Introduction
Lymphoepithelial cysts (LECs) are relatively rare
benign cystic lesions that occur in the salivary glands (1,2).
This cyst is also referred to as branchial cleft cyst. LECs are
presumed to originate from salivary gland inclusions within peri
salivary lymph nodes; however, several theories have been proposed
to explain the origin of these cysts (1,2).
This cyst is found in increasing numbers in patients infected with
human immunodeficiency virus (HIV) and is well known as a common
cause of neck enlargement in patients with HIV (1). LECs usually arise in adults as a
painless and gradually enlarging mass (1). The characteristic histopathological
features of LECs include presence of well-circumscribed unilocular
cysts containing serous fluid or mucoid contents surrounded by
dense lymphoid tissues composed of small lymphocytes and lymphoid
follicles with germinal centres (1,2).
These cysts are usually lined by squamous epithelium. However, a
variable combination of ciliated, columnar, and mucous epithelia
has also been noted (1,2).
Fine-needle aspiration (FNA) cytological examination
is a standard and useful pre-operative diagnostic method for
salivary gland tumours (3,4). Various reporting systems for
cytological diagnosis of salivary gland tumours have been proposed.
The Milan System for Reporting Salivary Gland Cytopathology
(MSRSGC) was created as a standardised and reproducible reporting
system for classifying salivary FNA cytology specimens in
2018(5). Since then, several
studies have addressed the usefulness of this system (6-12),
and it has been used worldwide in daily diagnostic practice of
salivary gland cytological examination. The second edition of
MSRSGC was published in 2023(13).
The philosophy of this system is risk-stratification based on the
assumed risk of malignancy (ROM) and recommendation of therapeutic
management for each category (13). MSRSGC is classified into seven
diagnostic categories according to the cytomorphological findings:
I, non-diagnostic; II, non-neoplastic; III, atypia of undetermined
significance (AUS); IVa, benign neoplasm; IVb, salivary gland
neoplasm of uncertain malignant potential (SUMP); V, suspicious of
malignancy; and VI, malignant (13).
Various types of neoplastic and non-neoplastic
salivary gland lesions can present as cystic lesions (14). For example, benign non-neoplastic
salivary gland lesions, including LEC, exhibit cystic features, and
benign salivary gland tumours sometimes exhibit cystic changes.
Warthin's tumour, the second most common salivary gland tumour,
frequently exhibits cystic changes. Moreover, malignant salivary
gland tumours, including low-grade mucoepidermoid carcinomas, can
also demonstrate the presence of cystic components (14,15).
Cytological diagnosis of cystic salivary gland lesions is
considered challenging due to the fact that cytological specimens
contain only watery or mucinous cystic contents with few or no
cellular components (14,15). Although diagnostic algorithms for
cytological examination of cystic lesions of the salivary gland
have been proposed (15), only one
article has addressed the application of MSRSGC in cytodiagnosis of
cystic salivary gland lesions (14). Moreover, only a few articles have
reported the cytological features of LECs of the salivary glands
(16), and detailed cytological
features of case series have not been reported yet. In the present
manuscript, we reviewed the cytological features of a case series
of LEC of the salivary gland and evaluated the application of the
second edition of MSRSGC for the first time.
Materials and methods
Patient selection
Consecutive patients who were diagnosed with LEC of
the salivary gland by postoperative pathological examination at
Osaka Medical and Pharmaceutical University Hospital (Osaka, Japan)
and also underwent preoperative FNA (from 1 January 2010 to 30 June
2023) formed our study population.
This retrospective, single-institution study was
conducted in accordance with the tenets of Declaration of Helsinki
and the study protocol was approved by the Institutional Review
Board of Osaka Medical and Pharmaceutical University Hospital
(Approval #2023-073). All data were anonymised. The Institutional
Review Board waived the requirement for informed consent due to the
retrospective study design, as medical records and archived samples
were used with no risk to the participants. Moreover, the present
study did not include minors. Information regarding this study,
such as the inclusion criteria and opportunity to opt out, was
provided through the institutional website (https://www.ompu.ac.jp/u-deps/path/img/file19.pdf).
