
for 2 h. The membranes were washed again with TBS, and the proteins
were visualized using an enhanced chemiluminescence assay kit
(Beyotime Institute of Biotechnology). Images were captured using a
Bio-Rad Chemodoc XRS+ system and the Image-lab software (Version
6.0; Bio-Rad Laboratories, Inc.). The test was repeated three
times.
Cell cycle analysis using flow
cytometry
The MDA-MB-231, SUM149 and SUM159 cell lines
(5x104 cells/well) were seeded in 6-well plates and
treated with GO (IC50: 3.78, 1.85 and 1.60 mmol/l,
respectively) for 24 h. Next, the cells were collected and stored
in pre-cooled alcohol overnight at 4˚C, then stained with PI
(Shanghai Yeasen Biotechnology, Co., Ltd.) for 15 min in the dark
at 4˚C. The samples were tested using a Guava EasyCyte Plus flow
cytometer (Merck KGaA) and FlowJo VX (Becton-Dickinson and Company)
was used to analyze the results. The test was repeated three
times.
Cell apoptosis analysis using flow
cytometry
The MDA-MB-231, SUM149 and SUM159 cell lines
(5x104 cells/well) were seeded in 6-well plates and
treated with GO (IC50: 3.78, 1.85 and 1.60 mmol/l,
respectively) for 24 h. The cells were then collected and stained
using the Annexin V/PI kit (Shanghai Yeasen Biotechnology, Co.,
Ltd.) for 15 min in the dark at 4˚C. The samples were tested using
a Guava EasyCyte Plus flow cytometer (Merck KGaA) and FlowJo VX
(Becton, Dickinson and Company) was used to analyze the results.
The test was repeated three times.
Statistical analysis
The data were expressed as the mean ± SD, and
unpaired t-tests or repeated measures one-way ANOVA followed by
Dunnett's multiple comparisons test were performed for statistical
analyses using GraphPad Prism (v8.0; GraphPad Prism Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
GO inhibits breast cancer cell
proliferation
MTT and crystal violet assays were used to
investigate the biofunctions of GO on cancer cell proliferation in
different breast cancer cell lines. The results demonstrated that
cell proliferation was inhibited in a concentration-dependent
manner. As the GO concentration increased, a greater inhibitory
effect was exerted in the breast cancer cell lines. Inhibition
