1. Introduction
Prostate cancer is the second most common cancer
affecting men worldwide, with 1.41 million cases in 2020 according
to the World Health Organization, and is responsible for 375000
deaths every year (1).
Optimal patient care is based on clinical features
as well as biological, histological and imaging data. With such
information, clinicians are better able to offer suitable
therapeutic options to their patient. Nuclear medicine is an
essential part of prostate cancer management concerning initial
staging, patient's follow-up and even therapy. In fact,
developments in metabolic imaging techniques, from bone
scintigraphy to positron emission tomography/computed tomography
(PET/CT), have led to more accurate initial diagnosis as well as
diagnosis of cancer recurrence during patients' follow-up
period.
In this article, we will discuss more specifically
the interest of prostate-specific membrane antigen (PSMA), a
promising radiotracer.
PSMA is a glutamate carboxypeptidase II
transmembrane glycoprotein expressed by 80% of prostatic cells
(2). Its interest resides in its
specificity for prostatic tissue, including carcinoma cells, with
the caveat that its fixation to some other non-prostatic
malignancies and benign lesions can result in false positives.
In recent years, the potential of prostate-specific
membrane antigen positron emission tomography/computed tomography
(PSMA PET/CT) has been widely recognized, both as a tool in the
diagnosis and follow-up of intermediate and high-risk prostate
cancer as well as theranostic applications. However, the risk of
false positives raises questions about its place in the management
of patients with prostate cancer.
Despite updates on prostate cancer management
guidelines from globally influential associations such as the
European Association of Urologists or the American Society of
Clinical Oncology, the place of PSMA PET/CT in everyday practice
remains unclear (3,4).
The aim of this article is to review the
contribution of 68Ga-PSMA PET/CT to initial staging and diagnosis,
to clarify its use in patients' follow-up and to discuss the
theranostic use of 177-Lutetium-PSMA (177Lu-PSMA). Finally, we will
consider possible resistance mechanisms and ways to overcome them,
along with other new radionuclides associated with PSMA.
2. Clinical staging and initial management
impact
T-staging
Clinical T staging is based initially on digital
rectal exam (5,6). In recent years, however, MRI has
clearly emerged as a staging tool, including initial staging, due
to its high sensitivity for local staging in intermediate and
high-risk prostate cancer and excellent negative predictive value
in low-risk patients (7). This is
an important consideration in the decision-making process with
regard to nerve sparing strategies in radical prostatectomy for
this group (8).
In a retrospective study assessing the accuracy of
68Ga-PSMA PET/CT compared with multiparametric MRI (mpMRI) in
primary prostate cancer lesions, Kalapara et al reported no
significant difference for the detection of any tumor (94% vs. 95%,
P>0.9) and localization of all tumors (91% vs. 89%, P=0.47)
(9).
N-staging
68Ga-PSMA PET/CT has proven its usefulness in lymph
node detection for intermediate and high-risk patients in several
studies (10).
In a meta-analysis including a total of 1,597
patients, Wu et al showed that 68Ga-PSMA had both higher
sensitivity and specificity [0.65 (95% CI: 0.49-0.79) and 0.94 (95%
CI: 0.88-0.97)] compared with MRI [0.41 (95% CI: 0.26-0.57) and
0.92 (95% CI: 0.86-0.95)] for the detection of lymph node
metastases in the staging workup for intermediate or high-risk
prostate cancer (11).
Similarly, Herlemann et al, comparing the
accuracy of 68Ga-PSMA and Computed Tomography (CT) in detecting
lymph node metastases, reported higher sensitivity (84% vs. 65%),
specificity (82% vs. 76%), positive predictive value (84% vs. 75%),
and negative predictive value (82% vs. 67%) with 68Ga-PSMA
(12).
However, the implications for patient care are not
entirely clear. A multicenter prospective phase 3 imaging trial by
Hope et al assessing the accuracy of 68Ga-PSMA PET/CT
compared with histopathology for the detection of pelvic lymph node
metastases in intermediate to high-risk prostate cancer found
relatively low sensitivity [0.40 (95% CI: 0.34-0.46)] and high
specificity [0.95 (95% CI: 0.92-0.97)] (13).
Given that other studies similarly report low
(24.4%) or moderate (41.5%) sensitivity, 68Ga-PSMA-PET/CT cannot
replace lymph node dissection in the staging of pelvic lymph nodes
(14,15).
M-staging
Distant metastases from prostate cancer are mostly
localized in bones. The classic work-up to evaluate the presence of
metastatic localizations in prostate cancer is CT and bone
scan.
The proPSMA study assessed the impact of 68GaPSMA
PET/CT on the diagnosis and management of patients with localized
high-risk prostate cancer (16).
68GaPSMA PET/CT clearly improves the accuracy of diagnosis and the
staging of the disease, given that PSMA PET-CT showed a 27% (95% CI
23-31) increase in accuracy compared to conventional imaging [92%
(88-95) vs. 65% (60-69); P<0.0001].
68GaPSMA PET/CT seems also suited to detect rarer
metastatic sites, for example brain metastasis as described in
Chakraborty et al case report (17).
The use of 68GaPSMA PET/CT in the context of disease
staging is thus well-established and recommended, especially for
high-risk disease with metastases and lymph node involvement.
But would more accurate staging change our
management strategies? And does it have an impact on the survival
of our patients?
Management impacts
Using 68Ga-PSMA PET/CT for initial staging could
clearly have an impact on the therapeutic management due to changes
in the staging of the disease.
Hofman et al reported a change of management
in 28% vs. 15% (P=0.008) of patients for 68GaPSMA PET/CT and
conventional imaging, respectively. Among its advantages, 68GaPSMA
PET/CT involves less radiation exposure, produces fewer equivocal
findings than conventional imaging and, finally, the cost is lower
given that PSMA PET/CT is a single test in addition to being more
accurate (16).
The PSMA dRT trial (NCT04457245), a phase 3
multicenter randomized trial, assessed the impact of 68GaPSMA
PET/CT on patient selection, radiotherapy planning and improvement
in patient outcomes (18).
Preliminary results, as presented at the American
Society of Clinical Oncology GU congress, showed a change between
the pre-randomization radiotherapy (RT) plan and the delivered RT
plan for 28% of patients in the control arm vs. 57% in the PSMA arm
(P=0.002). Initial RT was replaced by systematic therapy and/or
metastasis-directed RT in 3% vs. 17% (P=0.17), while dose
prescription and/or target volume delineation was changed in 3% vs.
26% (P=0.001) of the control and PSMA arms respectively (19).
Calais et al reported upstaging by
68Ga-PSMA-PET/CT in 43% of the patients initially diagnosed with
localized disease: 9.5% were M1 and 34% were N1, 16.5% had at least
1 positive lesion not covered by either the prostate consensus CTV
or the pelvic LN consensus CTV while radiation field required a
major change for 32% (20).
Likewise, in a prospective study of 197 patients
undergoing scans for various non-classical clinical indications,
Sonni et al reported that PSMA PET/CT led to a change in the
assessment of disease stage in 69% of patients (38% upstaging vs.
