Introduction
Because carcinoma of the breast arises from the
mammary glandular epithelium, it usually exhibits the features of
adenocarcinoma. However, in <5% of breast carcinomas, some or
all of the neoplastic cells acquire a nonglandular morphology and
growth pattern by a process known as metaplasia (1). Metaplastic breast carcinoma (MBC) is
a heterogeneous group of invasive breast carcinomas (IBCs)
characterized by the differentiation of the neoplastic epithelium
toward squamous cells and/or mesenchymal-looking elements (2). The clinical features of metaplastic
carcinoma are similar to those of ER-negative IBC of no special
type; however, metaplastic carcinomas are more likely to present at
an advanced stage (2). MBCs are
reported to have lower response rates to conventional adjuvant
chemotherapy (2) and a worse
clinical outcome compared to non-MBC carcinomas (3). We describe the pathological and
immunohistochemical features of a patient with a rare MBC that
produced prominent basal lamina with neuroendocrine (NE)
differentiation, and we explain the course of its treatment.
Case report
A 42-year-old Japanese woman with a history of
hyperlipidemia was referred to Sodegaura Satsukidai Hospital
(Sodegaura, Japan) in January 2010 with the chief complaint of a
left breast tumor. The physical examination revealed no
abnormalities except for a palpable elastic firm tumor in her left
breast. There was no abnormal data including tumor markers such as