rates were up to 78.95±0.05, 88.83±1.35, 87.49±1.11, 88.98±9.90 and
71.77±1.29% for the MDA-MB-231, SUM149, SUM159, EMT6, and MCF-7,
respectively (P<0.05 compared with cells without GO). However,
GO only slightly reduced the proliferation rate in the MCF-10A
normal breast cell lines, as the inhibition rate was <38.26%.
The IC50 values of the MDA-MB-231, SUM149, SUM159, EMT6,
MCF-7 and MCF-10A cell lines were 3.78, 1.85, 1.60, 1.29, 2.22, and
4.39 mmol/l, respectively (Fig.
1A). In addition, cellular morphology indicated cell death
after treatment with GO for 24 h (Fig.
1B). Furthermore, under the above general tendency, the crystal
violet assay showed that cell proliferation was notably suppressed
(Fig. 2A).
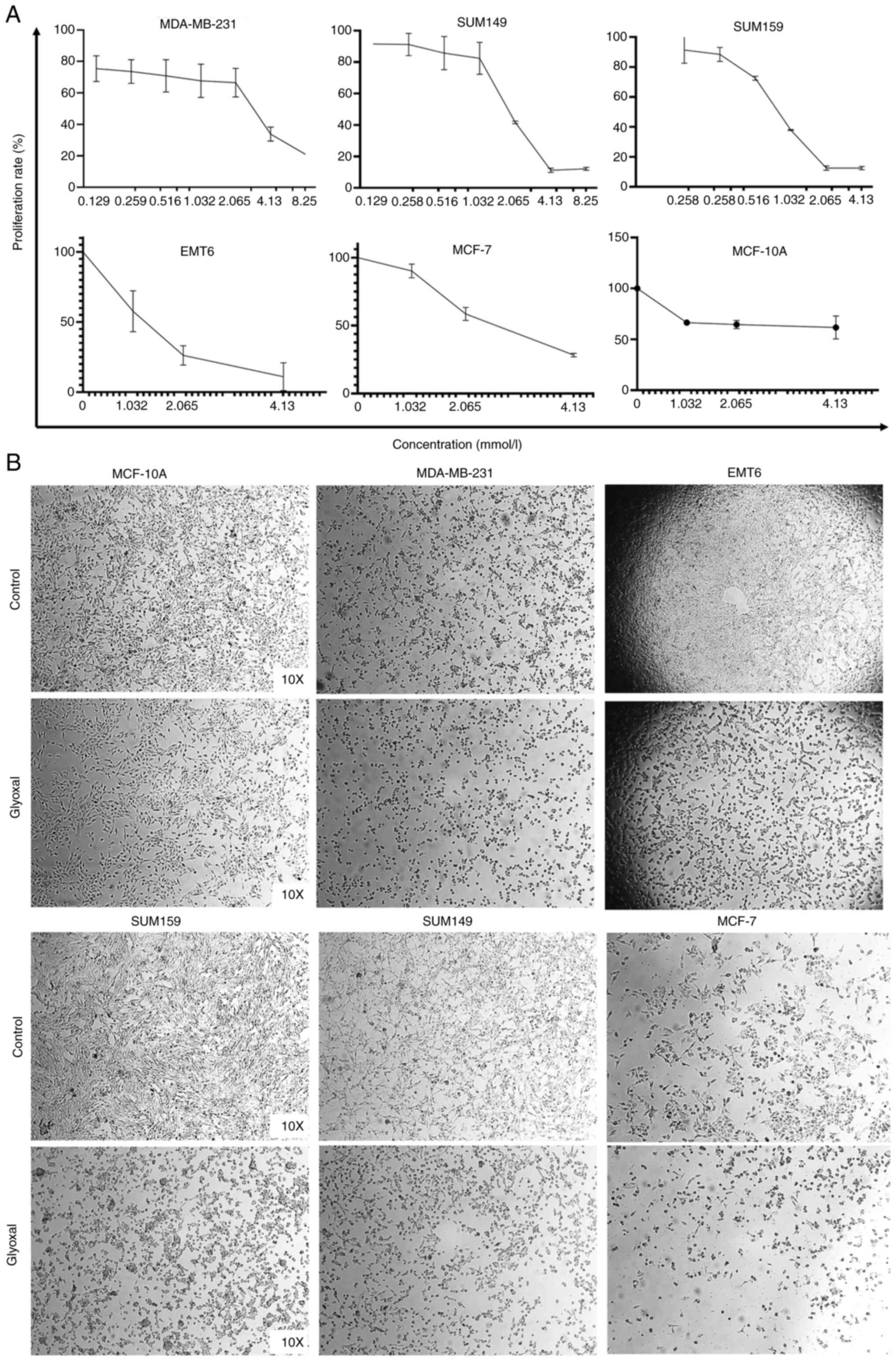 | Figure 1Inhibition ability at different doses
of GO was analyzed using the MTT and crystal violet assays in the
MDA-MB-231, SUM149, SUM159, EMT6, MCF-7 and MCF-10A cell lines. (A)
Under the above general tendency, the results from the MTT assay
showed that GO inhibited MDA-MB-231, SUM149, SUM159, EMT6 and MCF-7
cell proliferation in a concentration-dependent manner. GO at 4.13
mmol only notably inhibited the MCF-10A normal breast cell lines.
(B) The MDA-MB-231, SUM149, SUM159, EMT6 and MCF-7 cell lines were
treated with GO for 24 h and changes in cell morphology and cell
death were observed. However, the cell morphology of the MCF-10A
cell line was almost normal. GO, glyoxal. |
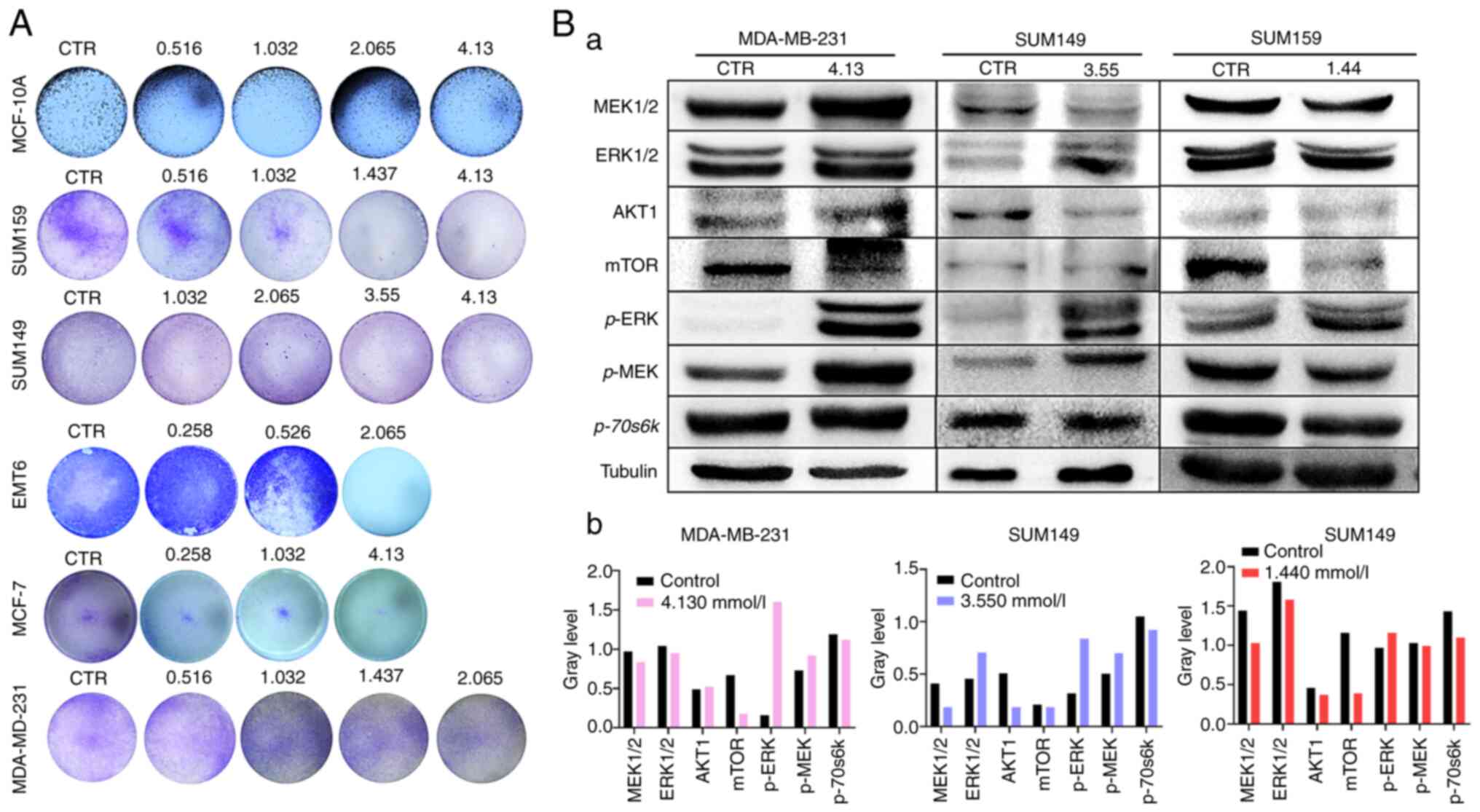 | Figure 2(A) GO notably reduced the colony
numbers in the MDA-MB-231, SUM159, MCF-7, MCF-10A and EMT6 cell
lines following treatment with different concentrations. (B) The
result of Western blot assay. a, TNBC cell lines were treated with
GO. The protein expression level of AKT1 decreased in the SUM149
and SUM159 groups, and the expression level of mTOR and P70-S6K
proteins decreased in all three cell lines. Meanwhile, p-ERK
protein expression increased in all three cell lines, and p-MEK
protein expression increased in MDA-MB-231 and SUM149 cell lines.
b, Analysis of Western blot bands in each cell line by Image J
software (National Institutes of Health, USA). GO, glyoxal; CTR,