30% downstaging) and a change in management for 57% (21).
The ORIOLE study, a phase 2 randomized study,
assessed the efficacy of stereotactic ablative radiotherapy (SABR)
in men with oligometastatic prostate cancer (22).
This study emphasized the importance of treating
lesions detected by 18Ga-PSMA. Indeed, for patients with no
untreated lesions median PFS was unreached, as opposed to 11.8
months for untreated lesions [HR 0.26 (95% CI: 0.09-0.76),
P=0.006], while the proportion of new metastatic lesions at 180
days was reduced from 62.5 to 15.8%.
However, caution must be taken given the lack of
available data concerning a potential improvement in patient
outcomes and the risk of false positives (23,24).
68Ga-PSMA: a prognostic factor?
Many studies have examined a potential correlation
between the clinical parameters of prostate cancer (Gleason score,
PSA level, lymph nodes metastasis or distant metastasis) with the
intensity of PSMA accumulation in the tumor (25-27).
Patients with PSA >10 ng/ml, Gleason score >7, lymph node
metastasis or distant metastasis had significantly higher SUVmax in
the primary tumor than their counterparts, for each factor.
Amiel et al assessed the predictive value of
68Ga-PSMA PET/CT for surgical response in patients with prostate
cancer, prior to radical prostatectomy (28). 68Ga-PSMA PET/CT found
extraprostatic disease sites in 14.1% of the patients. Among
patients with disease confined to the prostate, 82.9% achieved a
surgical response, compared to 28.6% in males with extraprostatic
disease identified with 68Ga-PSMA PET/CT (P<0.001).
Extraprostatic disease identified with 68Ga-PSMA PET/CT is thus
clearly a prognostic factor of poor surgical response (23).
Moreover, outcomes of patients with a positive
68-PSMA PET/CT but who would have been screen failures for the
VISION trial was assessed in a multicenter retrospective analysis
(29). These patients presented
worse outcomes with respect to PSA response rate, PSA-progression
free survival and overall survival than patients who were
classified as eligible for the VISION trial. Moreover, PSMA average
is a better prognosticator of overall survival than PSMAmax (HR:
0.959; P=0.047 vs. HR: 0.992; P=0.231), as reported by Seifert
et al (30).
3. Monitoring after local treatment
Detecting local recurrence or distant
metastases after biochemical recurrence
After local treatment, patients' follow-up is based
on clinical examination and PSA levels (3,5,6). In
case of biochemical recurrence, it is essential to distinguish
between local recurrence and distant metastases, with a
corresponding impact on the patient's management. Imaging
techniques have a decisive role in this setting.
Most of the time a minor rise in PSA levels does not
allow conventional imaging to detect local recurrence or distant
metastasis. 68Ga-PSMA PET/CT has demonstrated its superiority in
detecting recurrences compared to standard of care and particularly
in comparison with choline tracers (31). Because of the poor sensitivity of
choline tracers at low PSA levels, Morigi et al compared the
detection rates of 18F-fluoromethylcholine with those of 68Ga-PSMA
following radical prostatectomy and/or radiation treatment in 38
men with rising PSA who were candidates for targeted therapy.
Positive scan results were obtained in 26 patients (68%), of which
14 (54%) by 68Ga-PSMA alone, 11 (42%) by 68Ga-PSMA and
18F-fluoromethylcholine, while 18F-fluoromethylcholine alone
produced only a false positive (32). At PSA levels of <0.5, 0.5-2 and
>2 ng/ml, detection rates for 68Ga-PSMA vs.
18F-fluromethylcholine were respectively 50% vs. 12.5%, 69% vs. 31%
and 86% vs. 57%. The detection rate with 68Ga-PSMA it thus
significantly higher than with 18F-fluromethylcholine.
A meta-analysis by Hope et al assessed the
accuracy of 68Ga-PSMA PET/CT for the detection of prostate cancer
compared to histopathology. A total of 256 patients with
biochemical recurrence were enrolled across 15 studies, of which
233 were reported as true positive lesions (33). The predictive positive value was
0.99 (95% CI 0.96-1.00). The detection rate for 68Ga-PSMA increased
with the PSA levels from 0.63 (95% CI 0.55-0.70) with a PSA <2.0
ng/ml to 0.94 (95% CI 0.91-0.96) with a PSA >2.0 ng/ml.
Another study, a multicenter prospective clinical
trial by McCarthy et al evaluating the diagnostic
performances of 68Ga-PSMA PET/CT, focused on patients with
biochemical recurrence after local treatment and bone scan and
computed tomography (CT) showing no lesions or oligometastatic
disease (34). Among patients with
no lesions on CT and bone scan, 74% were found to have positive
lesions on 68Ga-PSMA PET/CT, with 57% oligometastatic disease.
Among patients with oligometastatic disease on the CT and bone
scan, 49% were confirmed as oligometastatic and 41% were upstaged
to polymetastatic. No notable adverse were observed. This study
thus demonstrates that 68Ga-PSMA PET/CT is not only safe, but
highly accurate, with a positive predictive value of 0.84 (95% CI,
0.75-0.90) to 0.92 (95% CI, 0.88-0.95), as confirmed by
histopathology and the composite reference standard. Furthermore, a
PSA drop of 50% or more in 80% of patients has been observed with
PET-directed focal therapy (35).
68Ga-PSMA PET/CT is becoming the standard imaging modality for the
management of patients with relapsed prostate cancer after local
therapy.
However, the specificity of 68Ga-PSMA is not perfect
and despite its ‘prostate specific’ naming, PSMA protein expression
can be found in normal tissue (like ganglia of the sympathetic
trunk) and in several benign or malignant tumors, leading to
potential false positive (36,37).
Management impacts
68Ga-PSMA PET/CT has a clear impact on the
management strategy for patients with biochemical recurrence of
prostate cancer.
Several studies, including a prospective survey of
physicians, have already shown that information from 68Ga-PSMA
PET/CT changes management in more than half of the patients with
biochemical recurrence of prostate cancer (38-40).
It has been established that this innovative imaging
modality could benefit patients, but randomized prospective trials
are needed to determine whether it can really improve outcomes.
More information on patient outcomes will hopefully
become available in the near future from studies such as the
ongoing randomized prospective phase 3 trial by Calais et al
which aims to evaluate how PSMA PET/CT findings may affect RT
planning and ultimately the success rate of salvage radiotherapy
for prostate cancer recurrence after prostatectomy (41).
4. A theranostic approach with
177Lu-PSMA-617 therapy
177Lu-PSMA-617 therapy outcomes
When it disintegrates, 177-Lutetium produces
β-radiation, used to induce cells death, and g radiations, used for
scintigraphy. This principle can be applied to prostate cancer
through PSMA.
The first case report on this innovative technology
was published in 2015. The patient presented a metastatic prostate
cancer with strong PSMA expression on his PET/CT (42). The patient's radiological response
was significant and his PSA level decreased from 38.0 to 4.6 ng/ml.
This case report encouraged clinical trials to investigate the
potential as a treatment for prostate cancer.
