Introduction
Cases of non-small cell lung carcinoma (NSCLC)
complicated by B-cell lymphoma are relatively rare. The majority of
previous reports regarding such cases included accidental diagnoses
or successful treatments through surgical resection (1). However, cases of advanced lung
carcinoma and B-cell lymphoma being treated by immune checkpoint
inhibitors (ICIs) have rarely been reported, except for two cases
with conflicting results involving treatment with sintilimab and
pembrolizumab (2,3).
The efficacy of ICIs has been demonstrated in
various types of malignant tumors, including NSCLC. Some clinical
trials have revealed that pembrolizumab improves progression-free
survival (PFS) and overall survival (OS) in treatment-naïve
patients with advanced NSCLC, particularly those with a high
programmed cell death ligand 1 (PD-L1) tumor proportion score (TPS)
(4,5). However, the efficacy of ICIs for
malignant lymphomas is unknown, with the exception of Hodgkin's
lymphoma and some types of B-cell lymphoma, such as
mucosa-associated lymphoid tissue (MALT) lymphoma. In particular,
in the case of indolent lymphomas, such as follicular lymphoma (FL)
or MALT lymphoma, the efficacy of ICIs is controversial (6,7).
Moreover, the safety of ICIs in B-cell lymphoma has
yet not been clarified. Some studies have reported the development
of malignant lymphomas, such as FL, during or after treatment with
ICIs (3,8,9); one
study suspected that the development of lymphoma might be caused by
ICIs (3). Thus, consensus on the
commencement of ICI treatment for patients with advanced lung
cancer in conjunction with B-cell lymphomas is not unanimous
because of fear of lymphoma progression.
Herein, we present the case of successful treatment
with pembrolizumab monotherapy following bendamustine and rituximab
(BR) therapy for a patient simultaneously diagnosed with advanced
NSCLC and MALT lymphoma, which could help determine the efficacy
and safety of ICI treatment for this demographic.
Case report
A 69-year-old female patient visited Kyoto
University Hospital (Kyoto, Japan) in March 2022 with an upper
gastrointestinal hemorrhage. She had no history of smoking and a
specific medical history, including Helicobacter pylori (HP)
infection. Upper gastrointestinal endoscopy revealed ulcerative
lesions in the stomach without active bleeding (Fig. 1A), and a biopsy of the lesions
revealed MALT lymphoma (positive for cluster of differentiate 20
(CD20) and negative for CD3). Serological evaluation for
immunoglobulin G (IgG) antibody against HP was negative, and MALT1
translocation was detected by fluorescence in situ
hybridization. Bone marrow biopsy did not indicate malignancy.
Computed tomography (CT) revealed stomach wall thickening, swollen
lymph nodes around the stomach and left pulmonary hilum, and
atelectasis in the middle lobe of the right lung, whereas the
positron emission tomography-CT (PET-CT) image (Fig. 1C) showed right adrenal gland
enlargement with 18F-fluorodeoxyglycose (18F-FDG) uptake. Magnetic
resonance imaging showed no brain metastases. Although the stomach
lesions were diagnosed as MALT lymphoma, the lesions in the lung
and adrenal glands were suspected to be complicated by other
diseases, such as lung cancer. Endobronchial ultrasound-guided
transbronchial needle aspiration of lymph nodes in the left
pulmonary hilum indicated NSCLC with suspected multiple distant
metastases to the adrenal gland and the opposite side of the lung
[cTxN1M1c, cStageⅣB, no oncogenic driver mutations, PD-L1 TPS ≥75%
(22C3)]. However, brush cytology detected atypical lymphoid cells,
indicating that the atelectasis was caused by MALT lymphoma. Based
on these findings, the patient was diagnosed with advanced NSCLC
complicated with MALT lymphoma. Although NSCLS should be treated
promptly, treatment for MALT lymphoma was prioritized, considering
stomach bleeding. We initiated one course of bendamustine (90
mg/m2) plus rituximab (375 mg/m2) therapy.