Cytological analysis
FNA specimens were stained using Papanicolaou and/or
Giemsa stains. Cytological characteristics of pre-operative FNA
specimens of salivary gland lesions, such as background features
(presence of proteinaceous contents and crystalloids) along with
presence and/or types of inflammatory and epithelial cells, were
evaluated. Moreover, the second edition of MSRSGC was used to
classify these FNA specimens into the following seven categories:
I, non-diagnostic; II, non-neoplastic; III, AUS; IVa, benign
neoplasm; IVb, SUMP; V, suspicious of malignancy; and VI, malignant
(summarised definitions and diagnostic criteria for cystic lesions
are shown in Table I) (13,14).
At least two researchers independently re-evaluated the cytological
features of all the specimens after the routine cytological
diagnosis.
 | Table IDiagnostic categories of the second
edition of Milan System for Reporting Salivary Gland Cytopathology
and its definition and criteria (13,14). |
Table I
Diagnostic categories of the second
edition of Milan System for Reporting Salivary Gland Cytopathology
and its definition and criteria (13,14).
| Category | Definition | Diagnostic criteria
for cystic lesions |
|---|
| I.
Non-diagnostic | Insufficient cellular
material for a cytological diagnosis | Non-mucinous cystic
fluid only |
| II.
Non-neoplastic | Benign entities, such
as chronic sialadenitis and infection | Benign appearing
acinar or ductal epithelial components, abundant inflammatory
cells, and/or inflammatory cells with amylase crystalloids |
| III. Atypia of
undetermined significance | Limited atypia and
indefinite for neoplasm | Abundant mucin with
or without rare epithelial cells, or rare atypical cells |
| IVA. Benign
neoplasm | Benign neoplasms
diagnosed based on established cytological criteria | Warthin tumor or
cystic pleomorphic adenoma |
| IVB. Salivary gland
neoplasm of uncertain malignant Potential | Diagnostic of
neoplasm, however, a diagnosis of a specific entity cannot be
made | Epithelial cells such
as oncocytic neoplasms with a cystic background |
| V. Suspicious of
malignancy | Showing features that
are highly suggestive of, but not unequivocal for malignancy | Atypical cells in a
mucinous background; suspicious for low-grade mucoepidermoid
carcinoma |
| VI. Malignant | Diagnostic of
malignancy | Malignant cells in a
cystic background |
Histopathological analysis
Surgically resected salivary gland specimens were
fixed in 10% buffered formalin, dehydrated, embedded in paraffin,
sectioned, and stained with haematoxylin and eosin. At least two
researchers independently evaluated the histopathological features
of all the specimens. The diagnostic criteria for LEC were:
presence of well-circumscribed unilocular cysts surrounded by dense
lymphoid tissues composed of small lymphocytes and lymphoid
follicles with germinal centres (1,2).
Histopathological diagnostic criteria of LEC are as follows: Cysts
are usually lined by squamous epithelium, and a variable
combination of ciliated, columnar, and mucous epithelia has also
been noted (1,2). Histopathological features, such as
type of epithelium and cystic fluid content, were re-evaluated and
compared with the cytological features of the FNA specimens after
the routine histopathological diagnosis.
No immunohistochemical analysis was performed in the
present study.
Results
Patient characteristics
Table II
summarises the clinicopathological features of the study cohort.
The cohort included 13 patients with LEC of the salivary gland. The
median age of the patients was 50 years (range: 25-76 years). The
study population comprised seven males and six females. The lesions
were located in the parotid gland in all the patients (six and
seven patients on the right and left sides, respectively). All the
patients tested negative for HIV infection. Patient 1 also had a
pleomorphic adenoma of the parotid gland on the same side; however,
no continuity was observed between the two lesions.
 | Table IIClinicopathological and cytological
features of lymphoepithelial cyst of the salivary gland. |
Table II
Clinicopathological and cytological
features of lymphoepithelial cyst of the salivary gland.
