Introduction
Acute myeloid leukemia (AML) is the most frequent
form of acute leukemia and the second most common leukemia subtype
in adults (1). In 2020, the
incidence of AML in the United States was estimated to be ~4 cases
per 100,000 adults (2). Over the
past two decades, results from studies has deepened the
understanding whilst providing valuable insights into the genomics
and pathophysiology of AML. In turn, these insights have assisted
in improvements in prognostic assessment techniques, which have and
contributed to the development of novel therapeutic strategies for
AML, including the use of second-generation FLT3 inhibitors and
venetoclax (targeting Bcl-2) (3,4). In
particular, FMS-like tyrosine kinase 3 (FLT3) gene mutation
has been identified to be a major prognostic indicator for AML
(5,6).
Internal tandem duplications (ITDs) in the
FLT3 gene have been detected in ~25% newly diagnosed AML
cases, with ~7% FLT3 mutations manifest as tyrosine kinase
domain (TKD) point mutations (7,8).
FLT3-ITD mutations are associated with an increased risk of
treatment resistance to chemotherapy, particularly in patients
receiving chemotherapy without an FLT3 inhibitor. By contrast,
individuals with FLT3-TKD mutations may have a lower disease burden
at diagnosis and they exhibit a superior response to chemotherapy
(4,9). However, this does not necessarily
imply that FLT3-TKD mutations will not develop chemotherapy
resistance. Previous studies have shown that although FLT3-TKD
mutations may respond better to chemotherapy initially, resistance
can still develop over time, particularly in the context of clonal
evolution or the acquisition of additional mutations during the
course of the disease (10,11).
FLT3 inhibitors are a class of tyrosine kinase
inhibitors and are categorized as first- and second-generation
inhibitors, with the inhibitors categorized on the basis of their
specificity and potency against the kinase (9). Midostaurin and sorafenib are examples
of first-generation FLT3 inhibitors, whereas quizartinib and
gilteritinib are examples second-generation inhibitors.
Second-generation inhibitors were designed with structural
modifications to improve selectivity and efficacy against mutant
FLT3. These inhibitors bind more specifically to the active or
inactive conformations of the FLT3 kinase domain, particularly
targeting FLT3-ITD and TKD mutations. Specifically, quizartinib is
tailored to inhibit mutant FLT3 while minimizing off-target effects
on other kinases, whereas gilteritinib is effective against both
FLT3-ITD and resistance-conferring D835 mutations, which are common
in relapsed/refractory AML (12-15).
Quizartinib and gilteritinib are both second-generation FLT3
inhibitors used to treat FLT3-mutated AML, but they target
different forms of the mutation. Quizartinib is a type II
inhibitor, meaning it primarily targets the FLT3-ITD mutation in
the inactive conformation of the FLT3 kinase. By contrast,
gilteritinib is a type I inhibitor, which is effective against both
FLT3-ITD and FLT3-TKD mutations in their active forms, providing
broader activity and potentially reducing the likelihood of
resistance due to TKD mutations (16).
To the best of our knowledge, only one study
(17) demonstrated improved
overall survival in patients with relapsed or refractory AML,
highlighting the efficacy of second-generation FLT3 inhibitors for
AML treatment. Therefore, the present meta-analysis was performed
to compare the clinical efficacy of such second-generation
inhibitors in patients with AML in terms of overall survival.
Additionally, the safety profiles of second-generation FLT3
inhibitors in relation to cardiac disorders, anemia, neutropenia,
thrombocytopenia, diarrhea and pneumonia were compared. For
clinical practice, this study suggests that second-generation FLT3
inhibitors are the optimal choice for treating patients with
FLT3-mutated AML.
Materials and methods
Study search and selection
A comprehensive search for relevant studies was
conducted across multiple databases, including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase
(https://ovidsp-dc1-ovid-com.lib.chimei.org.tw:8443/ovid-new-a/ovidweb.cgi),
the Cochrane Library (https://www.cochranelibrary.com/), Clinicaltril
(https://clinicaltrials.gov/) and Medline
(https://www.nlm.nih.gov/medline/medline_overview.html),
from inception until April 28, 2024. To identify relevant studies,
the following key words were used: ‘Quizartinib OR AC220’,
‘Gilteritinib OR ASP2215’ and ‘Acute Myeloid Leukemia’. The present
study exclusively considered randomized controlled trials (RCTs)
assessing the efficacy and safety of second-generation FLT3
inhibitors. Crenolanib was not considered for the present study due
to the absence of randomized controlled trial data. The present
meta-analysis strictly adhered to including only RCTs to ensure the
reliability and validity of the results. Without RCTs, the efficacy
and safety of crenolanib in comparison to other second-generation
FLT3 inhibitors could not be accurately assessed. Details regarding
the search strategy for the present systematic review and
meta-analysis are provided in Table
SI. In total, two authors (TSW and SYH) conducted a thorough,
independent screening and assessment of each study. In cases of
discrepancy regarding the inclusion of an article, a third author
(CMC) was consulted until a consensus was reached.
Inclusion and exclusion criteria
The population, intervention, comparison and outcome
(PICO) framework for the present meta-analysis was as follows: i)
P, human participants with AML and FLT3 mutations; ii) I, treatment
with second-generation FLT3 inhibitors; iii) C, control group; and
iv) O, overall survival time, heart-rate corrected QT interval
(QTc) prolongation, cardiovascular disorders, anemia, neutropenia,
thrombocytopenia, diarrhea and pneumonia.
In addition, the reference lists of the relevant
articles were manually reviewed to identify possible additional
eligible papers. No language restrictions were applied. RCTs
meeting the following criteria were included: i) Inclusion of
patients with a diagnosis of AML; ii) use of a second FLT3
inhibitor as monotherapy or in combination with other chemotherapy
as the intervention; iii) reporting study outcomes related to
overall survival; and iv) reporting study outcomes related to QTc
prolongation or cardiovascular disorders, anemia, neutropenia,
thrombocytopenia, diarrhea and pneumonia.
The following studies were excluded from the present
review and meta-analysis: i) non-RCTs; ii) studies focusing on
pharmacokinetic and pharmacodynamic analyses; iii) in vitro
or animal experimental studies; iv) studies lacking a control
group; and v) studies with participant overlap with previously
published trials.
Study quality and outcome
assessment
To assess the methodological quality of the included
studies, the Cochrane risk of bias tool for randomized trials
(version 2, RoB 2, https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool)
was employed. This tool can be used to evaluate study quality in
the following six key domains: Randomization process; adherence to
intervention; handling of missing outcome data; outcome
measurement; selective reporting; and overall risk of bias
(18).
In the present study, the primary outcome was the
efficacy of second-generation FLT3 inhibitors in improving overall
survival. The exclusion of RFS and DFS from the present primary
analysis was primarily due to the lack of available data on these
outcomes in the studies included. The secondary outcomes included
the risks of cardiovascular events, such as atrial fibrillation,
cardiac failure, cardiac arrest, myocardial infarction, acute
myocardial infarction and prolonged QT interval on
electrocardiogram. Additionally, common adverse events, such as
anemia, neutropenia, thrombocytopenia, diarrhea, pneumonia and
event-free survival, were also evaluated. Overall survival is
defined as the time from the initiation of treatment until death
from any cause. It considers only one endpoint-mortality.
Event-free survival, on the other hand, is defined as the time from
the initiation of treatment until the occurrence of any event that
signifies treatment failure, such as relapse, progression of the
disease or mortality from any cause (19). It captures a broader range of
outcomes, including both death and significant clinical events that
indicate the treatment is no longer effective. To account for cells
with zero events and to facilitate calculations, 0 was substituted
with the value of 0.5. The aforementioned outcomes were quantified
using odds ratios (ORs) (20).