control; p, phosphorylated. |
To further elucidate the mechanisms underlying the
action of GO, the protein expression level of the downstream
kinases of the MAPK and AKT/mTOR pathways were investigated using
western blot analysis. Consistent with the aforementioned results,
GO was found to be involved in the regulation of the MEK-ERK and
AKT/mTOR pathways. The results indicated that the protein
expression level of AKT1 was suppressed in SUM149 and SUM159 group,
and the expression level of mTOR and P70-S6K proteins was
suppressed in MDA-MB-231, SUM149, and SUM159 cells (Fig. 2B). By contrast, GO also increased
p-ERK protein expression in the same cell lines, and p-MEK protein
expression increased in MDA-MB-231 and SUM149 cell lines (Fig. 2B). In summary, the results indicated
that GO suppressed breast cancer cell proliferation by acting on
the MAPK and AKT/mTOR pathways.
GO inhibits breast cancer cell
migration
To investigate the effect of GO on breast cancer and
normal breast cell migration, differences in the wound healing rate
in the MDA-MB-231, SUM149, SUM159, EMT6, MCF-7 and MCF-10A cell
lines 48 h following GO treatment were observed. The results showed
that the relative scratch width of the GO group was significantly
wider compared with that in the control group at the 24 and 48 h
time points. In the MDA-MB-231 group, the migration inhibition
rates were 84.18 and 86.32% at 3.550 and 4.130 mmol/l,
respectively. In the SUM149 group, the migration inhibition rates
were 81.19 and 82.94% at 3.550 and 4.130 mmol/l, respectively. In
the SUM159 group, the migration inhibition rates were 67.65 and
80.00% at 1.437 and 2.065 mmol/l, respectively. In the EMT6 group,
the migration inhibition rate was 52.86 and 81.88% at 1.85 and 4.13
mmol/l, respectively. In the MCF-7 cells, the migration inhibition
rate was 77.33 and 80.42% at 1.032 and 4.13 mmol/l, respectively.
In the MCF-10A normal breast cells, the migration inhibition rate
was 44.20 and 44.92% at 1.032 and 4.13 mmol/l, respectively. Under
the above general tendency, the data indicated that GO suppressed
cell migration in a concentration-dependent manner at both 24 and
48 h (P<0.05) (Fig. 3).