PET-CT showed reduced 18F-FDG uptake in the stomach wall and
atelectasis in the right lung, except in other lesions (Fig. 1D). We initiated pembrolizumab (200
mg/kg body weight) therapy as a prognostic factor for NSCLC. Four
months after pembrolizumab initiation, PET-CT showed no 18F-FDG
uptake in any of the lesions (Fig.
1E). An erosive lesion was found in the stomach wall through
gastrointestinal endoscopy and a re-biopsy of the stomach wall
showed no residual lesions of MALT lymphoma, indicating
pathological remission of the MALT lymphoma (Fig. 1B). The patient's best response was
a good partial response (PR) for NSCLC and complete remission (CR)
for MALT lymphoma. The treatment effect was maintained for more
than 1 year without recurrence under continuous pembrolizumab
monotherapy (cut-off date: April 25, 2023).
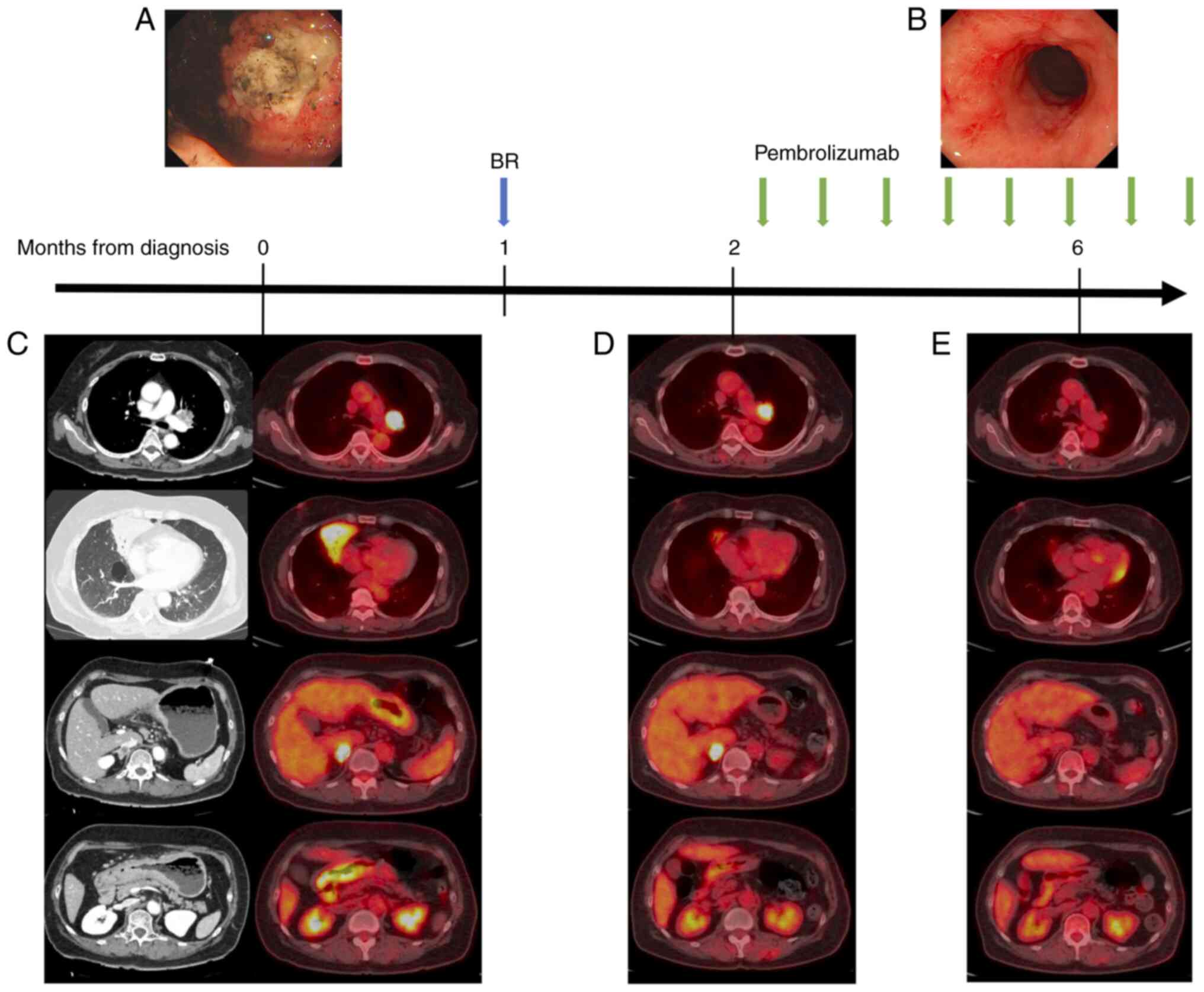 | Figure 1Clinical timeline and longitudinal
change of tumor lesions in the PET-CT image and gastrointestinal
endoscopy. (A) Ulcerative lesions formed by MALT lymphoma were
found during gastrointestinal endoscopy. (B) After 4 months from
the start of treatment, gastrointestinal endoscopy found an erosive
lesion in the stomach wall and the biopsy of this lesion found no
lymphoma cells. (C) At diagnosis, the CT image showed swelling of
left pulmonary hilar lymph node and right adrenal gland in addition
to atelectasis in the middle lobe of the right lung and thickness
in the stomach wall. The PET-CT image showed 18F-FDG uptake in
these lesions. (D) After one cycle of BR therapy, 18F-FDG uptake in
the atelectasis and stomach wall reduced, leaving other lesions.
(E) After 4 months from pembrolizumab commencement, 18F-FDG uptake
in the swelling of the hilar lymph node and adrenal gland were
reduced. PET-CT, positron emission tomography-computed tomography;
MALT, mucosa-associated lymphoid tissue; CT, computed tomography;
18F-FDG, 18F-fluorodeoxyglucose; BR, bendamustine and
rituximab. |
Discussion
Cases of lung cancers being complicated by B-cell
lymphoma are relatively rare. To date, only a few cases of advanced
lung cancer with B-cell lymphoma have been reported. Two of these
cases were successfully treated with alectinib or osimertinib
(10,11) whereas the other cases reported