Data extraction and general
guidelines
In total, two authors (TSW and SYH) independently
performed data extraction. The following data were extracted from
each study: i) Name of the first author; ii) publication year; and
iii) participant demographics, including age, sample size, specific
second-generation FLT3 inhibitor used in treatment, outcome
measures, efficacy in terms of overall survival and data on the
risk of cardiovascular events and QT prolongation on
electrocardiogram. The present meta-analysis was performed adhering
to the latest version of the PRISMA 2020 guidelines (21). The present study was registered in
INPLASY under the registration no. INPLASY202450141. It was not
required to obtain ethics review board approval or participant
informed consent.
Statistical analysis
Because of the heterogeneity in the types of
second-generation FLT3 inhibitors used across the included studies,
a random-effects model was used for the present meta-analysis
(22). The meta-analysis was
conducted using the Comprehensive Meta-Analysis software (version
4; Biostat, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
The primary study outcome was quantified by
estimating hazard ratios (HRs) with 95% CIs, whereas ORs with their
corresponding 95% CIs were calculated to analyze the secondary
outcomes. The heterogeneity among the studies was assessed using
the I2-values. I2-values of 25, 50 and 75%
were considered to indicate low, moderate and high heterogeneity,
respectively (23).
In addition, subgroup analyses according to the type
of AML and the specific second-generation FLT3 inhibitor used were
performed. Meta-regression analyses were performed to investigate
the association between the impact of treatment effects based on
age and overall survival outcomes, which were determined by the
aforementioned parameters. To ensure the reliability of the
meta-analysis, sensitivity analyses were conducted using the
one-study removal approach. In this approach, each trial is removed
from the analysis to determine whether exclusion of any specific
trial leads to a significant change in the summary effect size
experienced a statistically significant alteration when any
specific trial was excluded. This suggests that the exclusion of
individual trials can notably impact the overall results (20). Potential publication bias was
assessed using Comprehensive Meta-Analysis software (version 4;
Biostat, Inc.) in accordance with the guidelines provided in the
Cochrane Handbook for Systematic Reviews of Interventions (24). Funnel plots were created and
visually examined. In this study, bias was assessed using funnel
plots. Bias was defined as the asymmetry of the funnel plot, which
would suggest that smaller studies with non-significant results
were less likely to be published, leading to a potential
overestimation of the treatment effect. A symmetrical funnel plot
would indicate the absence of such bias (25).
Results
Study selection
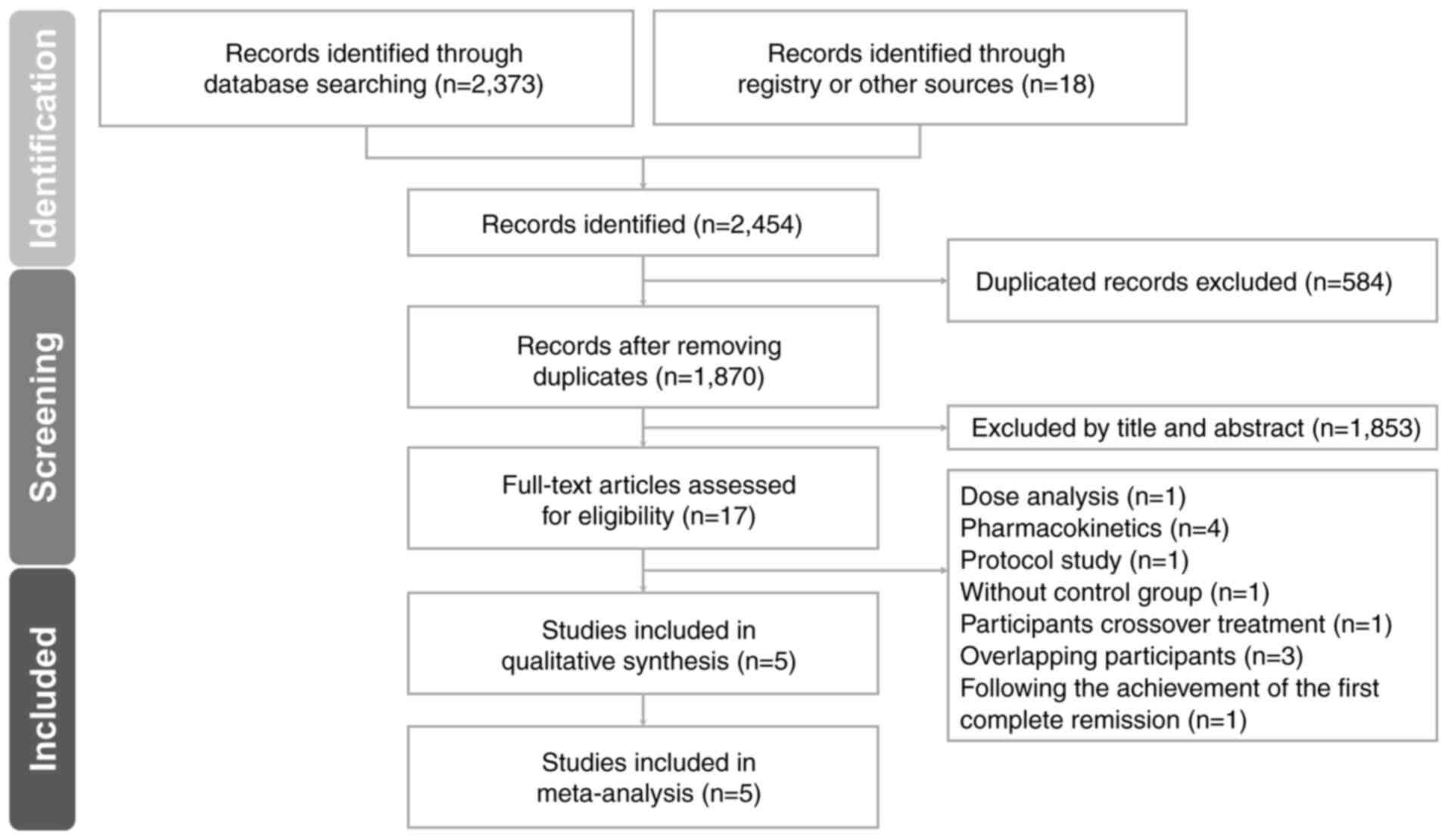
Fig. 1 illustrates
the PRISMA flowchart depicting the literature search process
(15). After elimination of
duplicate articles and exclusion of non-relevant articles through
title and abstract screening, 17 articles remained. Among these, 12
were excluded for the following reasons: i) 1 was related to dose
analysis (26); ii) 4 focused on
pharmacokinetics (27-30);
iii) 1 lacked the salvage chemotherapy control group, the commonly
used regimens include low-dose cytarabine (LoDAC), mitoxantrone,
etoposide and intermediate-dose cytarabine (MEC) or fludarabine,
cytarabine and granulocyte colony-stimulating factor (G-CSF) with
idarubicin (FLAG-IDA) (31); iv) 1
was a protocol study (32); v) 3
had overlapping participant populations (32-35);
and vi) 1 included patients achieving the first complete remission
(34). A total of 5 RCTs were
included in the final analysis (37-41).
The meta-analysis incorporated five eligible RCTs,
involving a collective cohort of 1,543 participants receiving
second-generation FLT3 inhibitors. These participants encompassed
individuals diagnosed with relapsed or refractory FLT3-ITD AML
(37,40) or with de novo or secondary
AML (38), in addition to those
with newly diagnosed FLT3-mutated AML (39,41).
