Introduction
Concurrent chemoradiotherapy (CCRT) is often used to
prevent locoregional recurrence and improve the overall survival of
patients with advanced lung cancer (1). Currently, two methods of irradiation
are commonly used in the treatment planning process for CCRT in
these patients: i) Elective nodal irradiation, which targets
microscopic mediastinal lymph node metastases that are not evident
on imaging, and ii) involved field radiation therapy (IFRT), which
targets only visible lesions on imaging studies. As systemic
therapies have progressed, the use of CCRT combined with IFRT has
become widespread in various institutions (2,3).
However, Yuan et al (4)
demonstrated that 7% of patients with lung cancer treated with IFRT
experience recurrence within the lymph node region. One possible
explanation is that the clinical target volume (CTV) in IFRT
planning, with reference to conventional imaging information, may
have been inadequate.
Imaging information, including non-enhanced computed
tomography (non-CE/CT), contrast-enhanced CT (CE/CT), and
[18F]-Fluorodeoxyglucose (FDG) positron emission tomography/CT
[(PET)/CT] images, is important for defining the CTV in IFRT
planning. In clinical practice, the CTV is generally delineated by
radiation oncologists with reference to these imaging data.
Although the quality of these images can affect the CTV
delineation, PET/CT images are particularly useful (5). In previous years, remarkable advances
have been made in PET/CT technology (6,7).
Semiconductor-based PET/CT is a new digital PET/CT (dPET/CT)
technique that has demonstrated improved tumor detection than
cPET/CT (8). The dPET/CT replaces
the photomultiplier tube of the cPET/CT with a semiconductor
optical sensor. The semiconductor optical sensor has a smaller
temporal fluctuation of the electrical signal, and the
time-of-flight temporal resolution is improved compared with the
cPET/CT. Therefore, the signal-to-noise ratio and contrast are
greatly improved, and even small lesions can be clearly visualized
(9).
However, the impact of dPET/CT on CTV delineation
remains unclear because radiation oncologists delineate the CTV
with reference to the aforementioned clinical information
(non-CE/CT, CE/CT, and PET/CT). Therefore, in the present study,
the influence of dPET/CT on CTV delineation in IFRT planning was
evaluated and compared with that of cPET/CT.
Materials and methods
Study protocol and cases
In total, 26 patients with lung cancer underwent
both cPET/CT and dPET/CT between April 2020 and September 2020 at
Ehime University Hospital (Toon, Japan). Out of all the patients,
those with early-stage lung cancer (n=15) and those with metastatic
lesions in the thoracic region (n=1) were excluded. Finally, the 10
remaining patients were included in the present study. The present
study was approved (approval. no. 2211016) by the Ethics Committee
of Ehime University Hospital (Matsuyama, Japan).
Image acquisition
Whole-body PET/CT was performed using an integrated
cPET/CT scanner (Discovery 600 PET/CT, GE Healthcare) and a dPET/CT
scanner (Discovery MI, GE Healthcare). The patients fasted for at
least 6 h and had a blood glucose level of 80-120 mg/dl before the
intravenous administration of 18F-FDG (3.7 MBq/kg). The order of
PET scans was randomly assigned to each patient. A total of 12
patients were first scanned using dPET followed by cPET
(dPET-first), and 14 patients were first scanned using cPET followed
by dPET (dPET-second). All dPET images were reconstructed using a
3-dimensional time-of-flight weighted line-of-response row-action
maximum-likelihood algorithm with attenuation correction using a CT
attenuation map. Integrated PET and CT images were reconstructed
and reviewed using Advantage Workstations Server 3.2 (Cytiva). The
display field of view was 60x60 cm and consisted of 256x256
matrices. The voxel size was 2.34x2.34x2.79 mm3.
CTV delineation
The data of the patients with lung cancer scanned
using non-CE/CT, CE/CT, cPET/CT, and dPET/CT were imported into
treatment planning systems (Eclipse, Varian Medical Systems,
Inc.).