| | Cytological
features | Histopathological
features |
|---|
| | | Inflammatory
cells | | | Type of
epithelium |
|---|
| Patient no. | Age | Sex | Location | Background | Crysta- lloids | Lymph- ocytes | Neutro- phils | Foamy cells | Giant cells | Type of
epithelium | MSRSGC category | Cystic content | Non-keratinizing
squamous epithelium | Keratinizing squamous
epithelium | Ciliated
epithelium | Mucus epithelium |
|---|
| 1 | 74 | F | Parotid | Proteinous | - | + | + | + | + | None | I | Proteinous | + | - | - | - |
| 2 | | M | Parotid | Proteinous | + | + | + | - | - | Epithelial cell
clusters | IVa | Proteinous | + | - | - | - |
| 3 | 49 | M | Parotid | Proteinous | - | + | - | + | + | Non- keratinizing
squamous cells | II | Proteinous | + | - | - | - |
| 4 | 37 | M | Parotid | Proteinous | - | + | - | - | - | Few epithelial
cells | III | Proteinous | + | - | - | - |
| 5 | 75 | F | Parotid | Proteinous | - | + | - | + | + | None | I | Proteinous | + | - | - | - |
| 6 | 76 | F | Parotid | Proteinous | - | + | - | + | + | None | I | Proteinous | + | - | - | - |
| 7 | 46 | M | Parotid | Proteinous | - | + | + | - | - | None | I | Proteinous | + | - | + | + |
| 8 | 74 | F | Parotid | Proteinous | - | + | - | + | + | None | I | Proteinous | + | - | - | - |
| 9 | 66 | F | Parotid | Proteinous | - | + | - | + | - | None | I | Proteinous | + | - | - | - |
| 10 | 25 | M | Parotid | Proteinous | - | + | - | - | + | Non- keratinizing
squamous cells | II | Proteinous | + | + | - | - |
| 11 | 49 | M | Parotid | Proteinous | - | + | - | + | - | None | I | Proteinous | - | - | + | - |
| 12 | 50 | F | Parotid | Proteinous | + | + | + | - | - | Non- keratinizing
squamous cells | II | Proteinous | + | - | - | - |
| 13 | 48 | M | Parotid | Proteinous | - | + | + | + | - | None | I | Proteinous | + | - | + | - |