The salvage chemotherapy regimens encompassed various treatment
protocols, including mitoxantrone, etoposide and cytarabine;
fludarabine, cytarabine, granulocyte colony-stimulating factor and
idarubicin; low-dose cytarabine; and azacitidine. Detailed
information from the retrieved trials is presented in Table I.
 | Table ISummary of the included randomized
clinical trials investigating use of second-generation FLT3
inhibitors. |
Table I
Summary of the included randomized
clinical trials investigating use of second-generation FLT3
inhibitors.
| Author, year | Age, years | Population | Number
patients | Second generation
FLT 3 | Control | Outcome
measurement | (Refs.) |
|---|
| Cortes et
al, 2019 | 56.0
(18-81)a | Relapsed or
refractory FLT3-internal tandem duplications AML | Qui, 245; Salvage
CT, 122 | Qui, 20 OR 30
mg | Salvage CT | OS, EFS, AE and
SAE | (37) |
| Dennis et
al, 2021 | 77
(60-89)a | De novo and
secondary AML | Qui, 72; Salvage
CT, 71 | Qui, 90 mg QD | Salvage CT | OS, AE and response
rate | (38) |
| Erba et al,
2023 |
54.0±12.93b | Newly Diagnosed
FLT3-mutated AML | Qui, 268; Salvage
CT, 271 | Qui, 40 mg QD | Salvage CT | OS, EFS, CR rate,
AE and SAE | (39) |
| Perl et al,
2019 | Gil 62
(20.0-84.0)a; Salvage
CT 61.5 (19.0-85.0)a | Relapsed or
Refractory FLT3-mutated AML | Gil, 247; Salvage
CT, 124 | Gil, 120 mg QD | Salvage CT | OS, EFS and AE | (40) |
| Wang et al,
2022 | Gil + AZA,
77.4±5.6b; AZA, 76.7
± 5.3b | Newly diagnosed
FLT3 mutated AML | Gil + Salvage CT,
74; Salvage CT, 49 | Gil, 120 mg QD +
Salvage CT | Salvage CT | OS, EFS, response
rates, AE and SAE | (41) |
Quality assessment of included
studies
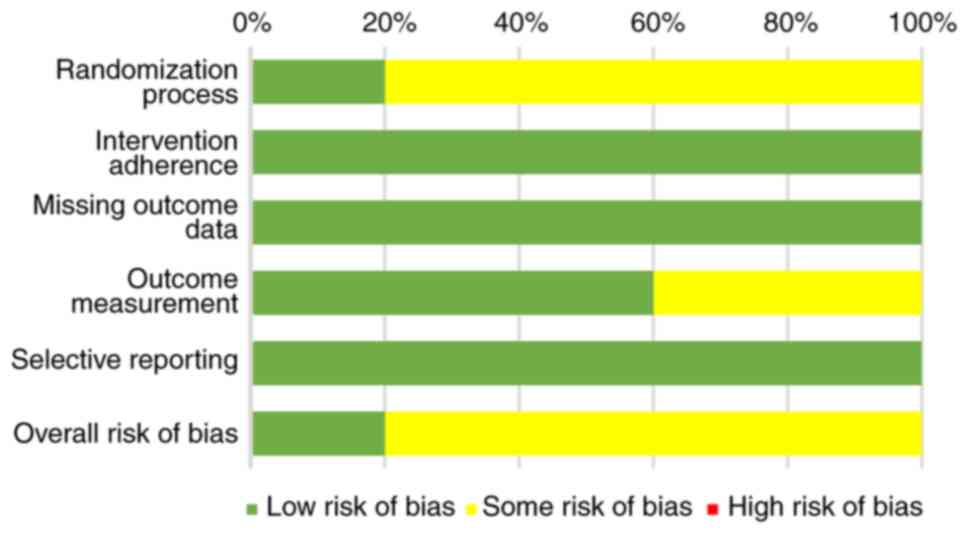
Regarding the overall methodological quality of the
included studies, the results revealed that 20.0% of the studies
exhibited a low risk of bias, whereas 80.0% had some degree of bias
but none were deemed to have a high risk of bias (Fig. 2). In a comprehensive evaluation,
three studies were categorized as having some risk of bias in
outcome measurement because of their open-label designs (37,40,41).
In addition, one study was classified as having some risk of bias
because allocation concealment details were not provided (38). The findings of the risk of bias
assessment are summarized in Table
II.
 | Table IIComprehensive quality assessment of
included studies as conducted using the Cochrane risk of bias 2
tool. |
Table II
Comprehensive quality assessment of
included studies as conducted using the Cochrane risk of bias 2
tool.
| First author,
year | Randomization
process | Intervention
adherence | Missing outcome
data | Outcome
measurement | Selective
reporting | Overall risk of
bias | (Refs.) |
|---|
| Cortes et
al, 2019 | Sa | L | L | S1 | L | S | (37) |
| Perl et al,
2019 | Sa | L | L | S1 | L | S | (40) |
| Dennis et
al, 2021 | Sb | L | L | L | L | S | (38) |
| Wang et al,
2022 | Sa | L | L | L | L | S | (41) |
| Erba et al,
2023 | L | L | L | L | L | L | (39) |
Effect of second-generation FLT3
inhibitors on overall survival
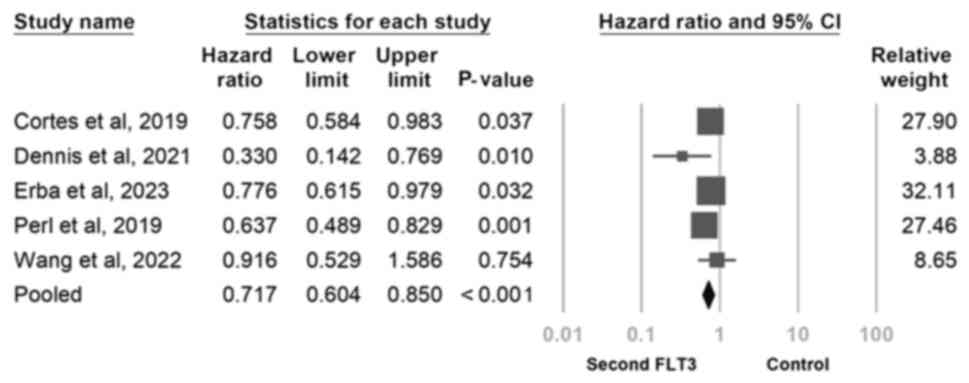
In the pooled analysis of the five trials (Fig. 3), second-generation FLT3 inhibitors
significantly improved overall survival (HR, 0.717; 95% CI,
0.604-0.850; P<0.001). However, low-to-moderate heterogeneity
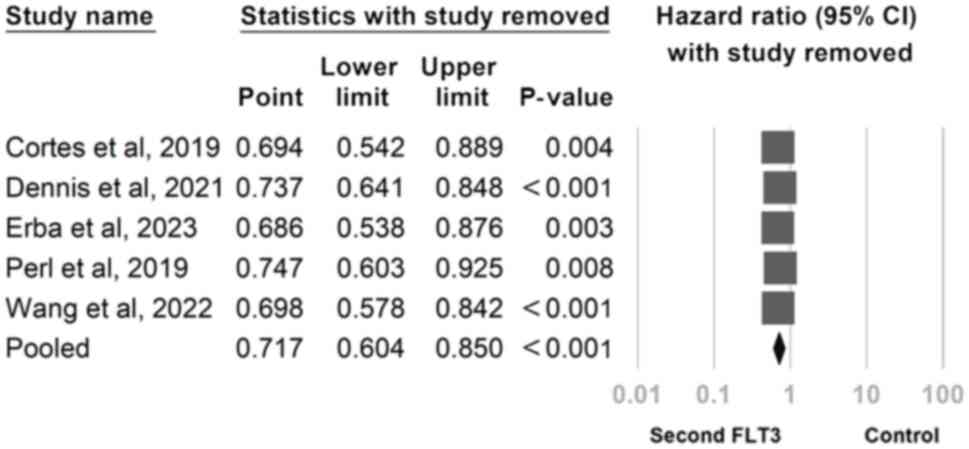
was noted. To address this, a sensitivity analysis was conducted by
using the one-study removal method. Second-generation FLT3
inhibitors was revealed to consistently exert a significant effect
on overall survival. Notably, the significance of these findings
remained unchanged after the exclusion of any of the included
studies (Fig. 4).