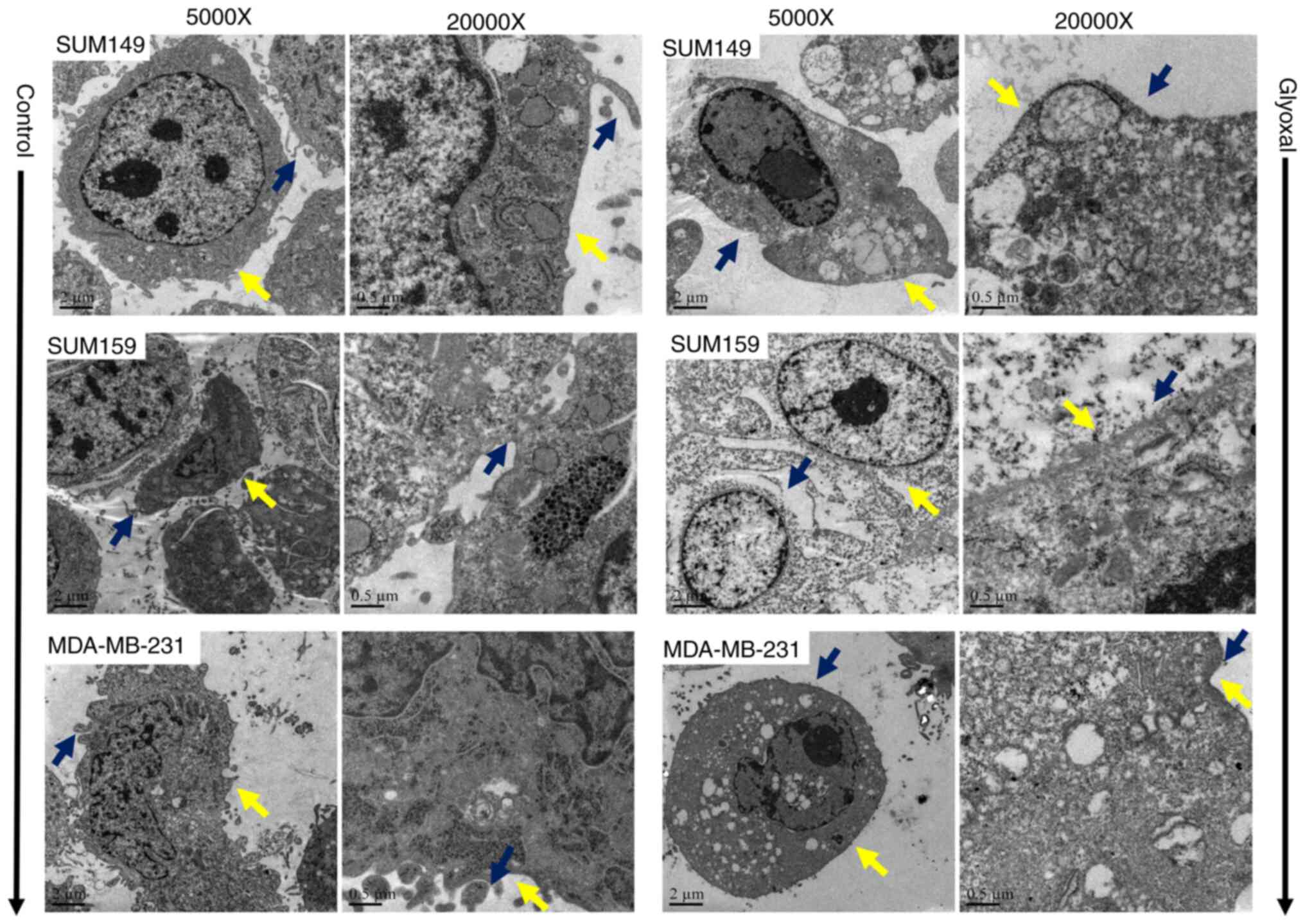
GO induces cellular ultrastructure and
changes morphology
The cellular ultrastructure was observed using TEM.
A previous study has indicated that typical morphological features
of apoptosis include chromatin condensation, nuclear fragmentation
and the disappearance of surface microvilli (16). As shown in Fig. 4, GO treatment for 24 h notably
altered the ultrastructure of the mitochondria, nucleus and
microvilli. Mitochondria appeared as enlarged organelles. The
cellular morphology became irregular and cytoplasmic vacuolization
was observed. In addition, rich microvilli, which were around the
cells, almost disappeared in the GO group, which is consistent with
cell migration inhibition. Chromatin also dissociated and appeared
around the edge of the nucleus after treatment with GO. However,
the cell membranes remained intact, and chromatin condensation and
nuclear fragmentation were not observed in either group.