Rahbar et al assessed efficacy and safety of
177Lu-PSMA-617 in a retrospective multicenter study with a cohort
of 145 patients. Biochemical response, defined as a ≥50% decline in
PSA, the primary endpoint of the study, was achieved in 45% of
patients, most of whom (58%) after a single cycle. Only 24 grade
3-4 adverse events were reported, most commonly anemia, and no
cases of therapy-related death (43).
Likewise, Heck et al evaluated the efficacy
and safety of 177Lu-PSMA-617 in 100 patients treated with
177Lu-PSMA-617 as a compassionate protocol. Median clinical PFS of
4.1 months and median overall survival of 12.9 months were both
extended for the subgroup of patients who achieved a ≥50% decline
in PSA. Hematologic grade ¾ toxicities were observed in 19% of
patients, but no other grade ¾ toxicities were noted (44).
These findings were confirmed by the LuPSMA trial, a
single-arm, single-center, phase 2 trial: 57% of the patients
treated with 177Lu-PSMA-617 achieved a PSA decline of at least 50%
(45).
Moreover, the benefits of rechallenge with
177Lu-PSMA-617 after progression have been demonstrated in another
study on long-term follow-up (46).
Two multicenter, open-label, randomized trials in
particular support the use of 177Lu-PSMA as a therapy in advanced
metastatic prostate cancer.
In the TheraP trial, as reported by Hofman et
al, patients with metastatic castration-resistant prostate
cancer were randomly assigned to receive either 177Lu-PSMA-617 or
cabazitaxel. Treatment with Lu-PSMA-617 resulted in a higher PSA
response (66% vs. 44% by treatment received, P=0.0016), fewer grade
3 or 4 adverse events (33% vs. 53%) and no deaths attributed to
Lu-PSMA-617(47).
The efficacy of 177Lu-PSMA-617 compared with
standard care (excluding chemotherapy, immunotherapy and
radium-223) was assessed by Sartor et al in the VISION
trial, the primary endpoints of which were progression-free
survival (imaging-based) and overall survival. Median PFS was 8.7
months in the 177Lu-PSMA-617 arm (vs. 3.4 months, HR
progression/death=0.40, P<0.001) while median overall survival
was 15.3 months (vs. 11.3 months; HR death=0.62, P<0.001). A
higher incidence of grade-3 adverse events was observed with
177Lu-PSMA-617 (52.7% vs. 38.0%), but did not affect quality of
life according to assessments using the Functional Assessment of
Cancer Therapy-Prostate (FACT-P) and Brief Pain Inventory-Short
Form (BPI-SF) (48).
Numerous studies to reveal the benefit of
177Lu-PSMA-617 in different scenarios are still under way.
The REALITY study, which also analyzed the efficacy
and safety of 177Lu-PSMA in 254 elderly, heavily pretreated
patients with late/end-stage disease, found a ≥50% decline in PSA,
median PSA-PFS of 5.5 (95% CI 4.4-6.6) months and median OS of 14.5
(95% CI 11.5-17.5) months (49).
The most common grade 3/4 adverse events were anemia 8.3% (95% CI
5.5-12.3), fatigue 7.1% (95% CI 4.5-10.9) and thrombocytopenia 4.3%
(95% CI 2.4-7.6), with no treatment-related deaths. In keeping with
findings by Heck et al, early biochemical response again
seems to be a significant prognostic factor (44).
The BULLSEYE trial is a multicenter, open-label,
randomized controlled trial that aims to prove the benefits of
177Lu-PSMA-617 in oligometastatic hormone sensitive prostate cancer
(50).
177Lu-PSMA-617 appears set to take its place in the
coming years as an effective, safe treatment for prostate cancer in
earlier stages.
Patient selection and resistance
mechanisms
68Ga-PSMA PET/CT parameters can assist in the
selection of responder patients.
Erdogan et al studied the predictive value of
68Ga-PSMA PET/CT parameters, SUVmax, PSMA TV, TL PSMA, before
177Lu-PSMA with respect to treatment response (51).
The AUC value for SUVmax was significant (AUC=0.677;
P<0.001).
From a therapeutic point of view, Peters et
al found that [68Ga]Ga-PSMA-PET can be used to predict the
absorbed dose of [177Lu]Lu-PSMA therapy in organs, and to a lesser
extent in lesions, which could help to personalize treatment by
maximizing doses without exceeding the threshold for at-risk organs
like the kidneys or liver (52).
With these results in mind, heterogeneity of PSMA
expression across metastases or within individual lesions could
still be detrimental for 177Lu-PSMA efficacy. This issue was raised
by Paschalis et al with heterogeneity detected between
metastases and even within the tumour (53). They reported that 42% of
castration-sensitive prostate cancer and 27% of mCRPC tissues
sampled had no detectable membranous PSMA. Demonstrating the
evolutivity of PSMA expression during the disease and influence by
different treatments, Lückerath et al have published a
preclinical study showing that androgen receptor blockade can
increase PSMA expression (54).
Based on the evidence of synergistic effects, the Enza-p trial, an
ongoing randomized phase 2 trial, assesses the efficacy of the
combination of enzalutamide and 177Lu-PSMA (55).
Mutational status seems to have an impact as well on
cancer response to radioligand therapy. In addition to its
usefulness as a prognostic factor for the development of distant
metastases, progression free survival and overall survival in
prostate cancer, p53 status could influence the response to
177Lu-PSMA (56). In a preclinical
study, Stuparu et al reported that p53 loss seems to make
prostate cancer resistant to radioligand therapy (57).
Likewise, tumors with defective DNA damage repair
had higher mPSMA expression which could incite further
investigations.
In the castration-resistant metastatic stage, small
cell transformation is possible (58). In order to detect both components
of the disease and its aggressiveness, it seems important to use
18F-FDG PET/CT and 68GaPSMA PET/CT. Parghane and Basu reported the
usefulness of dual tracer 68GaPSMA PET/CT and 18F-FDG PET/CT in
assessing this transformation (59).
Improving responses to 177Lu-PSMA
therapy
By knowing and understanding the resistance
mechanisms to 177Lu-PSMA therapy, we can find ways to improve
patients' response to this treatment.
As mentioned previously, androgen receptor blockage
can increase PSMA expression and increase the number of lesions
visualized on 68Ga-PSMA PET/CT (60). The Enza-p trial results will
provide more data on this subject.
Several ongoing trials are testing the benefit of
associating 177Lu-PSMA with other therapies as radiation
sensitizers: Olaparib for the LuPARP trial (NCT03874884), NOX66 for
the LuPIN trial (ACTRN12618001073291), or immunotherapy in
different trials like the PRINCE trial (NCT03658447) or the
EVOLUTION trial (NCT05150236). The results of these studies have
great potential to help personalize patients' care and improve the
response to radioligand therapy, and in particular 177Lu-PSMA-617
therapy.
5. New PSMA-associated radionuclides
The value of PSMA is due largely to its specificity
to prostatic cells, but other radionuclides than 68-Gallium and
177-Lutetium are being examined for use in imaging and therapy.
Several 18F-labeled PSMA ligands seem promising in
terms of diagnostic techniques.