The included studies were subsequently categorized
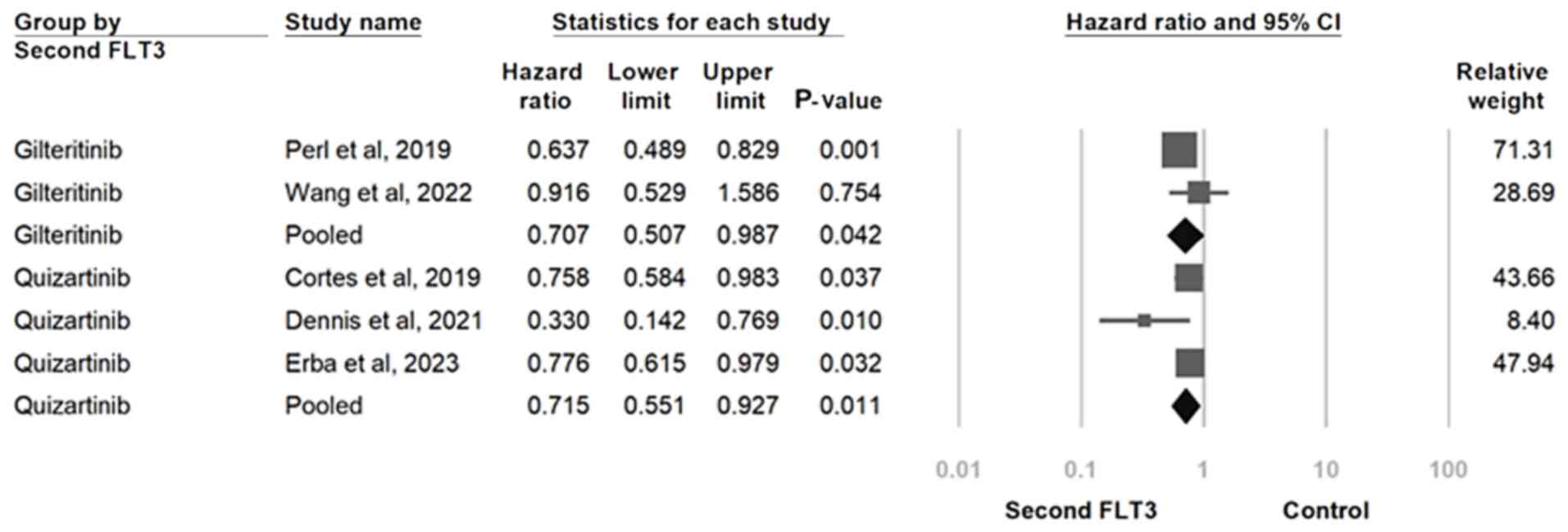
into two subgroups on the basis of the type of second-generation
FLT3 inhibitor used, whereby one group consisted of studies with
quizartinib use (30-32),
whereas the other consisted of studies with gilteritinib use
(40,41). The association between
second-generation FLT3 inhibitors and overall survival remained
consistent in both subgroups. Specifically, participants receiving
quizartinib (HR, 0.707; 95% CI, 0.502-0.987; P=0.042) and those
treated with gilteritinib (HR, 0.715; 95% CI, 0.551-0.927; P=0.011)
had consistent HRs with overlapping 95% CIs (Fig. 5).
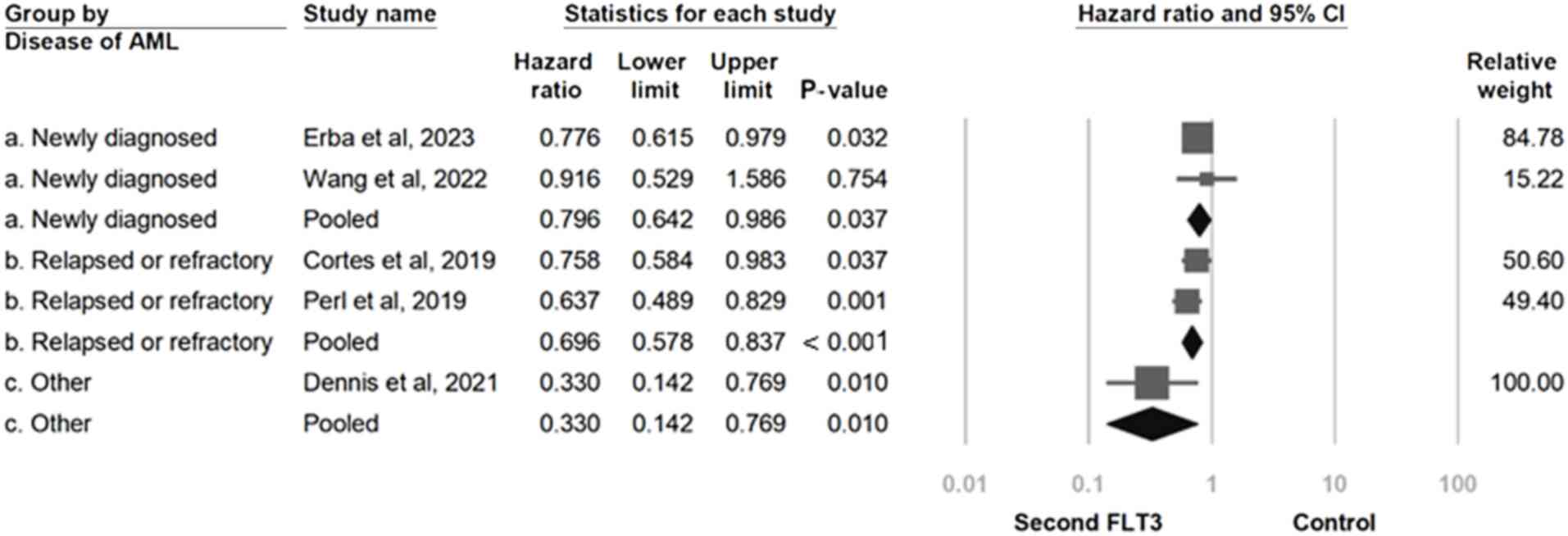
An additional subgroup analysis associated with the
form of AML was subsequently performed. The newly diagnosed group
experienced a significant overall survival benefit, indicating that
the use of second-generation FLT3 inhibitors has a favorable effect
in both clinically newly diagnosed and relapsed or refractory AML
patients (HR, 0.796; 95% CI, 0.642-0.986; P=0.037). Similarly, the
relapsed or refractory group (HR, 0.696; 95% CI, 0.578-0.837;
P<0.001) and the other group (HR, 0.330; 95% CI, 0.142-0.769;
P=0.010) experienced a significant overall survival effect
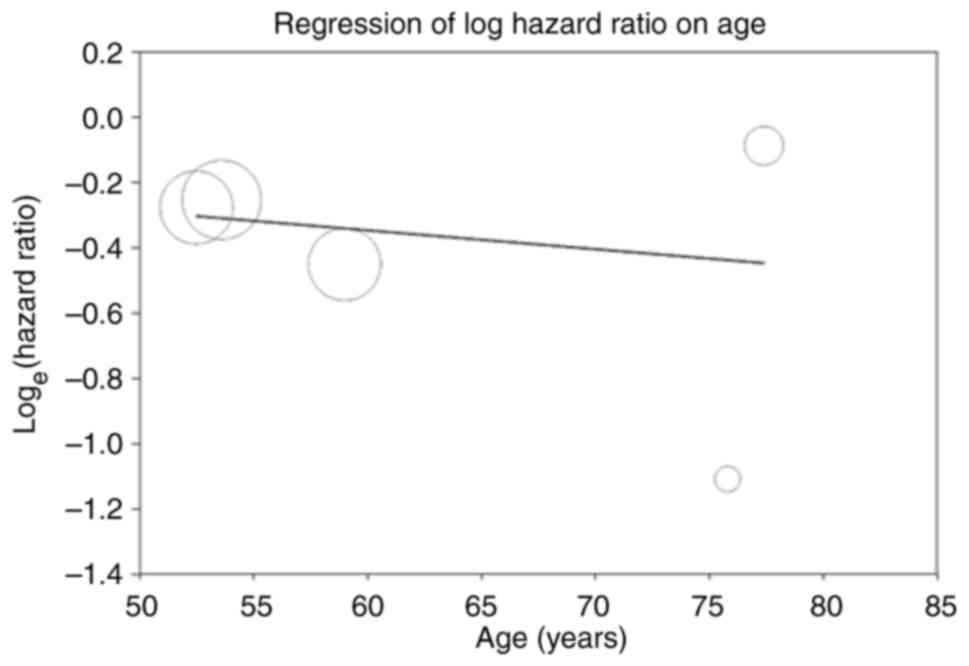
(Fig. 6). A meta-regression
analysis was next performed to assess the potential modification of
the overall survival effects by age. Age exhibited a statistically
significant but clinical trival correlation with overall survival
(coefficient=-0.0058; P<0.001; Fig.