CEA, CA15-3, and HER2 on laboratory findings. In the area that
showed focal asymmetric density by mammography, two separate
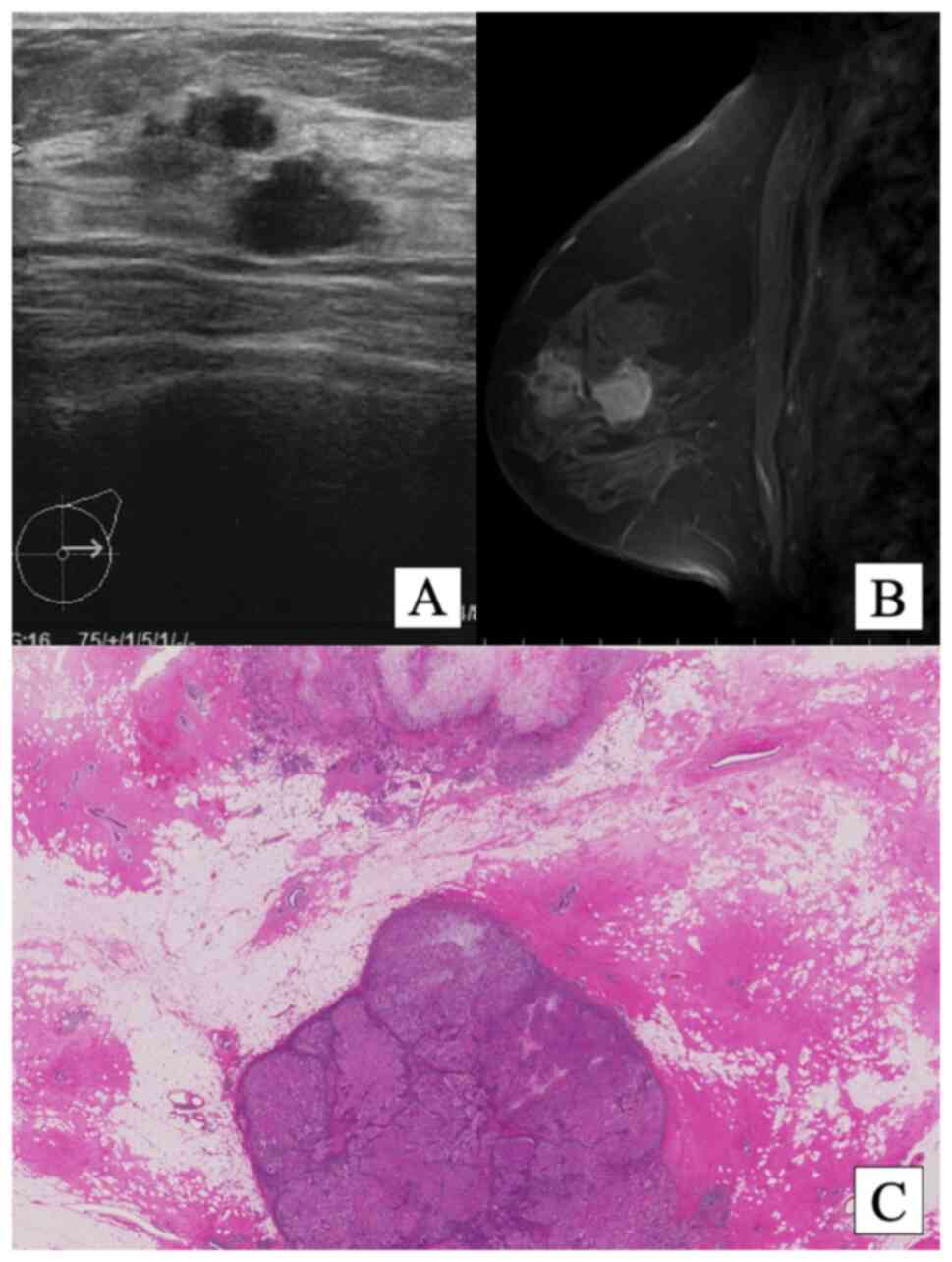
hypo-echoic masses with irregular surfaces were revealed by mammary
ultrasonography (Fig. 1A). The two
tumors detected by MRI with contrast medium were a 14x14-mm
internally heterogeneous ring-enhanced tumor under the nipple and a
16x15-mm internally homogenous enhanced tumor in the approximate
center of the left breast, without lymph node swelling (Fig. 1B). Both tumors had spiculated
margins and an irregular shape. An analysis of the shape of the
time/signal intensity curves of the tumors revealed a washout curve
in both tumors, and malignant tumors were thus suspected.
After fine-needle aspiration biopsy results
confirmed the presence of IBC, we performed a mastectomy along with
a sentinel lymph node biopsy for both tumors.
The patient's postoperative course was uneventful.
She was discharged on the 10th postoperative day. Beginning at 1
month post-surgery, she received six cycles of CMF
(cyclophosphamide 500 mg/m2, methotrexate 40
mg/m2, 5-fluorouracil 500 mg/m2). Thereafter,
she received four cycles of weekly paclitaxel (80
mg/m2). She also took a daily oral dose of
tegafur/gimeracil/oteracil potassium (300 mg/day) for 2 years.
Although >10 years have passed since the patient's surgery, she
is alive without recurrence.
On gross examination, the heterogeneous tumor was
12x11 mm, and the homogenous tumor was 17x15 mm (Fig. 1C). Microscopy ruled out lymph node