Two patterns of gross tumor volume (cGTVall=cGTVp +
cGTVn with reference to cPET/CT and dGTVall=dGTVp + dGTVn with
reference to dPET/CT) were determined based on the primary tumor
and lymph node metastases identified on PET/CT images. The CTV
(cCTVall=cCTVp + cCTVn with reference to cPET/CT and dCTVall=dCTVp
+ dCTVn with reference to dPET/CT) was determined by expanding 0.5
cm around the GTVs and excluding normal organs in principle.
Non-CE/CT and CE/CT images were referenced to delineate all
plans.
In total, 20 CTVs (10 cCTVp, n, all and 10 dCTVp, n,
all) were devised by five radiation oncologists as a reference for
cPET/CT and dPET/CT. All patients were blinded and randomized, and
one plan was created per month.
Statistical analysis
Statistical analyses were conducted using EZR
version 1.61 (Saitama Medical Center, Jichi Medical University), a
graphical user interface for R (version 3.5.0; The R Foundation for
Statistical Computing) (10).
Extreme values (outliers) were eliminated using Smirnov-Grubbs
analysis, which is a method of outlier detection that assumes the
data follow a normal distribution (11).
Results
Patients
After applying the exclusion criteria, 10 patients
[one with small cell lung cancer and nine with non-small cell lung
cancer; male/female, 9/1; age, 58-80 years (median, 65 years)] were
included in the analysis (Table
I). Out of all the patients only six patients had distant
metastases that were not present in the thoracic area (bone, one;
brain, two; bone/brain/liver, one; bone/liver, one; and
adrenal/pancreas, one).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Number of
patients | Percentage (%) |
|---|
| Age | | |
|
<65
years | 5 | 50.0 |
|
≥65
years | 5 | 50.0 |
| Sex | | |
|
Male | 9 | 90.0 |
|
Female | 1 | 10.0 |
| Stage | | |
|
3a | 4 | 40.0 |
|
4a | 2 | 20.0 |
|
4b | 4 | 40.0 |
| T stage | | |
|
1 | 2 | 20.0 |
|
2 | 4 | 40.0 |
|
4 | 4 | 40.0 |
| N stage | | |
|
1 | 3 | 30.0 |
|
2 | 4 | 40.0 |
|
3 | 3 | 30.0 |
| Metastasis | | |
|
Yes | 6 | 60.0 |
|
No | 4 | 40.0 |
| Primary site | | |
|
Upper
lobe | 5 | 50.0 |