7). The funnel plot generated for the five included trials
exhibited some asymmetry in the distribution of effect sizes
(Fig. S1). However, Egger's
regression test yielded P=0.450, indicating the absence of
publication bias.
Risk of prolongation of QTc interval
and cardiovascular disorders
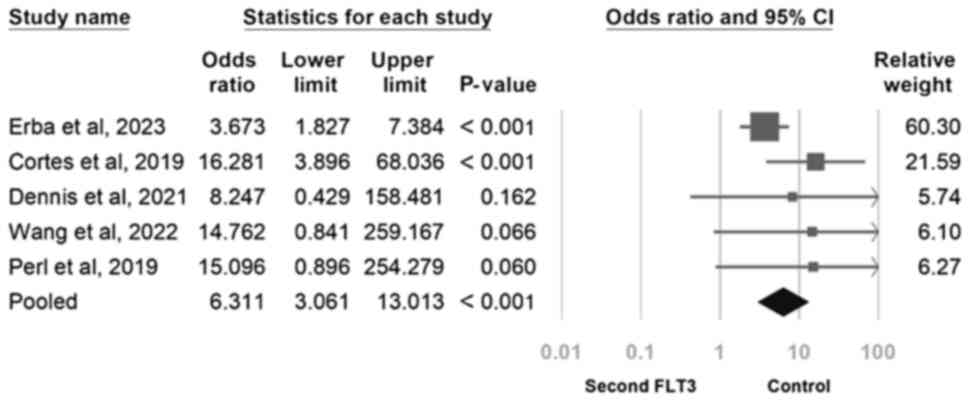
The overall risks associated with a prolonged QTc
interval and cardiovascular disorders were next examined. Compared
to the salvage chemotherapy control group, in the second-generation
FLT3 inhibitor group, the risk of a prolonged QTc interval was
found to be significant (OR, 6.311; 95% CI, 3.061-13.013;
P<0.001; Fig. 8). In addition,
the risk of cardiovascular disorders in the second-generation FLT3
inhibitor group was observed to be comparable to that in the
salvage chemotherapy control group (OR, 1.451; 95% CI, 0.538-3.911;
P=0.462; Fig. S2).
Risk of anemia, neutropenia,
thrombocytopenia, diarrhea and pneumonia
The risks of anemia, neutropenia, thrombocytopenia,
diarrhea and pneumonia were next assessed. Compared to the
second-generation FLT3 inhibitor group, the salvage chemotherapy
control group showed a significantly higher risk of anemia. (OR,
1.350; 95% CI, 1.021-1.786; P=0.035; Fig. S3). Additionally, the risk of
neutropenia observed in the second-generation FLT3 inhibitor group
was found to be similar to that in the salvage chemotherapy control
group (OR, 1.380; 95% CI, 0.808-2.359; P=0.238; Fig. S4). The risk of thrombocytopenia
was also comparable between the second-generation FLT3 inhibitor
group and the salvage chemotherapy control group (OR, 1.321; 95%
CI, 0.974-1.791; P=0.073; Fig.
S5), whereas the incidence of diarrhea was comparable between
the second-generation FLT3 inhibitor group and the salvage
chemotherapy control group (OR, 1.315; 95% CI, 0.684-2.527;
P=0.412; Fig. S6). No significant
difference could be observed in the incidence of pneumonia between
the second-generation FLT3 inhibitor group and the salvage
chemotherapy control group (OR, 1.271; 95% CI, 0.725-2.227;
P=0.403; Fig. S7).
Event free survival
Event-free survival was next assessed, which found
that second-generation FLT3 inhibitors significantly improved
event-free survival compared with that in the salvage chemotherapy
control group (HR, 0.755; 95% CI, 0.582-0.980; P<0.05; Fig. S8).
Discussion
In the present meta-analysis, second-generation FLT3
inhibitors was found to significantly improve overall survival. In
addition, the observed significant difference persisted in the
sensitivity analyses. Various second-generation FLT3 inhibitors,
such as Gilteritinib and Quizartinib, have been associated with
improved overall survival. This improvement was consistent across
different types of AML. Additionally, increasing age was correlated
with improvements in overall survival. Although Hedges (42) addressed the effect size and Deeks
et al (20) explained the
concept of low-to-moderate heterogeneity, to the best of our
knowledge, the present study is the first to synthesize these
insights in a systematic review and meta-analysis specifically
focused on second-generation FLT3 inhibitors. By quantifying the
heterogeneity observed across the included studies, the present
analysis further supports the consistency of the therapeutic
benefits of these inhibitors on overall survival across diverse
patient populations and settings.
Previous meta-analyses have demonstrated
improvements in overall survival to be associated with both
first-generation inhibitors (such as sorafenib, lestaurtinib and
midostaurin) and second-generation inhibitors (such as gilteritinib
and quizartinib) (37-39,43).
Mohebbi et al (17)
previously reported that second-generation FLT3 inhibitors can
improve overall survival in patients with relapsed or refractory
AML (17). These findings are
consistent with results from the present study. However, the study
by Mohebbi et al (17) on
second-generation FLT3 inhibitors was limited by the early
publication dates and two RCT studies by Cortes et al
(37) and Perl et al
(40). It is hoped that the
present meta-analysis can bridge this a gap in the literature by
providing an updated evaluation of the efficacy and safety of
second-generation FLT3 inhibitors.
FLT3, belonging to the receptor tyrosine kinase
family, exhibits a broad expression pattern in hematopoietic
progenitor cells and is commonly overexpressed in AML blasts
(44). Mutations in FLT3 are some
the most prevalent genomic aberrations in AML, being detected in
~33.3% of newly diagnosed adults (45). FLT3 mutations can occur in the
juxtamembrane domain, with such mutations commonly referred to as
ITD mutations (FLT3-ITD) (46) or
in the TKD (FLT3-TKD) (47,48).
Second-generation FLT3 inhibitors, such as quizartinib, selectively
inhibit FLT3 kinase activity, thereby preventing receptor
autophosphorylation. This inhibition leads to the suppression of
downstream FLT3 receptor signaling and arrest of the cell
proliferation process that is dependent on FLT3-ITD (13). Gilteritinib, another
second-generation FLT3 inhibitor, can also inhibit FLT3 receptor
signaling and subsequently proliferation using a mechanism similar
to that of quizartinib (49).
Orally administered gilteritinib was reported to induce apoptosis
in leukemic cells with FLT3-ITD mutations (50) and gilteritinib has been
demonstrated to effectively target both FLT3 mutation subtypes (ITD
and TKD) while both subtypes exhibit only weak activity against
c-Kit (51,52). Although both gilteritinib and
quizartinib are second-generation FLT3 inhibitors, they exhibit
different levels of activity against the c-Kit receptor.