partial efficacy of sintilimab treatment and exacerbation after
pembrolizumab treatment in patients with advanced lung cancer and
B-cell lymphoma (2) To the best of
our knowledge, this is the first report of pembrolizumab treatment
following one course of BR therapy for advanced NSCLC accompanied
by MALT lymphoma, which resulted in good disease control.
The antitumor efficacy of ICIs has been demonstrated
in many clinical trials. Pembrolizumab is a humanized IgG4 antibody
against the PD-1 receptor, and its efficacy in lung cancer has
previously been demonstrated. In particular, the Keynote-024
clinical trial revealed that pembrolizumab monotherapy for patients
with NSCLC expressing PD-L1 ≥50% significantly improved the OS and
PFS (4) The efficacy of ICIs for
malignant lymphomas has not been determined, with the exception of
Hodgkin's lymphoma. Clinical trials evaluating the efficacy of
nivolumab for relapse or recurrent (r/r) lymphoma showed an overall
response rate (ORR) of approximately 40% in r/r FL (6); however, phase 2 trials with nivolumab
treatment for r/r FL showed an ORR of only 4% (7) In contrast, pembrolizumab plus
rituximab therapy for FL showed relatively good ORR (67%) and CR
(50%), with long-term remission (12).
The efficacy of ICIs in treating MALT lymphoma
remains unknown, although a clinical study evaluating their
efficacy is ongoing (NCT04268277). Three reports have investigated
the treatment of B-cell lymphoma complicated with other solid
cancers with ICIs (Table I). Two
studies reported that MALT lymphoma with solid tumors was
controlled by ICI treatment followed by rituximab monotherapy or
rituximab-included chemotherapy (2,9) and
one of which achieved CR for MALT lymphoma and PR for lung cancer
by treatment with five courses of rituximab-included regimen,
followed by sintilimab monotherapy (2). Another study reported CR for MALT
lymphoma that developed during pembrolizumab treatment for
urothelial cell carcinoma and was treated with four courses of
rituximab (9) These cases suggest
that ICIs with rituximab-containing regimens may be effective in
these populations. However, the remaining patient reported the
development and exacerbation of FL after pembrolizumab monotherapy,
suggesting that lymphoma progression may be induced by ICI therapy
(3).