metastasis, and the smaller heterogeneous tumor revealed the
following unusual pathological characteristics. The shape of the
tumor cells with hyperchromatic nuclei was polygonal, and the tumor
cells' cytoplasm was eosinophilic. The tumor had eosinophilic
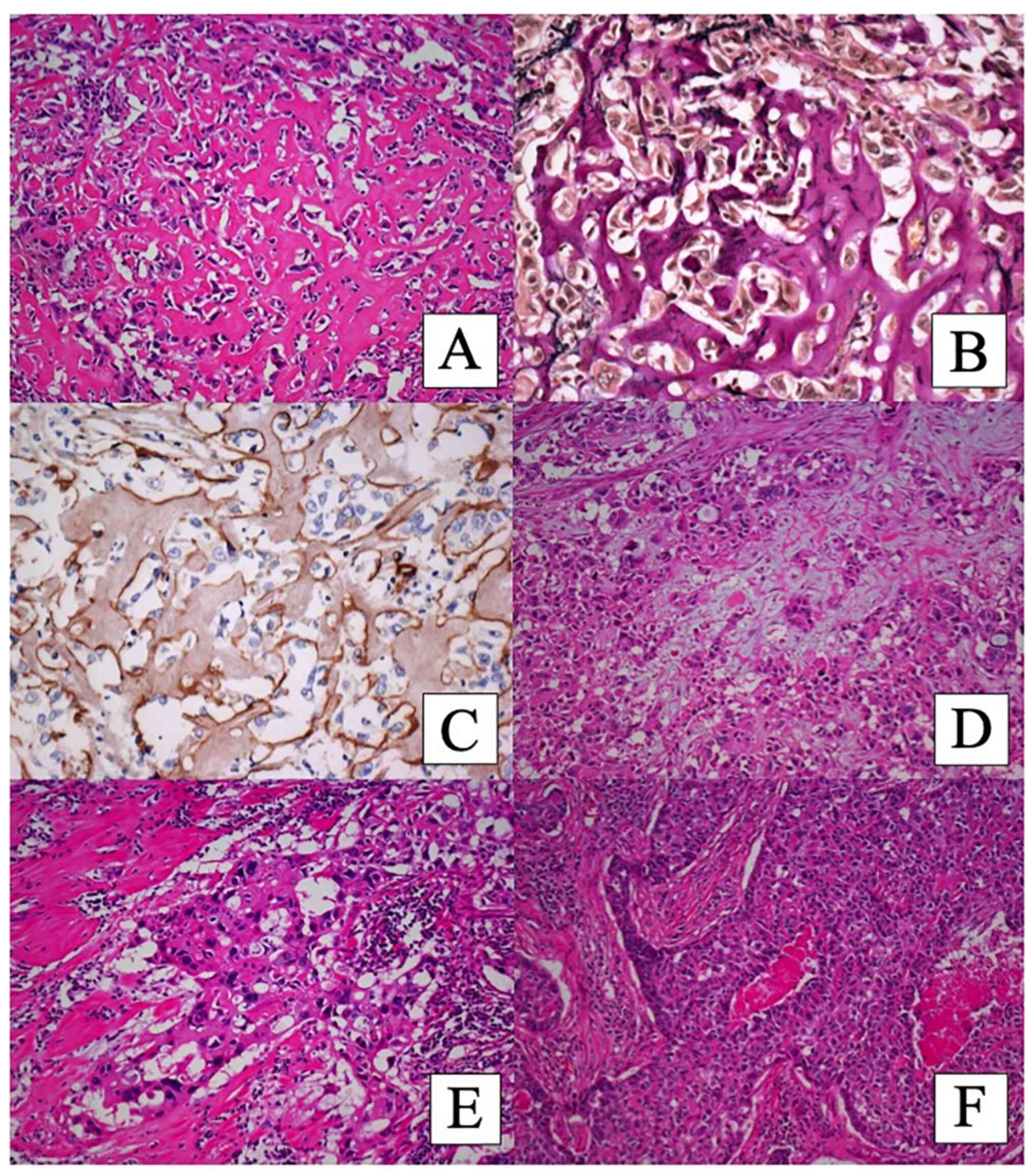
matrix with no apparent osteocytes or osteoblasts (Figs. 2A and 3A). No obvious chondromatous matrix was
observed. The eosinophilic matrix was hyalinized focally (Fig. 2A). In some areas, the tumor cells
had myxoid matrix (Fig. 2D). Some
tumor cells with dense eosinophilic cytoplasm, which had
intercellular bridges without keratinization, seemed to have
squamoid differentiation (Fig.
2E). In other areas, the tumor cells showed organoid nesting
with rosette formation, and the tumor cells at the periphery of the
nest were arranged in a palisading pattern (Fig. 2F). Necrosis was present in the
center of the nest. These findings suggested NE morphology, but
eosinophilic stroma was also observed.
The eosinophilic hyalinized matrix was positive for
periodic-acid Schiff (PAS) stain (Fig.
3C), Alcian blue stain, and Masson's trichrome stain. Elastic
and collagen fibers were stained with elastic Van Gieson's stain
(Figs. 2B and 3B). Congo Red staining was negative, and
the matrix did not show SATB2 immunostaining. The matrix was
positive for laminin (Fig. 2C) and
type IV collagen (Fig. 3D) but
negative for CAM5.2 (Fig. 3E) and
vimentin.