A phase 3 prospective multicenter study by Schuster
et al (SPOTLIGHT NCT04186845) assessing detection rate and
positive predictive value for 18F-rhPSMA-7.3 PET in 389 patients
with suspected prostate cancer recurrence found overall detection
and patient-level correct detection rates of 83 and 56.8%
respectively over a wide range of PSA levels (median 1.10 ng/ml,
range 0.03-134.6). Although PPV, at 59.7% (95% CI 54.7-64.7), did
not meet its prespecified threshold, this was attributed to the
high proportion of conventional imaging in the composite Standard
of Truth, whereas the threshold would have been reached if
histopathology alone were taken as Standard of Truth. These
findings thus encourage the use of 18F-rhPSMA-7.3 PET/CT in men
with recurrent prostate cancer (61).
18F-DCFPyl has also been evaluated through different
studies. The OSPREY trial assessed the accuracy of this radiotracer
with PET/CT for detecting metastatic prostate cancer. It presented
good specificity but a level of sensitivity that did not meet the
prespecified endpoint. Nevertheless, the high positive predictive
value suggests that 18F-DCFPyl could prove useful in staging
high-risk prostate cancers or detecting metastatic recurrences
(62).
Another phase 3 prospective multicenter study by
Morris et al assessing 18F-DCFPyL-PET/CT in patients with
suspected biochemical recurrence, CONDOR, reported disease
detection rates of 59.1-65.9% and correct localization rates of
84.8-87.0% among three independent readers. Findings obtained by
means of 18F-DCFPyL-PET/CT led to a change in management for 64% of
patients. Only one grade-3 adverse event was observed, and no
grade-4 events or deaths. These results thus confirm the usefulness
of 18F-DCFPyL-PET/CT to help detect and localize recurrent prostate
cancer (63).
As a last example, 18F-PSMA-1007 has showed a
significatively better overall correct detection rate than
18F-fluorocholine PET/CT (0.82 vs. 0.65 or 0.77 vs. 0.57 depending
on whether undetermined findings were considered respectively as
positive or negative for malignancy) leading to a more adequate
management of prostate cancer with biochemical recurrence in a
phase 3 prospective randomized multicenter study (64). Hoffmann et al showed
comparable performance for 18F-PSMA-1007 and 68Ga-PSMA PET/CT in
prostate cancer initial staging with a sensitivity, specificity,
positive predictive value and accuracy of 62, 85, 92 and 67%
respectively for 18F-PSMA-1007 and 54, 91, 93 and 66%% for
68Ga-PSMA (65).
In terms of therapeutic options, Terbium-161 has
been tested as a radionuclide associated with PSMA for radioligand
therapy. Müller et al compared 161Tb-PSMA to 177Lu-PSMA and
showed superior in vitro and in vivo results with
respect to tumor cell viability (66). Further studies will be needed to
support a clinical use. This is true for 225Ac-PSMA-617 as well,
which is currently undergoing evaluation in the AcTION trial, a
phase 1 study of 225Ac-PSMA-617 in PSMA-positive prostate cancer
with extensive skeletal metastases (NCT04597411).
6. Conclusion
Radiolabeled prostate-specific membrane antigen
(PSMA) is a specific radiotracer for prostate cancer. The
superiority of 68Ga-PSMA PET/CT in comparison with standard imaging
has been demonstrated in detecting metastatic recurrences for
patients with biochemical relapse even with very low PSA levels. It
also appears to be an excellent imaging modality for initial
staging both for nodal lymph nodes and distant metastases in
intermediate and high-risk prostate cancer. In both cases,
68Ga-PSMA PET/CT has implications for patients' management.
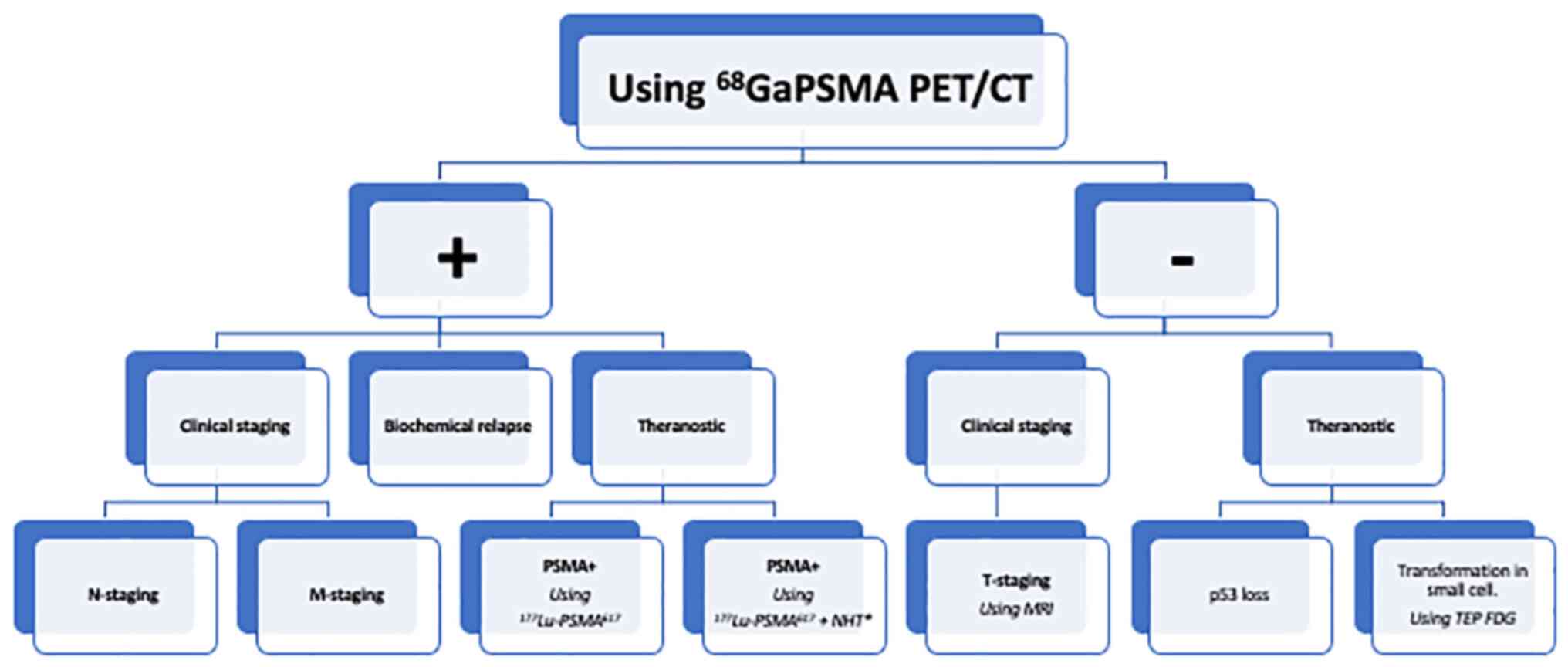
However, its impact on overall survival has yet to be determined
(Table I; Fig. 1).
 | Table IPlace of 68Ga-PSMA PET/CT in the care
of patients with prostate cancer. |
Table I
Place of 68Ga-PSMA PET/CT in the care
of patients with prostate cancer.