 | Table IB-cell lymphoma and other cancers
treated with ICIs. |
Table I
B-cell lymphoma and other cancers
treated with ICIs.
| First author,
year | Age/sex | Lymphoma | Coexisting
cancer | ICIs | Onset of
lymphoma | Clinical course | Safety of ICIs for
lymphoma | (Refs.) |
|---|
| Yuan, 2020 | 67/M | MALT lymphoma | LC (Ad) | Sintilimab | Before ICI
induction | During chemotherapy
with CBDCA, PTX, and bevacizumab, the MALT lymphoma was diagnosed
and treated with rituximab and lenalidomide, resulting in CR.
Sintilimab therapy for the progression of LC was initiated and
stable condition was maintained | Maintained CR after
the start of ICI treatment | (2) |
| Marumo, 2021 | 74/F | FL | LC (SQ) | Pembrolizumab | During ICI
treatment | Following the
commencement of pembrolizumab for LC, FL was developed and treated
with rituximab. After achieving CMR and resuming pembrolizumab for
LC, FL was exacerbated and R-CVP therapy was required to achieve
CMR | Development and
exacerbation of lymphoma might be induced by ICIs | (3) |
| Osumo, 2022 | 84/F | MALT lymphoma | Urothelial cell
carcinoma | Pembrolizumab | During ICI
treatment | Two years after the
start of pembrolizumab, the MALT lymphoma was diagnosed in the
right parotid gland. After the completion of rituximab therapy,
pembrolizumab monotherapy was resumed | Good response was
achieved after resuming ICI treatment | (9) |
However, the mechanisms underlying the development
and exacerbation of B-cell lymphoma by ICI remain unclear. Two
studies have reported the development of lymphoma after the
cessation of ICI therapy (8,13)
and one suggested that nivolumab treatment might inhibit the
development of lymphoma (8)
Moreover, the development of B-cell lymphoma after ICI treatment
requires a long time. Previous studies have reported 80 cycles of
treatment, 27 cycles of nivolumab, and approximately 2 years from
pembrolizumab commencement (8,9,14)
for the development of B-cell lymphoma, indicating that ICIs were
not a direct cause of lymphoma development. However, the
development and exacerbation of lymphoma during one case of
pembrolizumab treatment suggested that the mechanism of
exacerbation might be the depression of follicular helper T-cells
and follicular regulatory T-cells induced by anti-PD-1(3). Although the population at risk of
lymphoma development and exacerbation is unknown, it is important
to focus on this phenomenon during or after ICI treatment.
In our case, we observed CR for MALT lymphoma and
good PR for lung cancer treated with one course of BR therapy,
followed by pembrolizumab monotherapy. The efficacy of three
courses of BR therapy for MALT lymphoma has been reported to be
high, with an ORR of 100% and long-term response (15) One cycle of BR therapy may be
effective enough to prevent relapse or recurrence after
pembrolizumab initiation. Considering cases of MALT lymphoma with
no relapse or exacerbation after ICI treatment (2,9) the
initiation of ICI treatment may be safe after CR is achieved with a
rituximab-containing treatment.
Pembrolizumab treatment following a
rituximab-including regimen may be safely initiated in patients
with MALT lymphoma and solid cancers, such as lung cancer.
In conclusion, cases of non-small cell lung cancer
(NSCLC) simultaneously diagnosed with MALT lymphoma are rare.