Table I summarizes
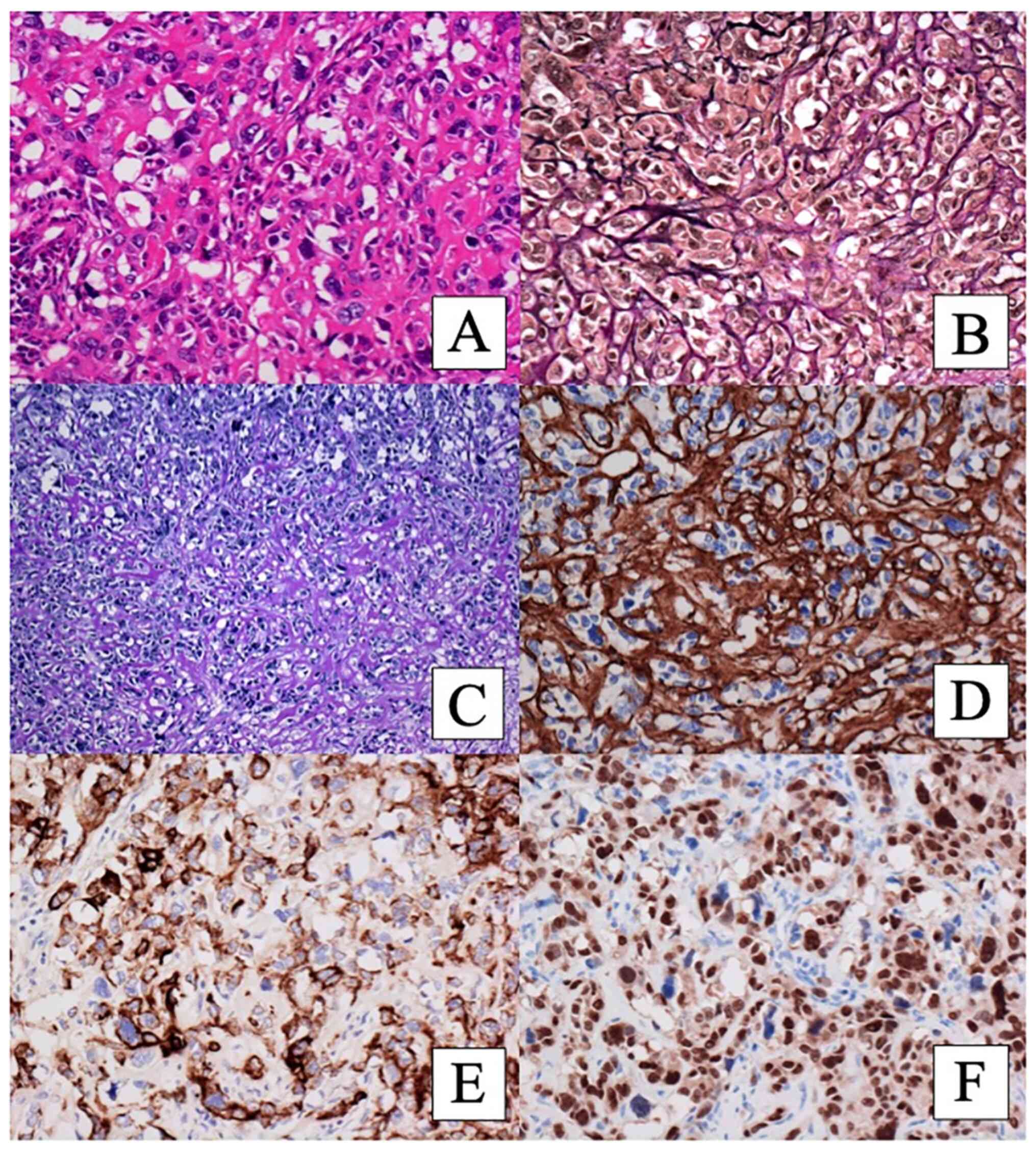
the immunohistochemistry results of the tumor cells. The tumor
cells with eosinophilic matrix exhibited diffuse immunoexpressions
of CAM5.2 (Fig. 3E), CK 34 beta
E12, S100, and SOX10 (Fig. 3F). CK
5/6, vimentin, CD56, alpha smooth muscle actin, and CD10 were
focally positive. GATA binding protein 3 (GATA-3) was negative. The
carcinoma with myxoid matrix showed CAM5.2, CK 34 beta E12, SOX10,
and CD56-positive staining and focally positive staining of CK5/6,
vimentin, and S100. The carcinoma with squamoid differentiation
revealed immunoexpressions of CAM5.2, CK 34 beta E12, CK 5/6, S100,
SOX10, and CD56; CK 5/6 was strongly positive and vimentin and CD10
were focally positive. However, p63 and desmocollin 3, which are
markers for squamous cell carcinoma (SQ), were negative.