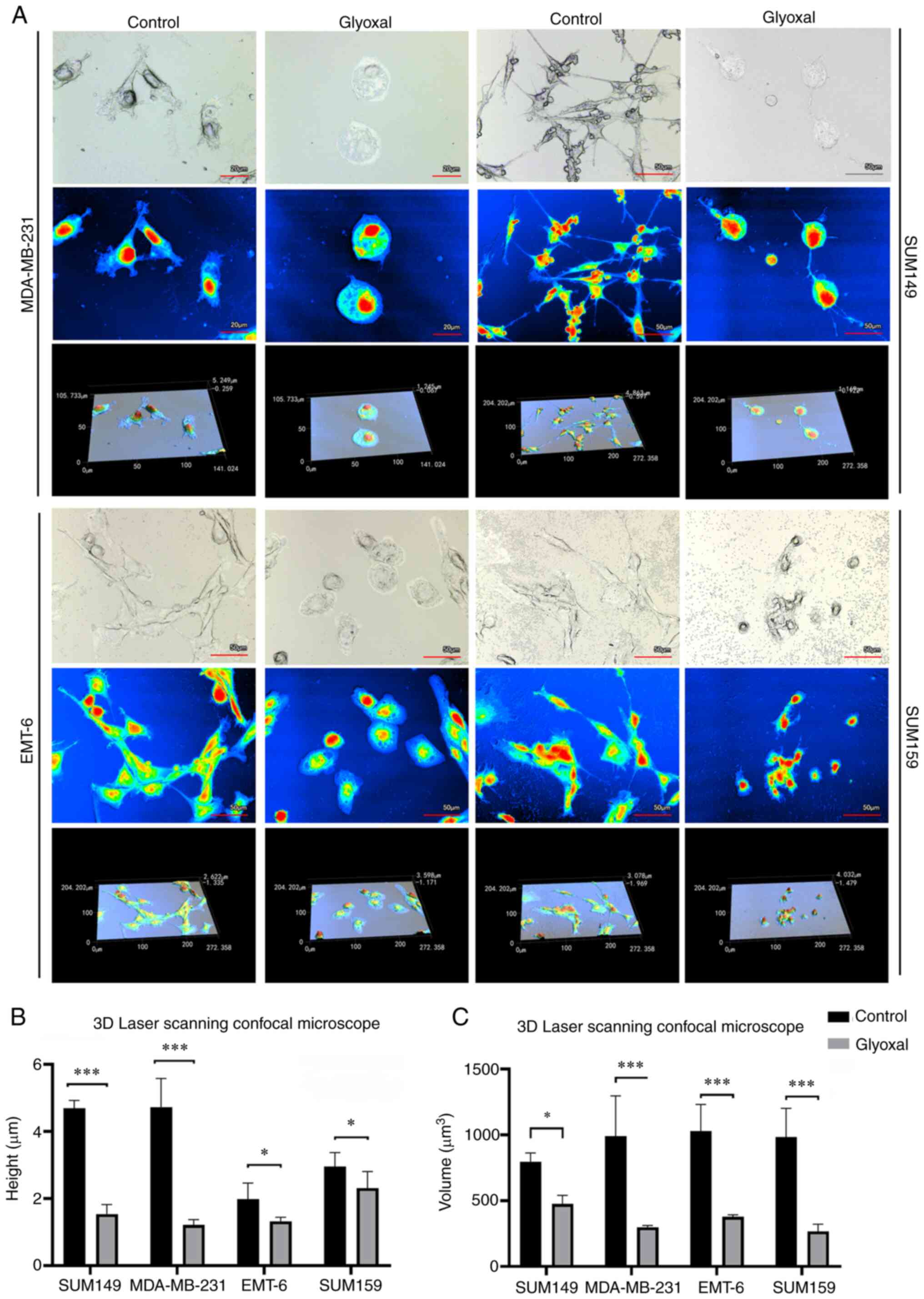
To improve the understanding into the changes in
cellular morphology following GO treatment, a 3D laser scanning
confocal microscope was used, which is a valuable tool for
obtaining high-resolution images and 3D reconstructions. Treatment
with GO for 24 h notably altered cellular morphology, height and
volume (Fig. 5A). Compared with
that in the control group, the cellular height of the SUM149,
MDA-MB-231, SUM159 and EMT6 cell lines decreased 67.22 (4.69±0.23
vs. 1.54±0.29 µm; P<0.001), 74.22 (4.73±0.86 vs. 1.22±0.15 µm;
P<0.001), 21.78 (2.96±0.41 vs. 2.32±0.49 µm; P <0.05) and
33.43% (1.98±0.48 vs. 1.32±0.12 µm; P<0.05) (Fig. 5B). In addition, the cellular volume
of the SUM149, MDA-MB-231, SUM159 and EMT6 cell lines decreased by
40.29 (797.28±66.43 vs. 476.06±65.02 µm3; P<0.05),
69.90 (991.09±305.26 vs. 298.30±13.42 µm3; P<0.001),
72.83 (982.57±218.70 vs. 266.92±54.35 µm3; P<0.001)
and 63.29% (1,028.85±203.27 vs. 377.96±15.55 µm3;
P<0.001) (Fig 5C), respectively.
As a result, the cellular morphology was altered, becoming more
circular and flatter, and the microvilli on the cell surface also
disappeared, which suggested that the cell skeleton was affected
and cell apoptosis occurred.
GO induces cell apoptosis and arrests
the cell cycle
Next, the effects of GO on cell apoptosis and the
cell cycle were investigated. As shown in Fig. 6, the proportion of Annexin V (+)/PI
(+) apoptotic cells increased significantly following treatment with
GO. The percentage of AnnexinV (+)/PI (+) cells in the GO group was
86.97±0.89, 31.8±0.28 and 31.15±3.61% compared with that in the
control 5.43±1.57, 15.6±7.21 and 14.65±2.76% for the MDA-MB-231
(P<0.001), SUM149 (P<0.05) and SUM159 (P<0.05) cell lines,
respectively (Fig. 6A). In
addition, GO arrested the cell cycle. A higher percentage of cells
in the G1 phase was accompanied by a decrease in the
proportion of cells in the G2/M phase following
treatment with GO for 24 h (7.01±1.73 vs. 23.82±2.24% for
MDA-MB-231; P=0.014; 10.75±2.33 vs. 83.50±4.10% for SUM149;
P=0.002; 42.60±1.41 vs. 67.40±5.94% for SUM159; P=0.029). Thus,
these results demonstrated that GO inhibited cell proliferation by
arresting the cell cycle in the G2 phase (Fig. 6B).