| A, Clinical staging
and initial management impact |
|---|
| First author,
year | Focus area | Summary of
findings | Should we use the
68Ga-PSMA PET/CT in this case? | (Refs.) |
|---|
| Kalapara et
al, 2020 | T-staging | •Digital rectal
exam | •No | (9) |
| | | •MRI for local
staging in intermediate and high-risk prostate cancer | | |
| | | •No significant
difference for the detection of tumors between 68Ga-PSMA PET/CT and
MRI | | |
| Wu et al,
2020; Herlemann et al, 2016; Hope et al, 2021 | N-staging | •68Ga-PSMA PET/CT
has proven better accuracy compared with MRI and CT for medium/high
risk prostate cancer | •Yes | (11-13) |
| | | •68Ga-PSMA-PET/CT
cannot replace lymph node dissection | | |
| Hofman et
al, 2020 | M-staging | •68GaPSMA PET/CT
improves the accuracy of metastasis detection for high- risk
prostate cancer and metastatic stage | •Yes | (16) |
| Hofman et
al, 2020; Calais et al, 2021; Calais et al, 2021;
Calais et al, 2018; Phillips et al, 2020 | Management
impacts | •Less radiation
exposure, fewer equivocal findings than conventional imaging, lower
cost | •Yes | (16,18-20,22) |
| | | •Leads to a change
in the assessment of disease stage in the majority of the patients
and therefore to a change in management | | |
| | | •Improvement in
patient outcomes? Risk of false positives? Prospective studies
needed. | | |
| Amiel et al,
2021; Hotta et al, 2022 | 68Ga-PSMA: a
prognostic factor? | •PSA >10 ng/ml,
Gleason >7, lymph node metastasis or distant metastasis: higher
SUVmax in the primary tumor | •Yes | (28,29) |
| | | •Extraprostatic
disease identified with 68Ga- PET/CT: a PSMA prognostic factor of
poor surgical response | | |
| | | •Worse outcomes for
patients with PSMA PET/ CT Screen Failure by VISION Criteria and
treated with 177Lu- PSMA Therapy | | |
| B, Monitoring after
local treatment |
| First author,
year | Focus area | Summary of
findings | Should we use the
68Ga-PSMA PET/CT in this case? | (Refs.) |
| Morigi et
al, 2015; Hope et al, 2019; McCarthy et al,
2019 | Biochemical
recurrence | •68Ga-PSMA PET/CT:
a safe and highly accurate way to detect local recurrence or
distant metastasis after biochemical recurrence | •Yes | (32-34) |
| Calais et
al, 2018; Calais et al, 2019 | Management
impacts | •68Ga-PSMA PET/CT
changes management in more than half of the patients with
biochemical recurrence | •Maybe | (39,41) |
| | | •Ongoing randomized
phase 3 trial to evaluate how PSMA PET/CT findings may affect
patient outcomes | | |
| C, 177Lu-PSMA-617
therapy |
| First author,
year | Focus area | Summary of
findings | Should we use the
68Ga-PSMA PET/CT in this case? | (Refs.) |
| Hofman et
al, 2018; Sartor et al, 2021; Khreish et al,
2022; Privé et al, 2021 | Outcomes | •High PSA response
and better overall survival rates with few adverse events in
patients with metastatic castration-resistant prostate cancer | •Yes for PSMA
positive patients | (36,48-50) |
| | | •Ongoing trials for
earlier stages of the disease | | |
| | | •Demonstrated
benefits of rechallenge with 177Lu-PSMA -617 after progression | | |
| Peters et
al, 2022; Paschalis et al, 2019; Rosar et al,
2020; Stuparu et al, 2021; Nadal et al, 2014 | Patient selection
and resistance mechanisms | •68Ga-PSMA PET/CT
parameters can assist in the selection of responder patients | •Maybe | (52,53,55,57,58) |
| | | •Heterogeneity of
PSMA expression across metastases or within individual lesions
could be detrimental for 177Lu-PSMA efficacy | | |
| | | •Androgen receptor
blockade could increase PSMA expression | | |
| | | •p53 status could
influence the response to 177Lu-PSMA | | |
| | | •Small cell
transformation after androgen deprivation therapy can lead to a
diminution of the tumor's avidity for 68Ga-PSMA: interest of a
dual-tracer PET/ CT with 18F-FDG? | | |
| Hope et al,
2017 | Improving responses
to 177Lu-PSMA therapy | Benefit of
associating177Lu- PSMA with other therapies as radiation
sensitizers? | •Yes | 60 |
| D, New
PSMA-associated radionuclides |
| First author,
year | Focus area | Summary of
findings | Should we use the
68Ga-PSMA PET/CT in this case? | (Refs.) |
| Schuster et
al, 2022; Morris et al, 2021; Olivier et al,
2022; Hoffmann et al, 2022 | Diagnostic | •Promising
18F-labeled PSMA ligands: 18F-rhPSMA-7.3, 18F-DCFPyl, 18F-PSMA-
1007 | •Yes | (61,63-65) |
| Müller et
al, 2019 | Therapy | •161Tb-PSMA:
superior in vitro and in vivo results with respect to
tumor cell viability compared to 177Lu-PSMA | | (66) |
| | | •225Ac-PSMA-617
undergoing evaluation in a phase 1 study | | |
| | | •Further studies
needed to support a clinical use | | |
On a therapeutic level, 177Lu-PSMA has proven its
benefits for advanced metastatic prostate cancer after ADT and
taxane-based chemotherapy with very few adverse events. It could,
depending on the results of ongoing studies, take a place in
earlier stage prostate cancer sometime in the close future. Many
resistance mechanisms to 177Lu-PSMA therapy have not yet been
elucidated but some solutions like androgen receptor blockage have
already shown great potential in improving responses. New
PSMA-associated radioligands are also being tested to enhance the
therapeutic and diagnostic arsenal. In sum, radiolabeled PSMA
undoubtedly has a promising future ahead of it.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS, DH, PC and CH contributed to the conception of
the work; the acquisition, analysis and interpretation of data; and
approved the submitted version. Data authentication is not
applicable. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization: Cancer Today.
Gco.iarc.fr. 2022. International Agency for Research
on Cancer. https://gco.iarc.fr/today/home.
|
|
2
|
Ghosh A, Wang X, Klein E and Heston WDW:
Novel role of prostate-specific membrane antigen in suppressing
prostate cancer invasiveness. Cancer Res. 65:727–731.
2005.PubMed/NCBI
|
|
3
|
EAU Guidelines. EAU Annual Congress
Amsterdam 2022. ISBN 978-94-92671-16-5.
|
|
4
|
Trabulsi EJ, Rumble RB, Jadvar H, Hope T,
Pomper M, Turkbey B, Rosenkrantz AB, Verma S, Margolis DJ,
Froemming A, et al: Optimum imaging strategies for advanced
prostate cancer: ASCO guideline. J Clin Oncol. 38:1963–1996.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Parker C, Castro E, Fizazi K, Heidenreich
A, Ost P, Procopio G, Tombal B and Gillessen S: ESMO Guidelines
Committee. Electronic address: simpleclinicalguidelines@esmo.org.