Cytological features
Table II
summarizes the cytological features of the 13 patients with LEC of
the salivary gland. Proteinaceous background was noted in all the
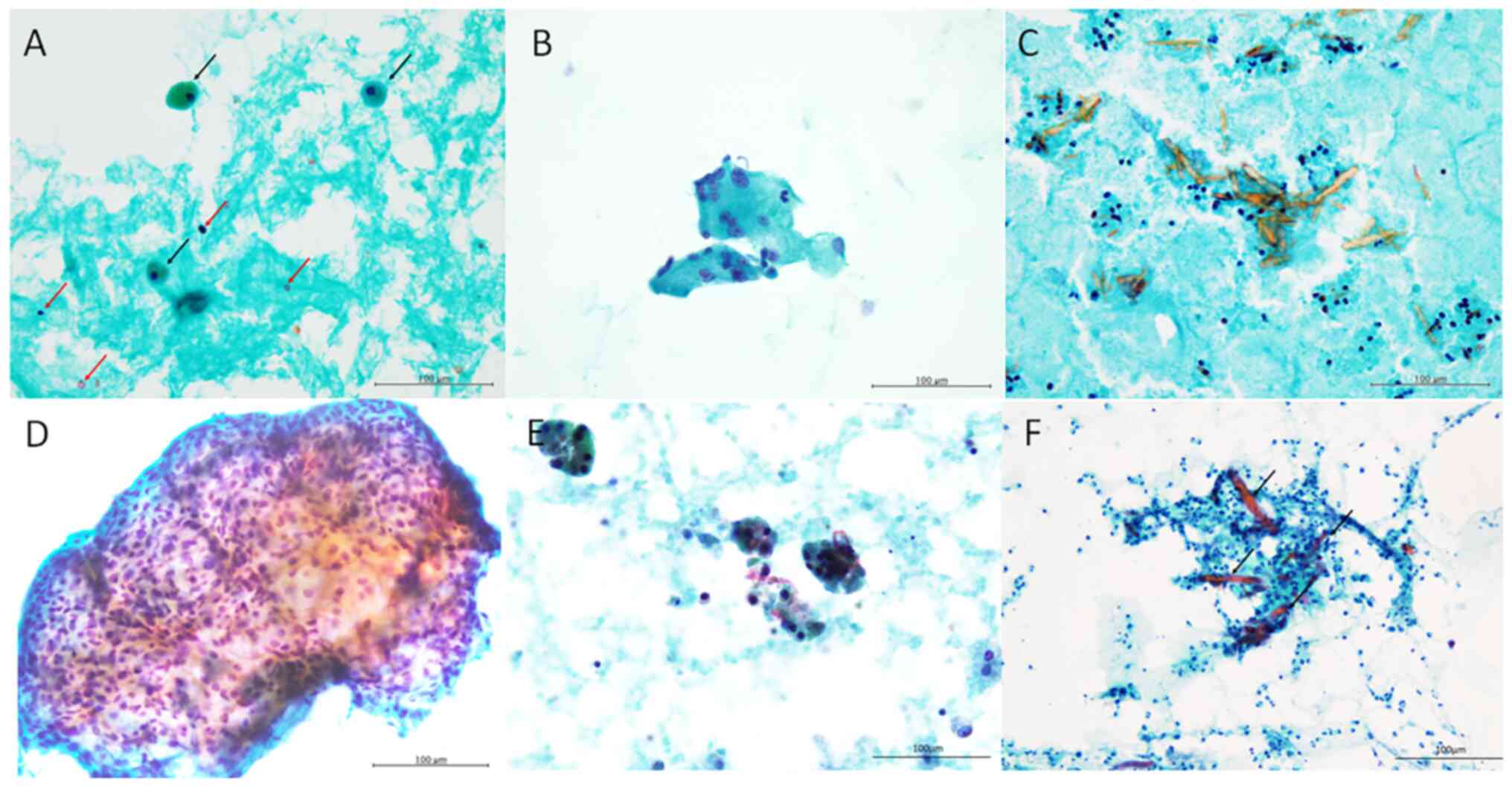
patients (Fig. 1A). Small-sized
lymphocytes were observed in all patients (Fig. 1A). Neutrophils were observed in
five patients. Foamy cells were observed in eight patients
(Fig. 1A) and giant cells in six
patients (Fig. 1B). Amylase
crystalloids, characterised by needle-shaped or rectangular
crystalline structures stained orange with Papanicolaou staining,
were observed in two patients (Fig.
1C).
Epithelial cells were absent in eight patients
(62.5%). Non-keratinising squamous cells without nuclear atypia
were observed in three patients (Fig.
1D). Only few epithelial cells with relatively rich cytoplasm
and round-to-oval nuclei were observed in Patient 4 (Fig. 1E). Epithelial cell clusters with
slightly enlarged nuclei and relatively rich cytoplasm were present
in neutrophilic and lymphocytic background along with amylase
crystalloids in Patient 2 (Fig.
1F).
Categorization of LECs according to
the second edition of MSRSGC
Eight, three, one, and one patients were categorised
as I, II, III, and IVa, respectively, according to the second
edition of MSRSGC (Table II). All
eight patients without epithelial cells were categorised as MSRSGC
I, and three with non-keratinising squamous cells without atypia
were categorised as MSRSGC II. Presence of few epithelial cells
without nuclear atypia was categorised as MSRSGC III (Patient 4).
Epithelial cell clusters with slightly enlarged nuclei and
relatively rich cytoplasm was categorised as MSRSGC IVa (Patient 2)
because Warthin tumour was suspected. None of the patient was
cytodiagnosed with LEC.