 | Table IThe immunohistochemistry results of
the two tumors. |
Table I
The immunohistochemistry results of
the two tumors.
| | Tumor 1 | |
|---|
| Antibodies | Basal lamina | Myxoid | Squamoid | NE | Tumor 2 |
|---|
| CAM 5.2 | + | + | + | + | + |
| CK 34 beta E12 | + | + | + | + | + |
| CK 5/6 | Focal | Focal | + | - | + |
| p63 | - | - | - | - | - |
| Desmocolin 3 | - | - | - | - | - |
| Vimentin | Focal | Focal | Focal | - | - |
| S100 | + | Focal | + | - | Focal |
| SOX10 | + | + | + | + | + |
| CD10 | Focal | - | Focal | - | Focal |
| SMA | Focal | - | - | - | - |
| CD56 | Focal | + | + | + | Focal |
| Synaptophysin | - | - | - | - | - |
| Chromogranin A | - | - | - | - | - |
| GATA3 | - | - | - | - | + |
| ER | - | - | - | - | - |
| PgR | Focal | - | - | - | - |
| HER2 | - | - | - | - | - |
| Type IV collagen | + | + | + | + | - |
| Laminin | + | + | + | + | - |
The tumor cells with NE morphology showed diffuse
and strong CD56 staining whereas synaptophysin and chromogranin
were negative. The tumor cells with NE morphology were also
positive for CAM5.2, CK 34 beta E12, and SOX10, and negative for CK
5/6 and vimentin. We diagnosed this component as carcinoma with NE
differentiation; carcinoma with squamoid differentiation was
excluded because CK5/6 was negative. Inflammatory cells including
plasma cells and lymphocytes proliferated in the stroma.
The matrix of this case was thought to be basement
lamina, because the laminin and type IV collagen were positive.
Squamoid differentiation was suspected based on the presence of
intercellular bridges, but SQ was excluded based on the negative
p63 and desmocollin 3 immunostaining. In light of these results, we
diagnosed this tumor as MBC producing prominent basal lamina with
NE differentiation. The following percentages were determined:
basal lamina-producing carcinoma, 45%; carcinoma with myxoid
matrix, 5%; carcinoma with squamoid differentiation, 20%; and
carcinoma with NE differentiation, 30%.
The larger, homogeneous tumor showed tubular
formation and scirrhous carcinoma and contained myxoid matrix, but
it did not contain eosinophilic hyalinized matrix. The tumor cells
were positive for CAM5.2, CK 34 beta E 12, and CK 5/6 but negative
for laminin, type IV collagen, alpha smooth muscle actin, and
vimentin. S100, CD10, and CD56 were focally positive. GATA-3 was
positive. We diagnosed this tumor as IBC of no special type.
Both tumors showed negative estrogen receptor (ER)
and human epidermal growth factor-2 (HER2) immunostaining and were
negative for progesterone receptor (PgR) with the exception of the
basal lamina-producing carcinoma, which exhibited focal
immunoexpression of PgR. The histological grade of both tumors was
grade 3. Because the smaller tumor produced basal lamina and was
GATA-3-negative but the larger tumor did not produce basal lamina
and was GATA-3-positive, we speculated that the patient had
multiple simultaneous ipsilateral primary carcinomas. The UICC
(Union for International Cancer Control) TNM classification was
stage Ia (T1cN0M0).