Gilteritinib demonstrates relatively weak inhibition of c-Kit, with
an IC50 of ~100 nM, meaning its impact on c-Kit is
minimal, reducing the likelihood of marrow suppression. By
contrast, quizartinib has stronger inhibitory activity against
c-Kit, which may contribute to a higher risk of myelosuppression in
clinical use (50).
In the separate subgroup analyses by use of
gilteritinib or quizartinib in patients with different forms of
AML, significant summary effect sizes were observed in both
subgroups. This observation may be attributable to the efficacy of
both treatments in improving overall survival in AML. Additionally,
subgroup analyses were conducted for different forms of AML,
including newly diagnosed, relapsed or refractory and other
(referring to de novo or secondary) AML. Regardless of the
AML subtype, second-generation FLT3 inhibitors were found to
significantly improve overall survival. These findings suggest that
second-generation FLT3 inhibitors led to a significant improvement
in overall survival across multiple different AML subtypes.
Furthermore, a statistically significant, though clinically
trivial, correlation between patient age and overall survival in
response to treatment was observed. These findings suggest that
second-generation FLT3 inhibitors have greater effectiveness in
improving overall survival in younger patients with AML with FLT3
mutations (coefficient=-0.0058 P<0.001).
Relapsed or refractory solid tumors in patients with
advanced-stage cancer can potentially influence the physiological
condition of the host. A possible reason is the disruption of the
blood-brain barrier (52),
allowing tumor cells to invade the brain. A previous study has
reported that melanoma, skin, ovarian, and lung tumor cells may
secrete hypothalamic hormones, pituitary hormones, steroids,
catecholamines, serotonin, N-acetylserotonin, melatonin and leptin.
The secretion of such neurohormonal modulators can ultimately
disrupt the body's homeostasis (53). For instance, in AML, FLT3
mutations, particularly FLT3-ITD, result in the constitutive
activation of downstream signaling pathways, such as the PI3K/Akt
and STAT5 pathways, which are crucial for cell survival and
proliferation (54,55). This activation can lead to the
dysregulation of various cellular processes, much like how solid
tumors disrupt homeostasis through the secretion of neurohormonal
modulators.
QTc prolongation is the most frequent adverse event
associated with the use of second-generation FLT3 inhibitors
(26,56). The present study revealed the
occurrence of QTc prolongation with the use of second-generation
FLT3 inhibitors, to suggest that administration of
second-generation FLT3 inhibitors may induce QTc prolongation due
to elevated concentrations in patients with FLT3 mutations
(57). Elevated concentrations of
second-generation FLT3 inhibitors, such as quizartinib, can occur
due to co-administration with cytochrome P450 3A4 inhibitors, which
impede the drug's metabolism, leading to reduced clearance. This
results in the accumulation of the drug in plasma, increasing its
concentration over time. Such interactions are common, especially
when patients are on multiple medications metabolized by the same
pathway (58,59). At higher concentrations, FLT3
inhibitors (such as quizartinib) may block the human
ether-a-go-go-related gene potassium channels, which are critical
for cardiac repolarization. Inhibition of these channels disrupts
the heart's electrical activity, leading to QTc prolongation. The
risk of this effect is dose-dependent, meaning the higher the
concentration of the drug, the more pronounced the QTc
prolongation. This has been demonstrated in clinical studies of
quizartinib, where elevated levels were directly associated with
QTc interval changes. Therefore, greater attention should be given
to such patients receiving these inhibitors (59,60).
Additionally, an analysis of drug-related cardiovascular adverse
events was conducted, but could not yield any significance. An
analysis of common adverse events, such as anemia, neutropenia,
thrombocytopenia, diarrhea, and pneumonia was also performed. A
significant difference was found in only the incidence of anemia
among patients treated with second-generation FLT3 inhibitors,
likely due to the impact of these inhibitors on reducing platelet
function (61). This finding
highlights the importance of monitoring anemia in patients
receiving second-generation FLT3 inhibitors. Additionally, the
effect of second-generation FLT3 inhibitors on event-free survival
compared with the control group was assessed. The results show that
the use of second-generation FLT3 inhibitors significantly improved
event-free survival, suggesting that second-generation FLT3
inhibitors can enhance event-free survival due to their activity
against both FLT3 mutation subtypes (ITD and TKD).
The present study has several limitations. The
second-generation FLT3 inhibitors used among the included trials
differed, potentially contributing to heterogeneity. Therefore, the
measurements were standardized using HRs, before applying a
random-effects model to combine the studies and perform subgroup
analyses to address heterogeneity in accordance with the standard
approach as recommended by the Cochrane Handbook (62,63).
In addition, variability was observed in the age distribution among
the trials, which may have influenced the estimated effects.
Therefore, a meta-regression analysis was performed to investigate
the presence of a linear relationship between age and overall
survival. The present study also did not include the
second-generation FLT3 inhibitor crenolanib due to the lack of
randomized controlled trials. Future research should include RCTs
involving crenolanib if data on such trials become available.
In summary, in the present study, second-generation
FLT3 inhibitors, such as gilteritinib and quizartinib, were found
to significantly improve overall survival, whereby age was
statistically significant, though clinically trivial, correlation
between patient age and overall survival in response to treatment.
However, clinicians should remain aware of potential QTc
prolongation when prescribing second-generation FLT3 inhibitors.
Further investigations are warranted to explore the combined effect
of crenolanib and second-generation FLT3 inhibitors, such as
gilteritinib and quizartinib, on overall survival.
Supplementary Material
Forest plot displaying the data from
five included trials, revealing some asymmetry in the distribution
of log hazard ratios.
Adverse event of cardiovascular
disorders associated with second-generation FLT3 inhibitors
compared to the control group. The relative weight indicates each
study's contribution to the overall effect in the meta-analysis.
FLT3, FMS-like tyrosine kinase 3.
Adverse event of anemia as an adverse
event associated with second-generation FLT3 inhibitors compared to
the control group. The relative weight indicates each study's
contribution to the overall effect in the meta-analysis. FLT3,
FMS-like tyrosine kinase 3.
Adverse event of neutropenia as an
adverse event associated with second-generation FLT3 inhibitors
compared to the control group. The relative weight indicates each
study's contribution to the overall effect in the meta-analysis.
FLT3, FMS-like tyrosine kinase 3.
Adverse event of thrombocytopenia as
an adverse event associated with second-generation FLT3 inhibitors
compared to the control group. The relative weight indicates each
study's contribution to the overall effect in the metaanalysis.
FLT3, FMS-like tyrosine kinase 3.
Adverse event of diarrhea as an
adverse event associated with second-generation FLT3 inhibitors
compared to the control group. The relative weight indicates each
study's contribution to the overall effect in the meta-analysis.
FLT3, FMS-like tyrosine kinase 3.
Adverse event of pneumonia as an
adverse event associated with second-generation FLT3 inhibitors
compared to the control group. The relative weight indicates each
study's contribution to the overall effect in the meta-analysis.
FLT3, FMS-like tyrosine kinase 3.
Summary of event-free survival effect
of second-generation FLT3 inhibitors. The relative weight indicates
each study's contribution to the overall effect in the
meta-analysis. FLT3, FMS-like tyrosine kinase 3.
Search strategy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by Chi Mei Medical Center,
Liouying (grant no. CLFHR11133).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SYH and YTH were responsible for designing the
research and extracting the data. CMC, WTL and CYL conducted the
statistical analysis and handled data visualization and
interpretation. SYH and TSW prepared the initial draft of the
manuscript. Both SYH and TSW performed critical revisions to the
manuscript, focusing on essential intellectual content, and
reviewed the data analysis. SYH and TSW confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cancer Stat Facts: Leukemia-acute myeloid
leukemia (AML). National Cancer Institute. https://seer.cancer.gov/statfacts/html/amyl.html.