Discussion
The morbidity rate of breast cancer has surpassed
that of lung cancer so that breast cancer is now the most malignant
tumor in the world. Thus, besides chemotherapy, targeted treatment
and endocrine treatment, more treatments are required for breast
cancer. Cytotoxic agents are no longer the only potential novel
anticancer drugs. The ‘Warburg effect’ suggests that even under
oxygen-sufficient conditions, tumor cells still take advantage of
glycolysis metabolism, using oxidative phosphorylation, which is
associated with the respiratory chain rather than producing ATP
(17). Therefore, changes in cancer
tissue metabolite levels can have important implications for cell
physiology (18), and the majority
of previous studies have focused on the role of mitochondria in the
regulation of cell proliferation and apoptosis (19-21).
During tumor metabolism processes, cytochrome c oxidase and
succinate dehydrogenase are increased, and the activity of glucose
transporters is enhanced. Furthermore, mitochondrial aerobic
oxidation is inhibited, disrupting the tricarboxylic acid cycle
(22). Zhou et al (23) indicated that mitochondrial function
plays an important role in the ‘bystander effect’ mediated by
radiation therapy, which relies on the
NF-κB/iNOS/COX-2/prostaglandin E2 pathways of mitochondria. In
addition, the cell energy metabolism level can be reduced by
avicins, which are triterpene compounds that act on the outer
mitochondrial membrane by closing voltage-dependent negative ion
channels (24).
In addition, tumor cell proliferation is
significantly inhibited by dichloroacetic acid, which inhibits
mitochondrial pyruvate dehydrogenase kinase and activates potassium
channels in all cancer cells, thereby inducing apoptosis (25). In addition, drugs that suppress
microvillus development and arrest the cell cycle at the
G0/G1, S or G2 phases, prevent
cancer proliferation (19,26-30).
In addition, King et al (31) summarized and discussed the fact that
the activities of succinate dehydrogenase (SDH) and fumaric acid
hydratase were associated with mitochondrial dysfunction in
malignant tumor cells. Notably, SDH is an important component of
the tricarboxylic acid cycle, that plays a key role in the
transition of ubiquinone to ubiquinol in the mitochondrial electron
transport chain (32). Thus,
changes in the electron transfer chain can alter the biological
behavior of tumors and interrupting electron transportation in cell
metabolism contributes to tumor progression, which is a potential
therapeutic target.
Furthermore, metabolic reprogramming is an important
hallmark of cancer cell proliferation (33). The preferential use of glycolysis
unavoidably generates methylglyoxal (MGO), which is associated with
the glycolysis reaction via the spontaneous dephosphorylation of
GAP and dihydroxyacetone phosphate (34). A dual role has been previously
demonstrated for MGO, which is favorable to neuron viability and
excitability at low levels, while high levels are cytotoxic
(33). At high concentrations, MGO
suppressed breast cancer cell proliferation via glycolysis, but low
doses of MGO promoted tumorigenesis even in the same tissue
(33,35,36).
Accordingly, the expression balance of MGO is more critical than
its expression level. By contrast, GO was associated with
glycolysis reactions; however, it is the smallest dialdehyde and
contains two adjacent reactive carbonyl groups, which form during
the oxidation-reduction reaction. These are referred to as reactive
electrophilic species and they are more effective than MGO
(13,37). Furthermore, GO-induced cytotoxicity
and protein carbonylation were more severe than for MGO (37); therefore, GO concentration must be
finely adjusted and maintained within a safe range in glycolytic
cancer cells. Therefore, GO was selected as the focus of the
present study, even though GO can cause mitochondrial toxicity
(14,38). In addition, GO plays important roles
in cellular responses to energy metabolism and T-cell activation by
regulating cell signaling pathways (39,40).
In addition, GO impairs the electron transport chain, mitochondrial
function and energy metabolism. For example, GO leads to advanced
lipoxidation and glycation end products, which are associated with
aging and age-related chronic diseases via mitochondrial
dysfunction (41).
Therefore, the present study aimed to further
investigate the effects and mechanisms of GO stress on breast
cancer cells. The results of functional analyses showed that GO
treatment notably induced a decrease in cell proliferation,
increased cell apoptosis, arrested the cell cycle in G2
phase and altered cellular ultrastructure. The western blot results
indicated that GO treatment was involved in the MAPK and mTOR
signaling pathways in breast cancer. Taken together, these findings
suggest that GO is a potential anti-cancer therapeutic agent that
inhibits breast cancer progression by regulating the MAPK and
AKT/mTOR signaling pathways. Notably, measuring the increase in
cellular levels of p-ERK has been shown to be an indirect indicator
of the increase in bioavailable copper that causes cell apoptosis
(42,43).