Discussion
The tumor cells of this patient's case were
polygonal-shaped, and there was an eosinophilic substance around
the tumor cells. Because SATB2 [which is specific for osteoblastic
differentiation (4)] was negative,
osseous differentiation was excluded. The negative Congo Red
staining result excluded the possibility of amyloid deposition. The
patient's breast carcinoma was confirmed to produce laminin and
type IV collagen around tumor cell nests and around each tumor
cell.
The intercellular accumulation of an enormous amount
of basal lamina material has been reported in adenoid cystic
carcinomas (5), pleomorphic
adenomas (6), myoepithelial
carcinomas (7), ovarian clear cell
carcinomas (8), hepatoid yolk sac
tumors (9), and skin tumors
(10). The tumor cells showed
myoepithelial characteristics and produced redundant basement
membrane (5-7).
The myoepithelial cells of the normal human breast gland contribute
to the formation of basement membrane, and the myogenic
differentiation of these cells is responsible for the contractile
phenotype (11). Extracellular
material is responsible for maintaining the proper polarity of the
epithelial cells (12).
Tumor-derived myoepithelial cells are negative for laminin and
deficient in the ability to confer polarity to luminal epithelial
cells (13). We have found no
English-language reports of breast carcinoma that mention
myoepithelial differentiation and redundant basement membrane in
the same patient. The present report thus appears to be the first
description in the English literature of breast carcinoma that
produced redundant basal lamina material. There are also no
comparative morphology or immunohistochemistry studies of this
topic.
The World Health Organization (WHO) classification
published in 2003 defined malignant myoepithelioma (myoepithelial
carcinoma) as an infiltrating tumor composed of myoepithelial cells
with identifiable mitotic activity (14). The immunohistochemistry of the
tumor cells of the present patient's case showed myoepithelial
differentiation such as expressions of CD10, SOX10, and S100. Two
different tumor-related matrices are noted in myoepithelial
carcinoma in a salivary gland: myxoid and hyalinized. Our patient's
tumor had both myxoid and hyalinized tumor-related matrices. These
findings suggested that the tumor was myoepithelial carcinoma. The
formation of type IV collagen-positive basal lamina around tumor
cells was reported in a patient with myoepithelial carcinoma of a
salivary gland (7); however,
myoepithelial carcinoma of the breast is merged with MBC in the
recently published WHO classification of breast tumors (2).
Historically, the term matrix-producing carcinoma
(MPC) was applied to a subgroup of MBCs defined as invasive breast
carcinoma with a direct transition of carcinoma to a cartilaginous
or osseous matrix without the presence of intervening spindle cells
(2). Such tumors are now
classified as MBC with heterologous mesenchymal components
(including chondroid, osseous, rhabdomyoid, and even neuroglial
differentiation) in the recently published WHO classification of
breast tumors (2). MBC with
heterologous mesenchymal differentiation was suspected in the
present patient, but its morphology differed from that of chondroid
differentiation, and the negative SATB2 immunostaining excluded the
possibility of osseous differentiation.
MBCs are a heterogeneous group of IBCs characterized
by differentiation of the neoplastic epithelium toward squamous
cells and/or mesenchymal-appearing elements, including but not
restricted to spindle, chondroid, and osseous cells (2). An MBC can be monophasic with only one
metaplastic component or biphasic with two or more components. The
tumor in the present case was a biphasic MBC with squamoid
differentiation of the tumor cells and redundant basal lamina
material around the tumor nests and around each tumor cell. This
case showed both the triple-negative (ER, PgR, HER-2)
immunophenotype that has been reported in the vast majority of MBCs
(15) and the negative GATA-3
expression that has been described in MBC (16).
MBC patients with a high proportion of
triple-negative breast carcinoma have shown lower overall survival
(OS) than non-MBC patients (3).
For MBC patients, the use of radiation therapy and chemotherapy is
associated with improved OS (17).