Accessed on September 1, 2024.
|
|
3
|
DiNardo CD, Jonas BA, Pullarkat V, Thirman
MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, et
al: Azacitidine and venetoclax in previously untreated acute
myeloid leukemia. N Engl J Med. 383:617–629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Daver N, Schlenk RF, Russell NH and Levis
MJ: Targeting FLT3 mutations in AML: Review of current knowledge
and evidence. Leukemia. 33:299–312. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kottaridis PD, Gale RE, Frew ME, Harrison
G, Langabeer SE, Belton AA, Walker H, Wheatley K, Bowen DT, Burnett
AK, et al: The presence of a FLT3 internal tandem duplication in
patients with acute myeloid leukemia (AML) adds important
prognostic information to cytogenetic risk group and response to
the first cycle of chemotherapy: Analysis of 854 patients from the
United Kingdom medical research council AML 10 and 12 trials.
Blood. 98:1752–1759. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Thiede C, Steudel C, Mohr B, Schaich M,
Schäkel U, Platzbecker U, Wermke M, Bornhäuser M, Ritter M,
Neubauer A, et al: Analysis of FLT3-activating mutations in 979
patients with acute myelogenous leukemia: Association with FAB
subtypes and identification of subgroups with poor prognosis.
Blood. 99:4326–4335. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Levis M: FLT3 mutations in acute myeloid
leukemia: What is the best approach in 2013? Hematology Am Soc
Hematol Educ Program. 2013:220–226. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wander SA, Levis MJ and Fathi AT: The
evolving role of FLT3 inhibitors in acute myeloid leukemia:
Quizartinib and beyond. Ther Adv Hematol. 5:65–77. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kennedy VE and Smith CC: FLT3 mutations in
acute myeloid leukemia: Key concepts and emerging controversies.
Front Oncol. 10(612880)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schlenk RF, Döhner K, Krauter J, Fröhling
S, Corbacioglu A, Bullinger L, Habdank M, Späth D, Morgan M, Benner
A, et al: Mutations and treatment outcome in cytogenetically normal
acute myeloid leukemia. N Engl J Med. 358:1909–1918.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eguchi M, Minami Y, Kuzume A and Chi S:
Mechanisms underlying resistance to FLT3 inhibitors in acute
myeloid leukemia. Biomedicines. 8(245)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Larrosa-Garcia M and Baer MR: FLT3
inhibitors in acute myeloid leukemia: Current status and future
directions. Mol Cancer Ther. 16:991–1001. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kampa-Schittenhelm KM, Heinrich MC, Akmut
F, Döhner H, Döhner K and Schittenhelm MM: Quizartinib (AC220) is a
potent second generation class III tyrosine kinase inhibitor that
displays a distinct inhibition profile against mutant-FLT3, -PDGFRA
and -KIT isoforms. Mol Cancer. 12(19)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tarver TC, Hill JE, Rahmat L, Perl AE,
Bahceci E, Mori K and Smith CC: Gilteritinib is a clinically active
FLT3 inhibitor with broad activity against FLT3 kinase domain
mutations. Blood Adv. 4:514–524. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ge SS, Liu SB and Xue SL: Developments and
challenges of FLT3 inhibitors in acute myeloid leukemia. Front
Oncol. 12(996438)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
The ASCO post: Tale of two FLT3 Inhibitors
in AML:Gilteritinib and quizartinib. https://ascopost.com/issues/april-25-2019/tale-of-two-flt3-inhibitors-in-aml-gilteritinib-and-quizartinib.
Accessed on September 5, 2024.
|
|
17
|
Mohebbi A, Shahriyary F, Farrokhi V,
Bandar B and Saki N: A systematic review of second-generation FLT3
inhibitors for treatment of patients with relapsed/refractory acute
myeloid leukemia. Leuk Res. 141(107505)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: A revised tool for assessing risk of bias in
randomised trials. BMJ. 366(l4898)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Verywell health: Event-free survival (EFS)
after treatment. https://www.verywellhealth.com/event-free-survival-efs-2252150.
Accessed on September 5, 2024.
|
|
20
|
Deeks JJ, Higgins JPT and Altman DG (eds):
Chapter 10: Analysing data and undertaking meta-analyses. In:
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ and
Welch VA (eds). Cochrane Handbook for Systematic Reviews of
Interventions version 6.4 (updated August 2023). Cochrane,
2023.
|
|
21
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA,
Hoffmann TC, et al: The PRISMA 2020 statement: An updated guideline
for reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Borenstein M, Hedges LV and Rothstein HR:
Fixed-effect versus random-effects models. In: Introduction to
Meta-Analysis. Wiley, Hoboken, NJ, 2009.
|
|
23
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Page MJ, Higgins JPT and Sterne JAC:
Chapter 13: Assessing risk of bias due to missing results in a
synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T,
Page MJ and Welch VA (eds). Cochrane Handbook for Systematic
Reviews of Interventions version 6.1 (updated September 2020).
Cochrane, 2020.
|
|
25
|
Sterne JAC, Sutton AJ, Ioannidis JPA,
Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM,
Schmid CH, et al: Recommendations for examining and interpreting
funnel plot asymmetry in meta-analyses of randomised controlled
trials. BMJ. 343(d4002)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cortes JE, Tallman MS, Schiller GJ, Trone
D, Gammon G, Goldberg SL, Perl AE, Marie JP, Martinelli G,
Kantarjian HM and Levis MJ: Phase 2b study of 2 dosing regimens of
quizartinib monotherapy in FLT3-ITD-mutated, relapsed or refractory
AML. Blood. 132:598–607. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
James AJ, Smith CC, Litzow M, Perl AE,
Altman JK, Shepard D, Kadokura T, Souda K, Patton M, Lu Z, et al:
Pharmacokinetic profile of gilteritinib: A novel FLT-3 tyrosine
kinase inhibitor. Clin Pharmacokinet. 59:1273–1290. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li J, Holmes M, Kankam M, Trone D, Mendell
J and Gammon G: Effect of food on the pharmacokinetics of
quizartinib. Clin Pharmacol Drug Dev. 9:277–286. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li J, Kankam M, Trone D and Gammon G:
Effects of CYP3A inhibitors on the pharmacokinetics of quizartinib,
a potent and selective FLT3 inhibitor, and its active metabolite.
Br J Clin Pharmacol. 85:2108–2117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li J, Trone D, Mendell J, O'Donnell P and
Cook N: A drug-drug interaction study to assess the potential
effect of acid-reducing agent, lansoprazole, on quizartinib
pharmacokinetics. Cancer Chemother Pharmacol. 84:799–807.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
No authors listed. Gilteritinib plus
azacitidine combination shows promise in newly diagnosed
FLT3-mutated AML. Oncologist. 26 (Suppl 1)(S10)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jaramillo S, Le Cornet L, Kratzmann M,
Krisam J, Görner M, Hänel M, Röllig C, Wass M, Scholl S, Ringhoffer
M, et al: Q-HAM: A multicenter upfront randomized phase II trial of
quizartinib and high-dose Ara-C plus mitoxantrone in
relapsed/refractory AML with FLT3-ITD. Trials.
24(591)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hosono N, Yokoyama H, Aotsuka N, Ando K,
Iida H, Ishikawa T, Usuki K, Onozawa M, Kizaki M, Kubo K, et al:
Gilteritinib versus chemotherapy in Japanese patients with
FLT3-mutated relapsed/refractory acute myeloid leukemia. Int J Clin
Oncol. 26:2131–2141. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Perl AE, Larson RA, Podoltsev NA,
Strickland S, Wang ES, Atallah E, Schiller GJ, Martinelli G,
Neubauer A, Wang ES, et al: Follow-up of patients with R/R
FLT3-mutation-positive AML treated with gilteritinib in the phase 3
ADMIRAL trial. Blood. 139:3366–3375. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pulte ED, Norsworthy KJ, Wang Y, Xu Q,
Qosa H, Gudi R, Przepiorka D, Fu W, Okusanya OO, Goldberg KB, et
al: FDA approval summary: Gilteritinib for relapsed or refractory
acute myeloid leukemia with a FLT3 mutation. Clin Cancer Res.