Pembrolizumab treatment following a rituximab-containing regimen
could be a treatment option in such cases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
DN wrote the original draft, and contributed to
conception, design, data acquisition and data analysis. YS and HO
contributed to supervision, writing, conception, design, data
analysis, and reviewed and edited the manuscript. CM contributed to
supervision, data acquisition, data analysis, and reviewed and
edited the manuscript. KS, TN, HA, HY and TH contributed to data
acquisition, conception, and reviewed and edited the manuscript. YS
and HO confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written and oral informed consent to publish this
report was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamasaki M, Takenaka T, Matsumoto N,
Asaoku H, Taniwaki M and Hattori N: Primary pulmonary collision
tumor comprising squamous cell carcinoma and mucosa-associated
lymphoid tissue lymphoma. Lung Cancer. 129:107–109. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yuan S, Hu X, Zhao Y and Wang Z: Case
report: PD-1 inhibitor is active in lung Adenocarcinoma with B cell
deficiency. Front Immunol. 11(563622)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Marumo Y, Kusumoto S, Masaki A, Nakashima
T, Kikuchi T, Mori F, Komatsu H, Inagaki H, Iida S and Inagaki A:
Newly diagnosed follicular lymphoma during pembrolizumab treatment
for lung cancer. Int J Hematol. 114:280–285. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-Positive
non-small-Cell Lung Cancer. N Engl J Med. 375:1823–1833.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lesokhin AM, Ansell SM, Armand P, Scott
EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ,
Lebovic D, et al: Nivolumab in patients with relapsed or refractory
hematologic malignancy: Preliminary results of a phase Ib study. J
Clin Oncol. 34:2698–2704. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Armand P, Janssens A, Gritti G, Radford J,
Timmerman J, Pinto A, Mercadal Vilchez S, Johnson P, Cunningham D,
Leonard JP, et al: Efficacy and safety results from CheckMate 140,
a phase 2 study of nivolumab for relapsed/refractory follicular
lymphoma. Blood. 137:637–645. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sengul Samanci N, Yılmaz U, Bedir S, Ozgur
Yurttas N, Oruc K, Durak Sahin Z, Celik E, Toplutas KN, Akı H,
Eskazan AE and Demirelli FH: Follicular lymphoma generating in a
patient with non-small cell lung cancer following nivolumab
discontinuation. J Oncol Pharm Pract. 26:2042–2046. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Osumo B, Radzevich J, Nashed N, Ustwani O
and Slotman G: Primary parotid non-Hodgkin's B-cell lymphoma in an
elderly woman on pembrolizumab for metastatic invasive papillary
urothelial cell carcinoma of the bladder. Am Surg. 88:799–801.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Okawa S, Rai K, Fujii N, Gion Y, Ninomiya
K, Kato Y, Taniguchi A, Kubo T, Ichihara E, Ohashi K, et al:
Marginal zone lymphoma and lung adenocarcinoma with an EGFR Exon 19
E746-S752del mutation in a patient with IgG4-related disease.
Intern Med. 60:2831–2837. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ashish S, Rodriguez RR, Sethi P, Yu F and
Raj M: Anaplastic lymphoma kinase (ALK) mutation-targeting
treatment with alectinib in lung adenocarcinoma and primary
cutaneous marginal zone B-cell lymphoma. Cureus.
14(e29922)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nastoupil LJ, Chin CK, Westin JR, Fowler
NH, Samaniego F, Cheng X, Ma MCJ, Wang Z, Chu F, Dsouza L, et al:
Safety and activity of pembrolizumab in combination with rituximab
in relapsed or refractory follicular lymphoma. Blood Adv.
6:1143–1151. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Castel M, Cotton C, Deschamps-Huvier A,
Commin MH, Marguet F, Jardin F, Duval-Modeste AB and Joly P:
Primary central nervous system lymphoma following immunotherapy for
metastatic melanoma. Ann Dermatol Venereol. 146:634–639.
2019.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
14
|
Boudou-Rouquette P, Grignano E, Arrondeau
J, Burroni B and Chouchana L: Diffuse large B-cell lymphoma after
nivolumab treatment for lung cancer: A case report and a World
Health Organization pharmacovigilance database review. Eur J
Cancer. 130:20–22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Salar A, Domingo-Domenech E, Panizo C,
Nicolás C, Bargay J, Muntañola A, Canales M, Bello JL, Sancho JM,
Tomás JF, et al: Long-term results of a phase 2 study of rituximab
and Bendamustine for mucosa-associated lymphoid tissue lymphoma.
Blood. 130:1772–1774. 2017.PubMed/NCBI View Article : Google Scholar
|