Histopathological features
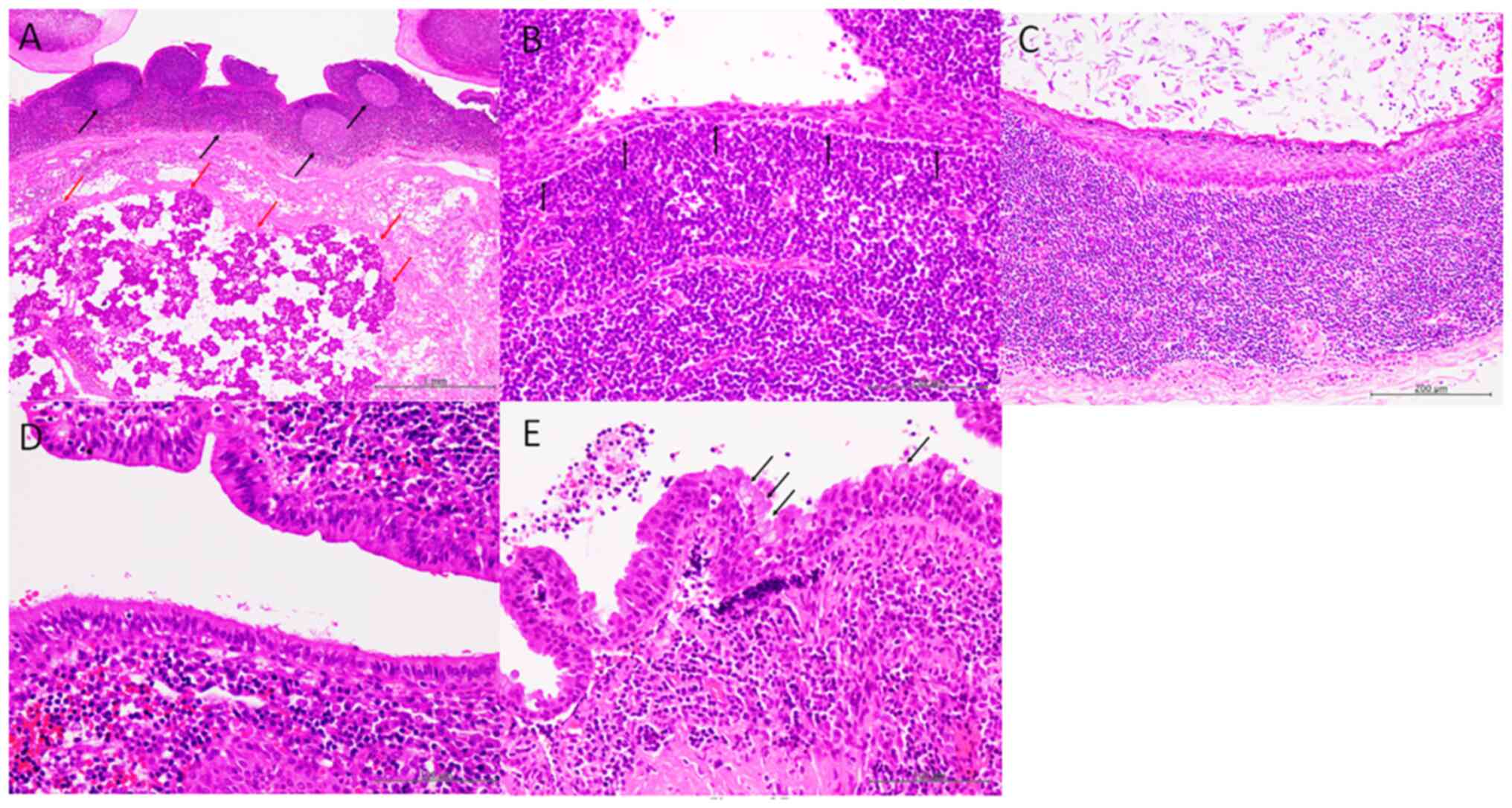
The lesions were characterized by well-circumscribed
unilocular cysts surrounded by fibrous tissue around the salivary
gland in all patients (Fig. 2A).
Dense lymphoid aggregates with lymphoid follicles accompanied by
reactive germinal centres were observed in the cyst wall (Fig. 2A). The types of epithelia covering
the cyst wall were non-keratinising squamous in 12 patients,
keratinising squamous in one, ciliated in three, and mucous in one
(Table II) (Fig. 2B-E). Epithelial cells showed no
nuclear atypia and mitotic figures were not observed (Fig. 2B-E).
Correlation between cytological and
histopathological features
Patients in whom cytological specimens demonstrated
non-keratinising squamous cells (Patients 3, 10, and 12) showed
non-keratinising squamous epithelium in the histopathological
specimens (Table II). Ciliated
epithelial cells were observed in the histopathological specimens
of three patients (Patients 7, 11, and 13), and mucus epithelium
was noted in one patient (Patient 7). However, these epithelial
components were not present in the cytological specimens of these
patients.
Discussion
In the present study, we reviewed the cytological
features of a case series of LEC of the salivary gland and analysed
the application of the second edition of MSRSGC to the features
elicited for the first time. Cytodiagnosis of LEC was difficult due
to the fact that eight of 13 patients (62.5%) in the present cohort
did not have epithelial component and were categorised as
non-diagnostic (MSRSGC I). Epithelial components were present in
five patients; and three, one, and one patient were categorised as
MSRSGC II, III, and IVa, respectively.