The MBC subtype (i.e., MPC, squamous, mixed squamous/spindle, and
spindle carcinoma) was reported to be an independent prognostic
variable associated with breast-cancer-specific survival (BCSS) and
the disease-free interval (DFI) (18). Notably, MPC was reported to have a
better prognosis than other subtypes of MBC. Among the subtypes of
MBC, both the mixed squamous/spindle type and the spindle type,
which have aggressive biological behavior, were associated with
worse prognosis (18). Future
advances in molecular biology and molecular genetics may elucidate
these differences in the prognosis of MBC subtypes.
Neuroendocrine carcinoma is an invasive carcinoma
characterized by high-grade NE morphology and a diffuse
immunoreactivity for NE markers. Because carcinoma with NE
differentiation is one of components of this tumor, we did not
diagnose this case as neuroendocrine carcinoma, but IBC with NE
differentiation (19,20). There is no specific therapy
targeting NE differentiation, and all IBCs with NE differentiation
are treated similarly to other IBCs (21). Patients with neuroendocrine tumors
(NETs) of the breast treated with endocrine therapy and
radiotherapy had longer OS than those who did not receive
treatment, whereas patients who were treated with chemotherapy had
lower OS than those who were not, because of the non-defined
regimen and the poor response itself (22).
After our patient's surgery, we chose to treat using
only chemotherapy with a regimen that had been reported to be
effective at that time (17). For
>10 years, the patient has lived without recurrence and is
considered to have had a high quality of life due to the long-term
treatment with anticancer drugs for this rare tumor. Chemotherapy
agents such as taxane, liposomal doxorubicin, and
molecular-targeted therapy drugs against PI3K, mTOR, and EGFR are
reported to provide a favorable response to MBC (3).
The prognoses of advanced MBCs are expected to
improve further with the continued advances in modalities such as
radiotherapy, immunotherapy (including immunotherapy using
immune-checkpoint inhibitors such as programmed death ligand 1
[PD-L1]), and new pharmacotherapies including gene therapy drugs
and anticancer drugs. The present case revealed that MBCs can show
stromal hyalinization that consists of basement membrane materials,
including laminin and type IV collagen. MBCs show differentiation
of the neoplastic epithelium towards squamous cells and/or
mesenchymal-looking elements. Our patient's case also indicates
that MBCs can show differentiation towards myoepithelial cells and
NE cells. A further accumulation of case reports will enable
analyses of more data of patients with these tumors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors (YF, KH, TW, HA, SK, MO, YN, AF and HY)
contributed to the conception and design of the present case
report. Material preparation and data collection was performed by
YF, KH, TW and HA. Pathological diagnosis was performed by KH, SK,
MO and YN. YF and KH confirm the authenticity of all the raw data.
The first draft of the manuscript was written by YF and KH. All
authors commented on drafts of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brogi E: Carcinoma with metaplasia and
low-grade adenosquamous carcinoma. Rosen's Breast Pathology. 4th
edition. Lippincott Williams & Wilkins, Philadelphia, PA,
pp547-pp598, 2014.
|
|
2
|
Reis-Filho JS, Gobbi H, McCart Reed AE,
Rakha EA, Shin SJ, Sotiriou C and Vincent-Salomon A: Metaplastic
carcinoma. WHO Classification of Tumours Editorial Board. Breast
Tumours. 5th edition. International Agency for Research on Cancer,
Lyon, pp134-pp138, 2019.
|
|
3
|
Ong CT, Campbell BM, Thomas SM, Greenup
RA, Plichta JK, Rosenberger LH, Force J, Hall A, Hyslop T, Hwang ES
and Fayanju OM: Metaplastic breast cancer treatment and outcomes in
2,500 patients: A retrospective analysis of a national oncology
database. Ann Surg Oncol. 25:2249–2260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ordonez NG: SATB2 is a novel marker of
osteoblastic differentiation and colorectal adenocarcinoma. Adv
Anat Pathol. 21:63–67. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mazur MT and Battifora HA: Adenoid cystic
carcinoma of the uterine cervix: Ultrastructure,
immunofluorescence, and criteria for diagnosis. Am J Clin Pathol.