|
Middle
lobe | 0 | 0.0 |
|
Lower
lobe | 5 | 50.0 |
Comparison between cCTV and dCTV
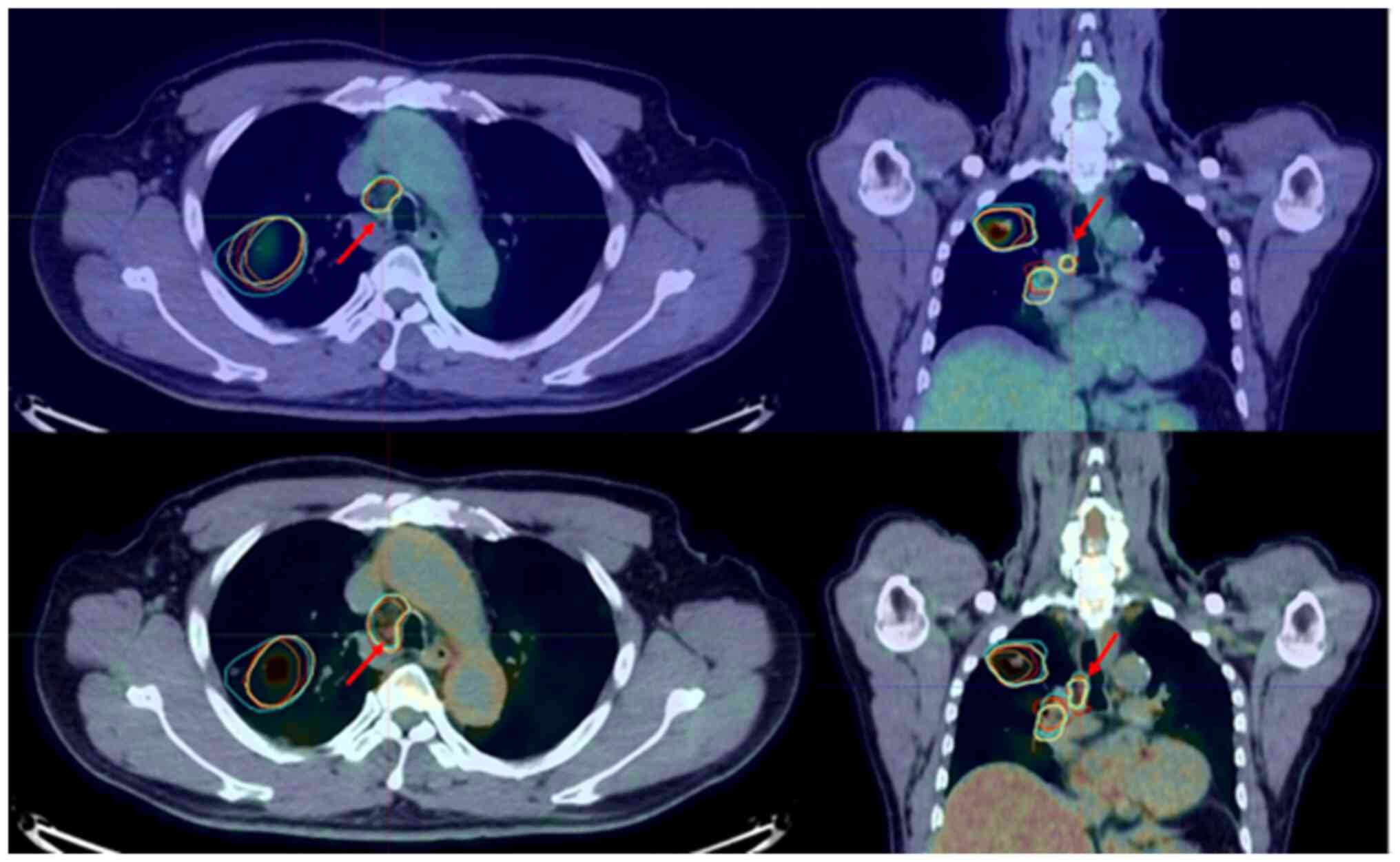
In the Smirnov-Grubbs analysis of the GTVn/CTVn
change ratio, one outlier was found (P<0.05; Fig. 1). From the results of this patient,
it was found that the dGTV divided by the cGTV and the dCTVn
divided by the cCTVn more than doubled (2.97 and 2.18 times,
respectively). The case with GTVn/CTVn outliers is illustrated in
Fig. 1. In this case, the size of
the 4R lymph node was less than 1 cm, and FDG uptake was found only
in the dPET/CT image. All radiation oncologists judged this lymph
node as GTVn/CTVn when they contoured the GTVn/CTVn with reference
to dPET/CT. By contrast, no outliers were found in the GTVp/CTVp or
GTVall/CTVall change ratios.
Discussion
The present study investigated the influence of
dPET/CT on GTV/CTV delineation during IFRT planning for advanced
lung cancers. The results of the present study indicated that
dPET/CT rarely brought about clinically significant changes in the
GTV/CTV for IFRT planning for advanced lung cancer. However, it was
observed that 10% (1/10) of the patients exhibited a large change
that more than doubled in the GTVn/CTVn delineation with reference
to the dPET/CT image compared with the cPET/CT image.
In the immune checkpoint inhibitor +
intensity-modulated radiation therapy era, IFRT is commonly used
for advanced lung cancer (3,12).
However, some patients were treated with IFRT experience lymph node
recurrence (4). The possible
explanation for this, is the lack of image detection of small lymph
node metastases. The lack of image detection with cPET/CT may have
inhibited the GTV/CTV delineation of the true target volumes. In
the present study, the GTVn/CTVn delineation with reference to
dPET/CT resulted in an increased GTVn/CTVn ratio in 10% (1/10) of
the patients. This suggested that the GTVn/CTVn delineation for
IFRT used in previous studies may have been inadequate in some
cases and that interpreting the results of previous studies using
IFRT demands caution (4).