Few previous cytological studies dealt with LEC of
the salivary gland as a differential diagnostic consideration or
one of the lists of diagnostic categories of cystic salivary gland
lesions or lesions containing cystic fluid (14,15).
However, detailed reports on cytological features of LEC are
limited (16). A recent case
report of LEC described a cytological specimen containing scattered
lymphocytes with few neutrophils and macrophages in a proteinaceous
background. However, the report provided no information regarding
availability of the epithelial component (16). The present study demonstrated that
the cytological features of LECs are not specific due to the fact
that cytological specimens contain proteinaceous fluid,
lymphocytes, and/or foamy cells, with occasional giant cells, and
less frequently, epithelial cell components, including
non-keratinising squamous cells. Moreover, LECs of the salivary
gland are usually associated with HIV infection (1); however, in the present cohort, none
of the 13 patients had HIV infection.
Several studies have addressed the usefulness of the
first edition of MSRSGC in cytodiagnosis of salivary gland lesions
(6-12).
Although it is well recognised that cytodiagnosis of cystic
salivary gland lesions is challenging because of the absence or
small number of epithelial cells, the utility of MSRSGC in cystic
salivary gland lesions has also been reported (14). Maleki et al analysed the
MSRSGC categorization (1st edition) of 178 cases of cystic salivary
gland lesions and proposed the cytological diagnostic criteria for
cystic salivary gland lesions (14). They showed that 29.2, 44.9, 19.7,
1.7, 1.7, 2.2, and 0.6% of the cases were categorised as MSRSGC I,
II, III, IVa, IVb, V, and VI, respectively (14). Of these, 51 patients (28.7%)
underwent surgical excision and were histopathologically diagnosed.
MSRSGC II category was the most common in their series (44.9%), and
all four LEC in their series were included in this category. None
of the LEC was categorized as MSRSGC I (14). LEC in their series contained
prominent lymphocytes in the cytological specimens; however, no
information regarding the types of epithelial cells was available
(14). In the present cohort,
eight of 13 patients were categorised as MSRSGC I due to the
presence of proteinaceous fluid and absence of epithelial cells in
the cytological specimens. Moreover, three patients of the present
cohort were categorised as MSRSGC II due to the presence of
non-keratinising squamous cells without atypia. One patient each
was categorised as MSRSGC III and IVa, respectively, due to the
presence of few epithelial cells (Patient 4) and suspicion of a
Warthin tumour (Patient 2). Therefore, it can be inferred that
absence of epithelial component in 62.5% of the specimens made
cytodiagnosis an arduous task. However, it was not difficult to
evaluate their benignity.
Presence of mucinous material in cytological
diagnosis of cystic salivary gland lesion is very important as it
indicates the possibility of mucoepidermoid carcinoma (5,14,17,18).
Therefore, presence of mucinous material without epithelial cells
is categorised as MSRSGC III (AUS) (5,14).
Allison et al analysed the cytological features of cystic
salivary gland lesions and classified them as mucinous,
non-mucinous and lymphocytic, and non-mucinous and non-lymphocytic
(18). LECs are classified into
the non-mucinous and lymphocytic category, which mostly includes
non-neoplastic entities. Moreover, Warthin tumour and
mucoepidermoid carcinoma can be also included in this category
(18).
An interesting finding in the present cohort was the
presence of amylase crystalloids in two patients. Although the
presence of crystalloids in salivary gland lesions is not a common
finding (19), amylase-,
tyrosine-rich-, and collagenous crystalloids have been reported
(19-21).
The most common type of crystalloid reported in salivary gland
lesions is amylase crystalloid, as was seen in the present cohort
(19,20). This type of crystalloid is
contemplated to be composed of condensed alpha-amylase in
supersaturated saliva and undergo crystallisation due to the fact
that this structure is recognised as the presence of cystic lesion
accompanying saliva stasis (20).
Amylase crystalloids have been reported to be associated with
non-neoplastic salivary gland lesions, including sialadenitis and
obstructive cysts. Moreover, they are also observed in benign
salivary gland neoplasms, such as pleomorphic adenoma and Warthin
tumour (19,20,22).