77:494–500. 1982.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jakobiec FA, Stagner AM, Eagle RC Jr,
Lally SE and Krane JF: Unusual pleomorphic adenoma of the lacrimal
gland: Immunohistochemical demonstration of PLAG1 and HMGA2
oncoproteins. Surv Ophthalmol. 62:219–226. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Asai S, Tang X, Ohta Y and Tsutsumi Y:
Myoepithelial carcinoma in pleomorphic adenoma of salivary gland
type, occurring in the mandible of an infant. Pathol Int.
45:677–683. 1995.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kato N, Takeda J, Fukase M and Motoyama T:
Alternate mucoid and hyalinized stroma in clear cell carcinoma of
the ovary: Manifestation of serial stromal remodeling. Mod Pathol.
23:881–888. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Al-Obaidy KI, Williamson SR, Shelman N,
Idrees MT and Ulbright TM: Hepatoid Teratoma, Hepatoid yolk sac
tumor, and hepatocellular carcinoma: A morphologic and
immunohistochemical study of 30 cases. Am J Surg Pathol.
45:127–136. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weber L, Krieg T, Müller PK, Kirsch E and
Timpl R: Immunofluorescent localization of type IV collagen and
laminin in human skin and its application in junctional zone
pathology. Br J Dermatol. 106:267–273. 1982.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Murrell TG: The potential for oxytocin
(OT) to prevent breast cancer: A hypothesis. Breast Cancer Res
Treat. 35:225–229. 1995.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lepucki A, Orlińska K, Mielczarek-Palacz
A, Kabut J, Olczyk P and Komosińska-Vassev K: The role of
extracellular matrix proteins in breast cancer. J Clin Med.
11(1250)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gudjonsson T, Rønnov-Jessen L, Villadsen
R, Rank F, Bissell MJ and Petersen OW: Normal and tumor-derived
myoepithelial cells differ in their ability to interact with
luminal breast epithelial cells for polarity and basement membrane
deposition. J Cell Sci. 115:39–50. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tavassoli FA and Soares J: Myoepithelial
lesions. In: Pathology and Genetics of Tumours of the Breast and
Female Genital Organs. Tavassoli FA and Devilee P (eds).
International Agency for Research on Cancer, Lyon, pp86-88,
2003.
|
|
15
|
Rakha EA, Coimbra ND, Hodi Z, Juneinah E,
Ellis IO and Lee AH: Immunoprofile of metaplastic carcinomas of the
breast. Histopathology. 70:975–985. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cakir A, Isik Gonul I, Ekinci O, Cetin B,
Benekli M and Uluoglu O: GATA3 expression and its relationship with
clinicopathological parameters in invasive breast carcinomas.
Pathol Res Pract. 213:227–234. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rayson D, Adjei AA, Suman VJ, Wold LE and
Ingle JN: Metaplastic breast cancer: Prognosis and response to
systemic therapy. Ann Oncol. 10:413–419. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rakha EA, Tan PH, Varga Z, Tse GM, Shaaban
AM, Climent F, van Deurzen CH, Purnell D, Dodwell D, Chan T and
Ellis IO: Prognostic factors in metaplastic carcinoma of the
breast: A multi-institutional study. Br J Cancer. 112:283–289.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Frachon S, Pasquier D, Treilleux I,
Seigneurin D, Ringeisen F, Rosier P, Bolla M and Boutonnat J:
Breast carcinoma with predominant neuroendocrine differentiation.
Ann Pathol. 24:278–283. 2004.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
20
|
Ding HY and Gao LX: Spindle cell carcinoma
of breast with neuroendocrine differentiation. Zhonghua Bing Li Xue
Za Zhi. 35:13–17. 2006.PubMed/NCBI(In Chinese).
|
|
21
|
Lai BS, Tsang JY, Poon IK, Shao Y, Chan
SK, Tam FK, Cheung SY, Shea KH and Tse GM: The clinical
significance of neuroendocrine features in invasive breast
carcinomas. Oncologist. 25:e1318–1329. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rosen SE and Gattuso P: Neuroendocrine
tumors of the breast. Arch Pathol Lab Med. 141:1577–1581.
2017.PubMed/NCBI View Article : Google Scholar
|