Prostate cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 31:1119–1134. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lowrance WT, Breau RH, Chou R, Chapin BF,
Crispino T, Dreicer R, Jarrard DF, Kibel AS, Morgan TM, Morgans AK,
et al: Advanced prostate cancer: AUA/ASTRO/SUO guideline PART I. J
Urol. 205:14–21. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ahmed HU, El-Shater Bosaily A, Brown LC,
Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG,
Freeman A, et al: Diagnostic accuracy of multi-parametric MRI and
TRUS biopsy in prostate cancer (PROMIS): A paired validating
confirmatory study. Lancet. 389:815–822. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Somford DM, Hamoen EH, Fütterer JJ, van
Basten JP, Hulsbergen-van de Kaa CA, Vreuls W, van Oort IM,
Vergunst H, Kiemeney LA, Barentsz JO and Witjes JA: The predictive
value of endorectal 3 Tesla multiparametric magnetic resonance
imaging for extraprostatic extension in patients with low,
intermediate and high risk prostate cancer. J Urol. 190:1728–1734.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kalapara AA, Nzenza T, Pan HYC, Ballok Z,
Ramdave S, O'Sullivan R, Ryan A, Cherk M, Hofman MS, Konety BR, et
al: Detection and localisation of primary prostate cancer using
68gallium prostate-specific membrane antigen positron
emission tomography/computed tomography compared with
multiparametric magnetic resonance imaging and radical
prostatectomy specimen pathology. BJU Int. 126:83–90.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peng L, Li J, Meng C, Li J, You C, Tang D,
Wei T, Xiong W and Li Y: Can 68Ga-prostate specific
membrane antigen positron emission tomography/computerized
tomography provide an accurate lymph node staging for patients with
medium/high risk prostate cancer? A diagnostic meta-analysis.
Radiat Oncol. 15(227)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu H, Xu T, Wang X, Yu YB, Fan ZY, Li DX,
Luo L, Yang XC, Jiao W and Niu HT: Diagnostic performance of
68gallium labelled prostate-specific membrane antigen
positron emission tomography/computed tomography and magnetic
resonance imaging for staging the prostate cancer with intermediate
or high risk prior to radical prostatectomy: A systematic review
and meta-analysis. World J Mens Health. 38:208–219. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Herlemann A, Wenter V, Kretschmer A,
Thierfelder KM, Bartenstein P, Faber C, Gildehaus FJ, Stief CG,
Gratzke C and Fendler WP: 68Ga-PSMA positron emission
tomography/computed tomography provides accurate staging of lymph
node regions prior to lymph node dissection in patients with
prostate cancer. Eur Urol. 70:553–557. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hope TA, Eiber M, Armstrong WR, Juarez R,
Murthy V, Lawhn-Heath C, Behr SC, Zhang L, Barbato F, Ceci F, et
al: Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal
metastasis detection prior to radical prostatectomy and pelvic
lymph node dissection: A multicenter prospective phase 3 imaging
trial. JAMA Oncol. 7:1635–1642. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yaxley JW, Raveenthiran S, Nouhaud FX,
Samartunga H, Yaxley AJ, Coughlin G, Delahunt B, Egevad L, McEwan L
and Wong D: Outcomes of primary lymph node staging of intermediate
and high risk prostate cancer with 68Ga-PSMA positron
emission tomography/computerized tomography compared to
histological correlation of pelvic lymph node pathology. J Urol.
201:815–820. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
van Kalmthout LWM, van Melick HHE,
Lavalaye J, Meijer RP, Kooistra A, de Klerk JMH, Braat AJAT,
Kaldeway HP, de Bruin PC, de Keizer B and Lam MGEH: Prospective
validation of gallium-68 prostate specific membrane
antigen-positron emission tomography/computerized tomography for
primary staging of prostate cancer. J Urol. 203:537–545.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hofman MS, Lawrentschuk N, Francis RJ,
Tang C, Vela I, Thomas P, Rutherford N, Martin JM, Frydenberg M,
Shakher R, et al: Prostate-specific membrane antigen PET-CT in
patients with high-risk prostate cancer before curative-intent
surgery or radiotherapy (proPSMA): A prospective, randomised,
multicentre study. Lancet. 395:1208–1216. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chakraborty PS, Kumar R, Tripathi M, Das
CJ and Bal C: Detection of brain metastasis with 68Ga-labeled PSMA
ligand PET/CT: A novel radiotracer for imaging of prostate
carcinoma. Clin Nucl Med. 40:328–329. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Calais J, Zhu S, Hirmas N, Eiber M,
Hadaschik B, Stuschke M, Herrmann K, Czernin J, Kishan AU, Nickols
NG, et al: Phase 3 multicenter randomized trial of PSMA PET/CT
prior to definitive radiation therapy for unfavorable
intermediate-risk or high-risk prostate cancer [PSMA dRT]: Study
protocol. BMC Cancer. 21(512)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Calais J, Zhu S, Hirmas N, Eiber M,
Hadaschik BA, Stuschke M, Herrmann K, Czernin J, Kishan AU, Nickols
NG, et al: Phase III randomized trial of PSMA PET prior to
definitive radiation therapy for unfavorable intermediate-risk or
high-risk prostate cancer [PSMA dRT]: Study protocol NCT04457245. J
Clin Oncol. 39 (Suppl 6)(TPS172)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Calais J, Kishan AU, Cao M, Fendler WP,
Eiber M, Herrmann K, Ceci F, Reiter RE, Rettig MB, Hegde JV, et al:
Potential impact of 68Ga-PSMA-11 PET/CT on the planning
of definitive radiation therapy for prostate cancer. J Nucl Med.
59:1714–1721. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sonni I, Eiber M, Fendler WP, Alano RM,
Vangala SS, Kishan AU, Nickols N, Rettig MB, Reiter RE, Czernin J
and Calais J: Impact of 68Ga-PSMA-11 PET/CT on staging
and management of prostate cancer patients in various clinical
settings: A prospective single-center study. J Nucl Med.