No association between amylase crystalloids and malignant neoplasms
has been described (17,19). Sun et al analysed the
incidence and types of crystalloids in cytological specimens of
salivary glands (19). They
reported that a total of 5.6% (37 of 663 patients) of the salivary
gland cytological specimens contained crystalloids, and the most
common type (75% of these cases) was amylase crystalloids, followed
by the tyrosine-rich (11%) and collagenous (3%) types. The most
common histology containing amylase crystalloids is oncocytic
cystadenoma/oncocytic cyst, followed by sialadenitis or ductal
obstructive changes (19). Only
one patient with LEC in their series demonstrated the presence of
amylase crystalloids (19). Only a
few reports on presence of amylase crystalloids in LEC have been
published (23,24). Most cases of amylase crystalloids
present with sialadenitis and obstructive changes, and LECs may
demonstrate this type of crystalloid in cytological specimens.
Therefore, LEC must be included in differential diagnostic
considerations for presence of amylase crystalloids in cytological
specimens (24).
In conclusion, the present study demonstrated that
62.5% of cytological specimens of patients with LEC had no
epithelial component. The remaining specimens had epithelial
components, including non-keratinising squamous epithelium in a
proteinaceous background with lymphocytes and/or foamy cells.
Eight, three, one, and one patients were categorised as MSRSGC I,
II, III, and IVa, respectively. Amylase crystalloids were present
in two patients and therefore, LEC must be included in differential
diagnostic considerations in the presence of this structure in
cytological specimens of salivary glands. Although cytodiagnosis of
LEC may be difficult, application of MSRSGC may be useful for the
cytological evaluation of ROS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated and analysed in this study are
included in this published article.
Authors' contributions
MI conceptualized and designed the study. MI, IK,
MT, HO, KA, MU, CD, SO, RT, and YH performed the cytological
analyses. MI and YH conducted the histopathological examinations.
MI, IK, MT, HO, KA, MU, CD, SO, RT, and YH acquired and analysed
the data. MI drafted the manuscript along with the tables and
figures. MI and YH confirmed the authenticity of all raw data. All
the authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
tenets of Declaration of Helsinki, and the study protocol was
approved by the Institutional Review Board of Osaka Medical and
Pharmaceutical University (protocol no. 2023-073; Takatsuki,
Japan). All data were anonymised. The Institutional Review Board
waived the requirement for informed consent due to the
retrospective design of the study with no risk of identity exposure
for patients. This study did not include any minors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joshi J, Shah S, Agarwal D and Khasgiwal
A: Benign lymphoepithelial cyst of parotid gland: Review and case
report. J Oral Maxillofac Pathol. 22:S91–S97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu L, Cheng J, Maruyama S, Yamazaki M,
Tsuneki M, Lu Y, He Z, Zheng Y, Zhou Z and Saku T: Lymphoepithelial
cyst of the parotid gland: Its possible histopathogenesis based on
clinicopathologic analysis of 64 cases. Hum Pathol. 40:683–692.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schmidt RL, Hall BJ, Wilson AR and
Layfield LJ: A systematic review and meta-analysis of the
diagnostic accuracy of fine-needle aspiration cytology for parotid
gland lesions. Am J Clin Pathol. 136:45–59. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eytan DF, Yin LX, Maleki Z, Koch WM,
Tufano RP, Eisele DW, Boahene KDO, Fakhry C, Bishop JA, Westra WH
and Gourin CG: Utility of preoperative fine needle aspiration in
parotid lesions. Laryngoscope. 128:398–402. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Faquin WC, Rossi ES, Baloch Z, Barkan GA,
Foschini MP, Kurtycz DF, Pusztaszeri M and Vielh P (eds): The Milan
System for Reporting Salivary Gland Cytopathology. Springer
International Publishing AG, Switzerland, 2018.
|
|
6
|
Tochtermann G, Nowack M, Hagen C, Rupp NJ,
Ikenberg K, Broglie MA, Saro F, Lenggenhager D and Bode PK: The
Milan system for reporting salivary gland cytopathology-A
single-center study of 2156 cases. Cancer Med. 12:12198–12207.