In the present study, although all GTV/CTVs were
contoured with reference to CE/CT and PET/CT images, the GTV/CTV
changed in 10% (1/10) of the patients. Similarly, Koopman et
al (8) revealed that dPET/CT
improves the detection of small lesions and the disease in some
cases [TNM upstaging with dPET/CT in 13% (4/30) of the cases].
Thus, although dPET/CT did not change the GTV/CTV in the majority
of patients with advanced lung cancer, dPET/CT appeared to have an
impact on GTVn/CTVn delineation in some cases, even when GTVn/CTVn
was contoured with reference to multiple imaging modalities. The
use of dPET/CT for GTVn/CTVn delineation may improve the outcomes
of IFRT in advanced lung cancer.
The present study had several limitations. First,
the sample size was small. Second, there were only a few cases of
stage III lung cancer. The present study included patients with
advanced lung cancer who underwent both cPET/CT and dPET/CT imaging
examinations, following the upgrade of the PET/CT machines at Ehime
University Hospital (Toon, Japan). The patients were randomly
selected, which resulted in fewer cases of stage III lung cancer.
Therefore, it was needed to include not only patients with stage
III lung cancer but also those with stage IV lung cancer that did
not affect the GTV/CTV delineation in the thoracic region. Third,
which image was correct when the lymph node metastatic lesions
depicted on dPET/CT differed from those depicted on cPET/CT was
unclear. Further prospective studies are required in the future.
Despite these limitations, it was considered by the authors that
the present study is important because it provides a crucial
perspective on the interpretation of the results of previous
studies, and the use of dPET/CT can potentially improve the
treatment outcomes of IFRT for advanced lung cancer. Furthermore,
various treatment modalities and tumor detection techniques are
currently being investigated (13-15).
Still, further studies are needed because the development of these
technologies may lead to more precise treatment methods for lung
cancer and contribute to improved treatment outcomes.
In conclusion, most GTV/CTV delineations with
reference to dPET/CT were unchanged compared with those from
GTV/CTV delineations with reference to cPET/CT. However, in some
cases, the GTVn/CTVn delineation with reference to dPET/CT is
larger than that of cPET/CT, which may have an impact on the
treatment outcome of IFRT for advanced lung cancer.
Acknowledgements
The authors would like to thank Dr Natsumi
Yamashita, Department of Clinical Research, National Hospital
Organization Shikoku Cancer Center (Matsuyama, Japan) for her
statistical support.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors had full access to the data in the
study, confirmed the authenticity of all the raw data, and take
responsibility for the integrity of the data and the accuracy of
the data analysis. KM designed the study. KM, YH, HK, KN, MM, NK,
TO, TKi and TKo collected patient data and drafted the manuscript.
KM, YH, HK, KN, MM, NK, TO, TKi and TKo collaborated on
discussions. KM prepared the manuscript, and YH, KH and MM edited
the manuscript. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study were
in accordance with the ethical standards of the Institutional
Research Committee and the 1964 Declaration of Helsinki and its
later amendments. The need for informed consent was waived due to
the retrospective nature of the study. The present study was
approved (approval. no. 2211016) by the Ethics Committee of Ehime
University Hospital (Toon, Japan).
Patient consent for publication
The patients treated at Ehime University Hospital
consented in writing for the use of their anonymous data for
research. In addition, Opt-out method was applied to obtain consent
in the present study.
Competing interests
TKo received an honorarium from MSD, Ono, Kyowa
Hakko Kirin, AstraZeneca, Boehringer Ingelheim, Chugai, TAIHO, Eli
Lilly, Bristol Myers Squibb, Pfizer, Merck Biopharma, Nippon
Kayaku, Novartis, Bayer, Sawai, and AMGEN; consulting fee from
Chugai, AstraZeneca, Ono, Pfizer, Daiichi-Sankyo, Bayer, and
Abbvie; and received research funding from MSD, Kyowa Hakko Kirin,
AstraZeneca, Eli Lilly, Pfizer, Chugai, TAIHO, Ono, Bristol-Myers,
Merck Biopharma, Daiichi-Sankyo, AbbVie, AMGEN, Sanofi, Eisai,
LabCorp Development, IQVIA Services, Gilead Sciences, Pfizer, and
Bayer. All other authors declare that they have no competing
interests.