61:1153–1160. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Phillips R, Shi WY, Deek M, Radwan N, Lim
SJ, Antonarakis ES, Rowe SP, Ross AE, Gorin MA, Deville C, et al:
Outcomes of observation vs. stereotactic ablative radiation for
oligometastatic prostate cancer: The ORIOLE phase 2 randomized
clinical trial. JAMA Oncol. 6:650–659. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Maurer T, Gschwend JE, Rauscher I,
Souvatzoglou M, Haller B, Weirich G, Wester HJ, Heck M, Kübler H,
Beer AJ, et al: Diagnostic efficacy of (68)gallium-PSMA positron
emission tomography compared to conventional imaging for lymph node
staging of 130 consecutive patients with intermediate to high risk
prostate cancer. J Urol. 195:1436–1443. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zarbiv Y, Peerless Y, Wygoda M, Orevi M,
Meir K, Gofrit ON, Yutkin V and Frank S: Real-world Israeli single
institution experience with PET-PSMA for staging of patients with
clinically staged localized prostate carcinoma. Cancer Rep
(Hoboken). 4(e1386)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Onal C, Torun N, Oymak E, Guler OC, Reyhan
M and Yapar AF: Retrospective correlation of 68ga-psma
uptake with clinical parameters in prostate cancer patients
undergoing definitive radiotherapy. Ann Nucl Med. 34:388–396.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Uprimny C, Kroiss AS, Decristoforo C,
Fritz J, von Guggenberg E, Kendler D, Scarpa L, di Santo G, Roig
LG, Maffey-Steffan J, et al: 68Ga-PSMA-11 PET/CT in
primary staging of prostate cancer: PSA and gleason score predict
the intensity of tracer accumulation in the primary tumour. Eur J
Nucl Med Mol Imaging. 44:941–949. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ergül N, Yilmaz Güneş B, Yücetaş U, Toktaş
MG and Çermik TF: 68Ga-PSMA-11 PET/CT in newly diagnosed prostate
adenocarcinoma. Clin Nucl Med. 43:e422–e427. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Amiel T, Würnschimmel C, Heck M, Horn T,
Nguyen N, Budäus L, Knipper S, Wenzel M, Rauscher I, Eiber M, et
al: Regional lymph node metastasis on prostate specific membrane
antigen positron emission tomography correlates with decreased
biochemical recurrence-free and therapy-free survival after radical
prostatectomy: A retrospective single-center single-arm
observational study. J Urol. 205:1663–1670. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hotta M, Gafita A, Czernin J and Calais J:
Outcome of patients with PSMA PET/CT screen failure by VISION
criteria and treated with 177Lu-PSMA therapy: A
multicenter retrospective analysis. J Nucl Med. 63:1484–1488.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Seifert R, Seitzer K, Herrmann K, Kessel
K, Schäfers M, Kleesiek J, Weckesser M, Boegemann M and Rahbar K:
Analysis of PSMA expression and outcome in patients with advanced
prostate cancer receiving 177Lu-PSMA-617 radioligand
therapy. Theranostics. 10:7812–7820. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Afshar-Oromieh A, Zechmann CM, Malcher A,
Eder M, Eisenhut M, Linhart HG, Holland-Letz T, Hadaschik BA,
Giesel FL, Debus J and Haberkorn U: Comparison of PET imaging with
a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for
the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol
Imaging. 41:11–20. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Morigi JJ, Stricker PD, van Leeuwen PJ,
Tang R, Ho B, Nguyen Q, Hruby G, Fogarty G, Jagavkar R, Kneebone A,
et al: Prospective comparison of 18F-fluoromethylcholine versus
68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA
after curative treatment and are being considered for targeted
therapy. J Nucl Med. 56:1185–1190. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hope TA, Goodman JZ, Allen IE, Calais J,
Fendler WP and Carroll PR: Metaanalysis of 68Ga-PSMA-11
PET accuracy for the detection of prostate cancer validated by
histopathology. J Nucl Med. 60:786–793. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
McCarthy M, Francis R, Tang C, Watts J and
Campbell A: A multicenter prospective clinical trial of
68gallium PSMA HBED-CC PET-CT restaging in biochemically
relapsed prostate carcinoma: Oligometastatic rate and distribution
compared with standard imaging. Int J Radiat Oncol Biol Phys.
104:801–808. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fendler WP, Calais J, Eiber M, Flavell RR,
Mishoe A, Feng FY, Nguyen HG, Reiter RE, Rettig MB, Okamoto S, et
al: Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent
prostate cancer: A prospective single-arm clinical trial. JAMA
Oncol. 5:856–863. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hofman MS, Hicks RJ, Maurer T and Eiber M:
Prostate-specific membrane antigen PET: Clinical utility in
prostate cancer, normal patterns, pearls, and pitfalls.
Radiographics. 38:200–217. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rischpler C, Beck TI, Okamoto S, Schlitter
AM, Knorr K, Schwaiger M, Gschwend J, Maurer T, Meyer PT and Eiber
M: 68Ga-PSMA-HBED-CC uptake in cervical, celiac, and
sacral ganglia as an important pitfall in prostate cancer PET
imaging. J Nucl Med. 59:1406–1411. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fendler WP, Ferdinandus J, Czernin J,
Eiber M, Flavell RR, Behr SC, Wu IK, Lawhn-Heath C, Pampaloni MH,
Reiter RE, et al: Impact of 68Ga-PSMA-11 PET on the
management of recurrent prostate cancer in a prospective single-arm
clinical trial. J Nucl Med. 61:1793–1799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Calais J, Fendler WP, Eiber M, Gartmann J,
Chu FI, Nickols NG, Reiter RE, Rettig MB, Marks LS, Ahlering TE, et
al: Impact of 68Ga-PSMA-11 PET/CT on the management of
prostate cancer patients with biochemical recurrence. J Nucl Med.
59:434–441. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rousseau C, Le Thiec M, Ferrer L, Rusu D,
Rauscher A, Maucherat B, Frindel M, Baumgartner P, Fleury V, Denis
A, et al: Preliminary results of a 68Ga-PSMA PET/CT
prospective study in prostate cancer patients with occult
recurrence: Diagnostic performance and impact on therapeutic
decision-making. Prostate. 79:1514–1522. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Calais J, Czernin J, Fendler WP, Elashoff
D and Nickols NG: Randomized prospective phase III trial of
68Ga-PSMA-11 PET/CT molecular imaging for prostate
cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer.
19(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kratochwil C, Giesel FL, Eder M,
Afshar-Oromieh A, Benešová M, Mier W, Kopka K and Haberkorn U:
[77Lu] Lutetium-labelled PSMA ligand-induced remission
in a patient with metastatic prostate cancer. Eur J Nucl Med Mol
Imaging. 42:987–988. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rahbar K, Ahmadzadehfar H, Kratochwil C,
Haberkorn U, Schäfers M, Essler M, Baum RP, Kulkarni HR, Schmidt M,
Drzezga A, et al: German multicenter study investigating
177Lu-PSMA-617 radioligand therapy in advanced prostate cancer
patients. J Nucl Med. 58:85–90. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Heck MM, Tauber R, Schwaiger S, Retz M,
D'Alessandria C, Maurer T, Gafita A, Wester HJ, Gschwend JE, Weber
WA, et al: Treatment outcome, toxicity, and predictive factors for
radioligand therapy with 177Lu-PSMA-I&T in
metastatic castration-resistant prostate cancer. Eur Urol.
75:920–926. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hofman MS, Violet J, Hicks RJ, Ferdinandus
J, Thang SP, Akhurst T, Iravani A, Kong G, Ravi Kumar A, Murphy DG,
et al: [177Lu]-PSMA-617 radionuclide treatment in
patients with metastatic castration-resistant prostate cancer
(LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet
Oncol. 19:825–833. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Violet J, Sandhu S, Iravani A, Ferdinandus
J, Thang SP, Kong G, Kumar AR, Akhurst T, Pattison DA, Beaulieu A,
et al: Long-term follow-up and outcomes of retreatment in an
expanded 50-patient single-center phase II prospective trial of
177Lu-PSMA-617 theranostics in metastatic
castration-resistant prostate cancer. J Nucl Med. 61:857–865.