2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Higuchi K, Urano M, Akiba J, Nogami M,
Hirata Y, Zukeran Y, Moriyoshi K, Tada Y, Fukushima M, Obayashi M,
et al: A multi-institutional study of salivary gland cytopathology:
Application of the Milan system for reporting salivary gland
cytopathology in Japan. Cancer Cytopathol. 130:30–40.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shahi AK, Sharma S, Singh B, Tandon A,
Kumar A and Chandra S: Assessment of risk of malignancy of
fine-needle aspiration cytology in salivary gland lesions using the
milan system for reporting salivary gland cytopathology
categorization: A systematic review and meta-analysis. J Contemp
Dent Pract. 23:1039–1056. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Velez Torres JM, Tjendra Y, Zuo Y,
Garcia-Buitrago M, Jorda M, Kerr DA and Gomez-Fernandez CR:
Application of the milan system for reporting salivary gland
cytopathology: A single institutional experience of 354 cases with
cytologic-histologic correlation. Acta Cytol. 66:467–474.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jalaly JB, Farahani SJ and Baloch ZW: The
Milan system for reporting salivary gland cytopathology: A
comprehensive review of the literature. Diagn Cytopathol.
48:880–889. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Maleki Z, Baloch Z, Lu R, Shafique K, Song
SJ, Viswanathan K, Rao RA, Lefler H, Fatima A, Wiles A, et al:
Application of the milan system for reporting submandibular gland
cytopathology: An international, multi-institutional study. Cancer
Cytopathol. 127:306–315. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Viswanathan K, Sung S, Scognamiglio T,
Yang GCH, Siddiqui MT and Rao RA: The role of the milan system for
reporting salivary gland cytopathology: A 5-year institutional
experience. Cancer Cytopathol. 126:541–551. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Faquin WC and Rossi ED (eds): The Milan
System for Reporting Salivary Gland Cytopathology. Springer
International Publishing AG (2nd ed.), Switzerland, 2023.
https://link.springer.com/book/10.1007/978-3-031-26662-1.
|
|
14
|
Maleki Z, Allison DB, Butcher M, Kawamoto
S, Eisele DW and Pantanowitz L: Application of the milan system for
reporting salivary gland cytopathology to cystic salivary gland
lesions. Cancer Cytopathol. 129:214–225. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pantanowitz L, Thompson LDR and Rossi ED:
Diagnostic approach to fine needle aspirations of cystic lesions of
the salivary gland. Head Neck Pathol. 12:548–561. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Choudhury S: Benign lymphoepithelial cyst
of parotid gland: A pathologist's perspective. J Cytol. 40:217–219.
2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gomez M, Yu W and Sneige N: The milan
system for reporting salivary gland cytopathology: The experience
of a tertiary cancer center with emphasis on non-mucinous cysts and
improving diagnostic results. J Am Soc Cytopathol. 13:59–66.
2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Allison DB, McCuiston AM, Kawamoto S,
Eisele DW, Bishop JA and Maleki Z: Cystic major salivary gland
lesions: Utilizing fine needle aspiration to optimize the clinical
management of a broad and diverse differential diagnosis. Diagn
Cytopathol. 45:800–807. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Sun T, Faquin WC and Torous VF:
Crystalloids in FNA specimens of salivary gland lesions: A
retrospective study in a single large institute. Cancer Cytopathol.
129:432–438. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pantanowitz L, Goulart RA and Cao QJ:
Salivary gland crystalloids. Diagn Cytopathol. 34:749–750.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Okano K, Arimoto T, Ishida M, Sandoh K,
Fujisawa T, Iwai H and Tsuta K: Collagenous crystalloids in
pleomorphic adenoma of the parotid gland. Diagn Cytopathol.
47:612–613. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Hubbard EW, Winkler M and Davis T: Amylase
crystalloids in nonneoplastic salivary gland: A distinct finding.
Diagn Cytopathol. 43:835–837. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
López-Ríos F, Díaz-Bustamante T,
Serrano-Egea A, Jiménez J and de Agustín P: Amylase crystalloids in
salivary gland lesions: Report of a case with a review of the
literature. Diagn Cytopathol. 25:59–62. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
López-Ríos F, Ballestín C,
Martínez-González MA, Serrano R and de Agustín PP: Lymphoepithelial
cyst with crystalloid formation. Cytologic features of two cases.
Acta Cytol. 43:277–280. 1999.PubMed/NCBI View Article : Google Scholar
|