In conclusion, several compounds affect breast
cancer cell lines via glycolysis, such as MGO and GO. As GO is the
smallest dialdehyde and contains two adjacent reactive carbonyl
groups, GO can cause increased cytotoxicity, and protein
carbonylation than MGO. However, GO causes severe side-effects and
toxicity than MGO; therefore, the concentration of GO must be
strictly adjusted to be kept in a safe range. The results from the
present study showed that GO could be a potential therapeutic agent
for breast cancer; however, additional research is required to gain
a more in-depth understanding of its mechanisms.
Acknowledgements
Not applicable.
Funding
Funding: No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PR and LY designed the study. GN, LY and LQ
contributed to the cell culture and experiments. PR and LX analyzed
the data. LX and LY wrote the original paper. PR, LY, GN, LQ and LX
confirm the authenticity of all the raw data. All authors have read
and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for participation
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kunkler L: United stand crucial in breast
cancer battle. In: China Daily European Weekly. Europe: China
Daily, 2011.
|
|
2
|
Calado A, Neves PM, Santos T and Ravasco
P: The effect of flaxseed in breast cancer: A literature review.
Front Nutr. 5(4)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vander Heiden MG and DeBerardinis RJ:
Understanding the intersections between metabolism and cancer
biology. Cell. 168:657–669. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
DeBerardinis RJ and Chandel NS:
Fundamentals of cancer metabolism. Sci Adv.
2(e1600200)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hung YP, Albeck JG, Tantama M and Yellen
G: Imaging cytosolic NADH-NAD(+) redox state with a genetically
encoded fluorescent biosensor. Cell Metab. 14:545–554.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Haddad M, Perrotte M, Khedher MRB,
Demongin C, Lepage A, Fülöp T and Ramassamy C: Methylglyoxal and
glyoxal as potential peripheral markers for MCI diagnosis and their
effects on the expression of neurotrophic, inflammatory and
neurodegenerative factors in neurons and in neuronal
derived-extracellular vesicles. Int J Mol Sci.
20(4906)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
da Silva G: Hydroxyl radical regeneration
in the photochemical oxidation of glyoxal: Kinetics and mechanism
of the HC(O)CO + O(2) reaction. Phys Chem Chem Phys. 12:6698–6705.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Banerjee S: Effect of glyoxal modification
on a critical arginine residue (Arg-31α) of hemoglobin:
Physiological implications of advanced glycated end product an in
vitro study. Protein Pept Lett. 27:770–781. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Quan KK, Kusewitt DF and Hudson LG:
Glyoxal leads to defective keratinocyte migration and
down-regulation of Snai2. J Dermatol Sci. 73:166–169.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Acevedo KM, Hayne DJ, McInnes LE, Noor A,
Duncan C, Moujalled D, Volitakis I, Rigopoulos A, Barnham KJ,
Villemagne VL, et al: Effect of structural modifications to
glyoxal-bis(thiosemicarbazonato)copper(II) complexes on cellular
copper uptake, copper-mediated ATP7A trafficking, and
P-glycoprotein mediated efflux. J Med Chem. 61:711–723.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Palanimuthu D, Shinde SV, Somasundaram K
and Samuelson AG: In vitro and in vivo anticancer activity of
copper bis(thiosemicarbazone) complexes. J Med Chem. 56:722–734.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee C and Park C: Bacterial responses to
glyoxal and methylglyoxal: Reactive electrophilic species. Int J
Mol Sci. 18(169)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goudarzi M, Kalantari H and Rezaei M:
Glyoxal toxicity in isolated rat liver mitochondria. Hum Exp
Toxicol. 37:532–539. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rangiah K, Tippornwong M, Sangar V, Austin
D, Tétreault MP, Rustgi AK, Blair IA and Yu KH: Differential
secreted proteome approach in murine model for candidate biomarker
discovery in colon cancer. J Proteome Res. 8:5153–5164.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li X, Li X, Wang J, Ye Z and Li JC:
Oridonin up-regulates expression of P21 and induces autophagy and
apoptosis in human prostate cancer cells. Int J Biol Sci.
8:901–912. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.PubMed/NCBI
|
|
18
|
Luengo A, Abbott KL, Davidson SM, Hosios
AM, Faubert B, Chan SH, Freinkman E, Zacharias LG, Mathews TP,
Clish CB, et al: Reactive metabolite production is a targetable
liability of glycolytic metabolism in lung cancer. Nat Commun.
10(5604)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Antico Arciuch VG, Elguero ME, Poderoso JJ
and Carreras MC: Mitochondrial regulation of cell cycle and
proliferation. Antioxid Redox Signal. 16:1150–1180. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Antico Arciuch VG, Galli S, Franco MC, Lam
PY, Cadenas E, Carreras MC and Poderoso JJ: Akt1 intramitochondrial
cycling is a crucial step in the redox modulation of cell cycle
progression. PLoS One. 4(e7523)2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gabrielson M, Reizer E, Stål O and Tina E:
Mitochondrial regulation of cell cycle progression through
SLC25A43. Biochem Biophys Res Commun. 469:1090–1096.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Luo XJ and Cao Y: Research progress on
bioenergy metabolic mechanism of cancer*. Prog Biochem Biophy.