27:3515–3521. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
NCT02927262: A Study of ASP2215
(Gilteritinib), administered as maintenance therapy following
induction/consolidation therapy for subjects with FMS-like tyrosine
kinase 3 (FLT3/ITD) acute myeloid leukemia (AML) in first complete
remission. Journal, 2022.
|
|
37
|
Cortes JE, Khaled S, Martinelli G, Perl
AE, Ganguly S, Russell N, Krämer A, Dombret H, Hogge D, Jonas BA,
et al: Quizartinib versus salvage chemotherapy in relapsed or
refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): A
multicentre, randomised, controlled, open-label, phase 3 trial.
Lancet Oncol. 20:984–997. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dennis M, Thomas IF, Ariti C, Upton L,
Burnett AK, Gilkes A, Radia R, Hemmaway C, Mehta P, Knapper S, et
al: Randomized evaluation of quizartinib and low-dose ara-C vs
low-dose ara-C in older acute myeloid leukemia patients. Blood Adv.
5:5621–5625. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Erba HP, Montesinos P, Kim HJ, Patkowska
E, Vrhovac R, Žák P, Wang PN, Mitov T, Hanyok J, Kamel YM, et al:
Quizartinib plus chemotherapy in newly diagnosed patients with
FLT3-internal-tandem-duplication-positive acute myeloid leukaemia
(QuANTUM-First): A randomised, double-blind, placebo-controlled,
phase 3 trial. Lancet. 401:1571–1583. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Perl AE, Martinelli G, Cortes JE, Neubauer
A, Berman E, Paolini S, Montesinos P, Baer MR, Larson RA, Ustun C,
et al: Gilteritinib or chemotherapy for relapsed or refractory
FLT3-mutated AML. N Engl J Med. 381:1728–1740. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang ES, Montesinos P, Minden MD, Lee JH,
Heuser M, Naoe T, Chou WC, Laribi K, Esteve J, Altman JK, et al:
Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for
newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy.
Blood. 140:1845–1857. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hedges LV: Distribution theory for glass's
estimator of effect size and related estimators. J Educ Stat.
6:107–128. 1981.
|
|
43
|
Majothi S, Adams D, Loke J, Stevens SP,
Wheatley K and Wilson JS: FLT3 inhibitors in acute myeloid
leukaemia: Assessment of clinical effectiveness, adverse events and
future research-a systematic review and meta-analysis. Syst Rev.
9(285)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Carow CE, Levenstein M, Kaufmann SH, Chen
J, Amin S, Rockwell P, Witte L, Borowitz MJ, Civin CI and Small D:
Expression of the hematopoietic growth factor receptor FLT3
(STK-1/Flk2) in human leukemias. Blood. 87:1089–1096.
1996.PubMed/NCBI
|
|
45
|
Papaemmanuil E, Gerstung M, Bullinger L,
Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F,
Bolli N, et al: Genomic classification and prognosis in acute
myeloid leukemia. N Engl J Med. 374:2209–2221. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Nakao M, Yokota S, Iwai T, Kaneko H,
Horiike S, Kashima K, Sonoda Y, Fujimoto T and Misawa S: Internal
tandem duplication of the flt3 gene found in acute myeloid
leukemia. Leukemia. 10:1911–1918. 1996.PubMed/NCBI
|
|
47
|
Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R,
Kodera Y, Miyawaki S, Asou N, Kuriyama K, Yagasaki F, Shimazaki C,
et al: Activating mutation of D835 within the activation loop of
FLT3 in human hematologic malignancies. Blood. 97:2434–2439.
2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Abu-Duhier FM, Goodeve AC, Wilson GA, Care
RS, Peake IR and Reilly JT: Identification of novel FLT-3 Asp835
mutations in adult acute myeloid leukaemia. Br J Haematol.
113:983–988. 2001.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Inc. AP: Xospata (gilteritinib) tablets
package insert. https://astellas.us/docs/xospata.pdf. Accessed on
October 4, 2024.
|
|
50
|
Lee LY, Hernandez D, Rajkhowa T, Smith SC,
Raman JR, Nguyen B, Small D and Levis M: Preclinical studies of
gilteritinib, a next-generation FLT3 inhibitor. Blood. 129:257–260.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mori M, Kaneko N, Ueno Y, Yamada M, Tanaka
R, Saito R, Shimada I, Mori K and Kuromitsu S: Gilteritinib, a
FLT3/AXL inhibitor, shows antileukemic activity in mouse models of
FLT3 mutated acute myeloid leukemia. Invest New Drugs. 35:556–565.
2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Benmimoun B and Spéder P: Breaking down
barriers: Tumors make a leaky brain. Dev Cell. 56:2683–2685.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Slominski RM, Raman C, Chen JY and
Slominski AT: How cancer hijacks the body's homeostasis through the
neuroendocrine system. Trends Neurosci. 46:263–275. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ruglioni M, Crucitta S, Luculli GI,
Tancredi G, Del Giudice ML, Mechelli S, Galimberti S, Danesi R and
Del Re M: Understanding mechanisms of resistance to FLT3 inhibitors
in adult FLT3-mutated acute myeloid leukemia to guide treatment
strategy. Crit Rev Oncol Hematol. 201(104424)2024.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Takahashi S: Downstream molecular pathways
of FLT3 in the pathogenesis of acute myeloid leukemia: Biology and
therapeutic implications. J Hematol Oncol. 4(13)2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ballesta-López O, Solana-Altabella A,
Megías-Vericat JE, Martínez-Cuadrón D and Montesinos P:
Gilteritinib use in the treatment of relapsed or refractory acute
myeloid leukemia with a FLT3 mutation. Future Oncol. 17:215–227.
2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Novatcheva ED, Anouty Y, Saunders I,
Mangan JK and Goodman AM: FMS-like tyrosine kinase 3 inhibitors for
the treatment of acute myeloid leukemia. Clin Lymphoma Myeloma
Leuk. 22:e161–e184. 2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wiśniowska B, Tylutki Z, Wyszogrodzka G
and Polak S: Drug-drug interactions and QT prolongation as a
commonly assessed cardiac effect-comprehensive overview of clinical
trials. BMC Pharmacol Toxicol. 17(12)2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kang D, Ludwig E, Jaworowicz D, Huang H,
Fiedler-Kelly J, Cortes J, Ganguly S, Khaled S, Krämer A, Levis M,
et al: Concentration-QTc analysis of quizartinib in patients with
relapsed/refractory acute myeloid leukemia. Cancer Chemother
Pharmacol. 87:513–523. 2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Baracaldo-Santamaría D, Llinás-Caballero
K, Corso-Ramirez JM, Restrepo CM, Dominguez-Dominguez CA,
Fonseca-Mendoza DJ and Calderon-Ospina CA: Genetic and molecular
aspects of drug-induced QT interval prolongation. Int J Mol Sci.
22(8090)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Suknuntha K, Choi YJ, Jung HS, Majumder A,
Shah S, Slukvin I and Ranheim EA: Megakaryocytic expansion in
gilteritinib-treated acute myeloid leukemia patients is associated
with AXL inhibition. Front Oncol. 10(585151)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Higgins JPT, Eldridge S and Li T: Chapter
23: Including variants on randomized trials [last updated October
2019]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T,
Page MJ and Welch VA (eds). Cochrane Handbook for Systematic
Reviews of Interventions version 6.5. Cochrane, 2024.
|
|
63
|
Higgins JPT, Li T and Deeks JJ (eds):
Chapter 6: Choosing effect measures and computing estimates of
effect [last updated August 2023]. In: Higgins JPT, Thomas J,
Chandler J, Cumpston M, Li T, Page MJ and Welch VA (ed). Cochrane
Handbook for Systematic Reviews of Interventions version 6.5.
Cochrane, 2024.
|