References
|
1
|
Aupérin A, Le Péchoux C, Rolland E, Curran
WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus
R, et al: Meta-analysis of concomitant versus sequential
radiochemotherapy in locally advanced non-small-cell lung cancer. J
Clin Oncol. 28:2181–2190. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Topkan E, Ozdemir Y, Guler OC, Kucuk A,
Besen AA, Mertsoylu H, Sezen D, Akdemir EY, Sezer A, Bolukbasi Y,
et al: Comparison of involved field radiotherapy versus elective
nodal irradiation in stage IIIB/C non-small-cell lung carcinoma
patients treated with concurrent chemoradiotherapy: A propensity
score matching study. J Oncol. 2020(7083149)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Abe T, Iino M, Saito S, Aoshika T, Ryuno
Y, Ohta T, Igari M, Hirai R, Kumazaki Y, Miura Y, et al:
Feasibility of intensity modulated radiotherapy with involved field
radiotherapy for Japanese patients with locally advanced non-small
cell lung cancer. J Radiat Res. 62:894–900. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yuan S, Sun X, Li M, Yu J, Ren R, Yu Y, Li
J, Liu X, Wang R, Li B, et al: A randomized study of involved-field
irradiation versus elective nodal irradiation in combination with
concurrent chemotherapy for inoperable stage III nonsmall cell lung
cancer. Am J Clin Oncol. 30:239–244. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Konert T, Vogel W, MacManus MP, Nestle U,
Belderbos J, Grégoire V, Thorwarth D, Fidarova E, Paez D, Chiti A
and Hanna GG: PET/CT imaging for target volume delineation in
curative intent radiotherapy of non-small cell lung cancer: IAEA
consensus report 2014. Radiother Oncol. 116:27–34. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shiga T, Morimoto Y, Kubo N, Katoh N,
Katoh C, Takeuchi W, Usui R, Hirata K, Kojima S, Umegaki K, et al:
A new PET scanner with semiconductor detectors enables better
identification of intratumoral inhomogeneity. J Nucl Med.
50:148–155. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nguyen NC, Vercher-Conejero JL, Sattar A,
Miller MA, Maniawski PJ, Jordan DW, Muzic RF Jr, Su KH, O'Donnell
JK and Faulhaber PF: Image quality and diagnostic performance of a
digital PET prototype in patients with oncologic diseases: Initial
experience and comparison with analog PET. J Nucl Med.
56:1378–1385. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Koopman D, van Dalen JA, Stevens H, Slump
CH, Knollema S and Jager PL: Performance of digital PET compared
with high-resolution conventional PET in patients with cancer. J
Nucl Med. 61:1448–1454. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
López-Mora DA, Carrió I and Flotats A:
Digital PET vs analog PET: Clinical implications? Semin Nucl Med.
52:302–311. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grubbs FE: Sample criteria for testing
outlying observations. Ann Math Statist. 21:27–58. 1950.
|
|
12
|
Laurans M, Botticella A, Moukasse Y, Lévy
A and Le Péchoux C: Lung cancer and elective nodal irradiation: A
solved issue? Cancer Radiother. 23:701–707. 2019.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
13
|
Wang L, Zhang M, Pan X, Zhao M, Huang L,
Hu X, Wang X, Qiao L, Guo Q, Xu W, et al: Integrative serum
metabolic fingerprints based multi-modal platforms for lung
adenocarcinoma early detection and pulmonary nodule classification.
Adv Sci (Weinh). 9(e2203786)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Y, Bao Q, Yang S, Yang M and Mao C:
Bionanoparticles in cancer imaging, diagnosis, and treatment. VIEW.
3(20200027)2022.
|
|
15
|
Huang J, Bao H, Li X and Zhang Z: In vivo
CT imaging tracking of stem cells labeled with Au nanoparticles.
VIEW. 3(20200119)2021.
|