38:585–592. 2011.
|
|
23
|
Zhou H, Ivanov VN, Lien YC, Davidson M and
Hei TK: Mitochondrial function and nuclear factor-kappaB-mediated
signaling in radiation-induced bystander effects. Cancer Res.
68:2233–2240. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Haridas V, Li X, Mizumachi T, Higuchi M,
Lemeshko VV, Colombini M and Gutterman JU: Avicins, a novel
plant-derived metabolite lowers energy metabolism in tumor cells by
targeting the outer mitochondrial membrane. Mitochondrion.
7:234–240. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bonnet S, Archer SL, Allalunis-Turner J,
Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta
L, Bonnet S, et al: A mitochondria-K+ channel axis is suppressed in
cancer and its normalization promotes apoptosis and inhibits cancer
growth. Cancer Cell. 11:37–51. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu Q, Fan C, Chen T, Liu C, Mei W, Chen S,
Wang B, Chen Y and Zheng W: Microwave-assisted synthesis of arene
ruthenium(II) complexes that induce S-phase arrest in cancer cells
by DNA damage-mediated p53 phosphorylation. Eur J Med Chem.
63:57–63. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xia L, Shen C, Fu Y, Tian L and Chen M:
MGC29506 induces cell cycle arrest and is downregulated in gastric
cancer. Cell Immunol. 281:31–36. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang L, Cao H, Lu N, Liu L, Wang B, Hu T,
Israel DA, Peek RM Jr, Polk DB and Yan F: Berberine inhibits
proliferation and down-regulates epidermal growth factor receptor
through activation of Cbl in colon tumor cells. PLoS One.
8(e56666)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu J, Peng Y, Wu LC, Xie Z, Deng Y, Hughes
T, He S, Mo X, Chiu M, Wang QE, et al: Curcumin down-regulates DNA
methyltransferase 1 and plays an anti-leukemic role in acute
myeloid leukemia. PLoS One. 8(e55934)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liang Y, Yin D, Hou L, Zheng T, Wang J,
Meng X, Lu Z, Song X, Pan S, Jiang H and Liu L: Diphenyl
difluoroketone: A potent chemotherapy candidate for human
hepatocellular carcinoma. PLoS One. 6(e23908)2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
King A, Selak MA and Gottlieb E: Succinate
dehydrogenase and fumarate hydratase: Linking mitochondrial
dysfunction and cancer. Oncogene. 25:4675–4682. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang S and Millar AH: Succinate
dehydrogenase: The complex roles of a simple enzyme. Curr Opin
Plant Biol. 16:344–349. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Radu BM, Dumitrescu DI, Mustaciosu CC and
Radu M: Dual effect of methylglyoxal on the intracellular
Ca2+ signaling and neurite outgrowth in mouse
sensory neurons. Cell Mol Neurobiol. 32:1047–1057. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Phillips SA and Thornalley PJ: The
formation of methylglyoxal from triose phosphates. Investigation
using a specific assay for methylglyoxal. Eur J Biochem.
212:101–105. 1993.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nokin MJ, Durieux F, Bellier J, Peulen O,
Uchida K, Spiegel DA, Cochrane JR, Hutton CA, Castronovo V and
Bellahcène A: Hormetic potential of methylglyoxal, a side-product
of glycolysis, in switching tumours from growth to death. Sci Rep.
7(11722)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bellahcène A, Nokin MJ, Castronovo V and
Schalkwijk C: Methylglyoxal-derived stress: An emerging biological
factor involved in the onset and progression of cancer. Semin
Cancer Biol. 49:64–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang K, Qiang D, Delaney S, Mehta R, Bruce
WR and O'Brien PJ: Differences in glyoxal and methylglyoxal
metabolism determine cellular susceptibility to protein
carbonylation and cytotoxicity. Chem Biol Interact. 191:322–329.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shangari N, Bruce WR, Poon R and O'Brien
PJ: Toxicity of glyoxals-role of oxidative stress, metabolic
detoxification and thiamine deficiency. Biochem Soc Trans.
31:1390–1393. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee C, Kim I and Park C: Glyoxal
detoxification in Escherichia coli K-12 by NADPH dependent
aldo-keto reductases. J Microbiol. 51:527–530. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Corbett AJ, Eckle SB, Birkinshaw RW, Liu
L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, et
al: T-cell activation by transitory neo-antigens derived from
distinct microbial pathways. Nature. 509:361–365. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Moldogazieva NT, Mokhosoev IM, Mel'nikova
TI, Porozov YB and Terentiev AA: Oxidative stress and advanced
lipoxidation and glycation end products (ALEs and AGEs) in aging
and age-related diseases. Oxid Med Cell Longev.
2019(3085756)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen X, Lan X, Mo S, Qin J, Li W, Liu P,
Han Y and Pi R: p38 and ERK, but not JNK, are involved in
copper-induced apoptosis in cultured cerebellar granule neurons.
Biochem Biophys Res Commun. 379:944–948. 2009.PubMed/NCBI View Article : Google Scholar
|