2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hofman MS, Emmett L, Sandhu S, Iravani A,
Joshua AM, Goh JC, Pattison DA, Tan TH, Kirkwood ID, Ng S, et al:
[177Lu]Lu-PSMA-617 versus cabazitaxel in patients with
metastatic castration-resistant prostate cancer (TheraP): A
randomised, open-label, phase 2 trial. Lancet. 397:797–804.
2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sartor O, de Bono J, Chi KN, Fizazi K,
Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N,
El-Haddad G, et al: Lutetium-177-PSMA-617 for metastatic
castration-resistant prostate cancer. N Engl J Med. 385:1091–1103.
2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Khreish F, Ghazal Z, Marlowe RJ, Rosar F,
Sabet A, Maus S, Linxweiler J, Bartholomä M and Ezziddin S: 177
Lu-PSMA-617 radioligand therapy of metastatic castration-resistant
prostate cancer: Initial 254-patient results from a prospective
registry (REALITY study). Eur J Nucl Med Mol Imaging. 49:1075–1085.
2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Privé BM, Janssen MJR, van Oort IM,
Muselaers CHJ, Jonker MA, van Gemert WA, de Groot M, Westdorp H,
Mehra N, Verzijlbergen JF, et al: Update to a randomized controlled
trial of lutetium-177-PSMA in oligo-metastatic hormone-sensitive
prostate cancer: The BULLSEYE trial. Trials. 22(768)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Erdogan M, Sengul SS, Cetin B, Avcı M,
Yagci S, Ozkoç I, Barikan DE and Yildiz M: The role of
Ga68 PSMA PET/CT imaging in Lu177 PSMA
treatment planning in metastatic castration-resistant prostate
cancer. Ann Nucl Med. 36:562–569. 2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Peters SMB, Hofferber R, Privé BM, de
Bakker M, Gotthardt M, Janssen M, de Lange F, Muselaers CHJ, Mehra
N, Witjes JA, et al: [68Ga]Ga-PSMA-11 PET imaging as a
predictor for absorbed doses in organs at risk and small lesions in
[177Lu]Lu-PSMA-617 treatment. Eur J Nucl Med Mol
Imaging. 49:1101–1112. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Paschalis A, Sheehan B, Riisnaes R,
Rodrigues DN, Gurel B, Bertan C, Ferreira A, Lambros MBK, Seed G,
Yuan W, et al: Prostate-specific membrane antigen heterogeneity and
DNA repair defects in prostate cancer. Eur Urol. 76:469–478.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lückerath K, Wei L, Fendler WP,
Evans-Axelsson S, Stuparu AD, Slavik R, Mona CE, Calais J, Rettig
M, Reiter RE, et al: Preclinical evaluation of PSMA expression in
response to androgen receptor blockade for theranostics in prostate
cancer. EJNMMI Res. 8(96)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Rosar F, Dewes S, Ries M, Schaefer A,
Khreish F, Maus S, Bohnenberger H, Linxweiler J, Bartholomä M,
Ohlmann C and Ezziddin S: New insights in the paradigm of
upregulation of tumoral PSMA expression by androgen receptor
blockade: Enzalutamide induces PSMA upregulation in
castration-resistant prostate cancer even in patients having
previously progressed on enzalutamide. Eur J Nucl Med Mol Imaging.
47:687–694. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Grignon DJ, Caplan R, Sarkar FH, Lawton
CA, Hammond EH, Pilepich MV, Forman JD, Mesic J, Fu KK, Abrams RA,
et al: p53 status and prognosis of locally advanced prostatic
adenocarcinoma: A study based on RTOG 8610. J Natl Cancer Inst.
89:158–165. 1997.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Stuparu AD, Capri JR, Meyer CAL, Le TM,
Evans-Axelsson SL, Current K, Lennox M, Mona CE, Fendler WP, Calais
J, et al: Mechanisms of resistance to prostate-specific membrane
antigen-targeted radioligand therapy in a mouse model of prostate
cancer. J Nucl Med. 62:989–995. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nadal R, Schweizer M, Kryvenko ON, Epstein
JI and Eisenberger MA: Small cell carcinoma of the prostate. Nat
Rev Urol. 11:213–219. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Parghane R and Basu S: Small cell
transformation of metastatic prostate adenocarcinoma diagnosed by
dual-tracer PET/CT (68Ga-PSMA and 18F-FDG):
Potential clinical utility in therapeutic decision making and
treatment monitoring. J Nucl Med Technol. 47:85–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hope TA, Truillet C, Ehman EC,
Afshar-Oromieh A, Aggarwal R, Ryan CJ, Carroll PR, Small EJ and
Evans MJ: 68Ga-PSMA-11 PET imaging of response to androgen receptor
inhibition: First human experience. J Nucl Med. 58:81–84.
2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Schuster DM: SPOTLIGHT Study Group.
Detection rate of 18F-rhPSMA-7.3 PET in patients with
suspected prostate cancer recurrence: Results from a phase 3,
prospective, multicenter study (SPOTLIGHT). J Clin Oncol. 40
(Suppl)(S9)2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pienta KJ, Gorin MA, Rowe SP, Carroll PR,
Pouliot F, Probst S, Saperstein L, Preston MA, Alva AS, Patnaik A,
et al: A phase 2/3 prospective multicenter study of the diagnostic
accuracy of prostate specific membrane antigen PET/CT with
18F-DCFPyL in prostate cancer patients (OSPREY). J Urol.
206:52–61. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Morris MJ, Rowe SP, Gorin MA, Saperstein
L, Pouliot F, Josephson D, Wong JYC, Pantel AR, Cho SY, Gage KL, et
al: Diagnostic performance of 18F-DCFPyL-PET/CT in men
with biochemically recurrent prostate cancer: Results from the
CONDOR phase III, multicenter study. Clin Cancer Res. 27:3674–3682.
2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Olivier P, Giraudet AL, Skanjeti A, Merlin
C, Weinmann P, Rudolph I, Hoepping A and Gauthé M: Phase III study
of 18F-PSMA-1007 versus 18F-fluorocholine
PET/CT for localization of prostate cancer biochemical recurrence:
A prospective, randomized, cross-over, multicenter study. J Nucl
Med: Nov 23, 2022 (Epub ahead of print).
|
|
65
|
Hoffmann MA, Müller-Hübenthal J, Rosar F,
Fischer N, von Eyben FE, Buchholz HG, Wieler HJ and Schreckenberger
M: Primary staging of prostate cancer patients with
[18F]PSMA-1007 PET/CT compared with
[68Ga]Ga-PSMA-11 PET/CT. J Clin Med.
11(5064)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Müller C, Umbricht CA, Gracheva N, Tschan
VJ, Pellegrini G, Bernhardt P, Zeevaart JR, Köster U, Schibli R and
van der Meulen NP: Terbium-161 for PSMA-targeted radionuclide
therapy of prostate cancer. Eur J Nucl Med Mol Imaging.
46:1919–1930. 2019.PubMed/NCBI View Article : Google Scholar
|