1. Introduction
Opioids, such as morphine, fentanyl, oxycodone and
methadone, are a class of psychoactive compounds targeting central
and peripheral opioid receptors, are widely applied in the
management of cancer-associated pain due to their potent analgesic
effects (1-3),
including the management of moderate to severe cancer pain and the
management of pain at the site of primary and metastasis (4-6).
The use of opioids have significantly improved the quality of life
of patients with cancer (7,8).
However, recent studies suggest that opioids may interact with
immune cells within the tumor microenvironment, potentially
affecting tumor progression and treatment responses (9,10).
Although there is ample evidence indicating certain
immunomodulatory effects of opioids (10,11),
including their effects of reducing T cell activity, suppressing
the activity of NK cells and inhibiting B cell activity to reduce
antibody production. Notably, these effects appear to vary across
opioid types, with morphine showing the most pronounced effects.
However, the specific mechanisms by which they can influence the
tumor immune microenvironment remain to be fully elucidated. There
are great knowledge gaps regarding the immunomodulatory effects of
different opioids on various tumor models and outcomes of cancer
treatments. Additionally, the interactions between opioids and
novel immunotherapies, such as immune checkpoint inhibitors,
require further exploration.
The present review aims to systematically evaluate
the effects of opioids on the tumor immune microenvironment,
emphasizing their potential immunomodulatory roles across different
tumor types and their potential interactions with immunotherapies.
The present review also integrates recent research findings to
offer actionable insights for the clinical use of opioids,
particularly in achieving effective pain management whilst
preserving immune function in patients with cancer.
To the best of our knowledge, current literature has
only rarely delved into the dose-dependent effects of opioids on
the immune system, as they mainly instead focused on the effects of
opioids on pain management. A number of the previous studies have
mentioned the dose-dependent effects of opioids (11,12).
In the present review, by elucidating the dose dependent effects
and dual effect of opioids on the tumor immune microenvironment at
different doses and cancer types, with particular attention to
threshold concentrations observed in clinical and experimental
practice, it explored the mechanisms by which opioids can regulate
tumor progression under different conditions. In addition, the
effects of opioids on immune cell function at different doses were
discussed in detail. From this perspective, the present review
hopes to reveal the potential immunosuppressive mechanism of
opioids in patients with cancer.
2. Methods
The present review systematically retrieved the
materials from PubMed (https://pubmed.ncbi.nlm.nih.gov), using key words
‘opioids’, ‘immune system’, ’T cells’, ’monocytes’, ’macrophages’,
‘lymphocytes’, ‘natural killer cell’, ‘immunotherapy’, ‘immune cell
function’ and ‘dose dependent effect’.
Inclusion criteria: i) The present review included
all original studies, systematic reviews and meta-analyses
associated with cancer immunotherapy, opioid mechanisms of action
and their effects on the immune system; ii) Studies involving the
effects of opioids on the immune system, cancer growth or
immunotherapy effects, including in vitro cell experiments,
animal experiments and clinical studies, were all included; iii)
studies must address the use of opioids and analyze their effects
on immune cells [including T cells, macrophages, natural killer
(NK) cells] or immune-related molecules (such as IL-2 and IFN-γ);
iv) studies need to have control groups (such as individuals or
cell/animal models not using opioids) to compare the effects of
opioids on the immune system; v) at least one indicator of immune
function (such as cytokine level, immune cell activity and tumor
microenvironment change) must be reported; and vi) Literature
between January 2000 and November 2024.
Exclusion criteria: i) The content of the study was
not relevant, namely if the study did not explore the interaction
between opioids and the immune system or if it placed too much
focus on the analgesic effects of opioids but did not involve
immune regulation; ii) insufficient or low-quality data, such as if
the study did not provide complete experimental data, lacked
control groups, had too small sample sizes or were of low
methodological quality (such as drop-out rate >20%); and iii)
non-English literatures, to avoid translation bias.
3. Mechanisms of opioids and their impact on
the tumor immune microenvironment
Mechanisms of opioids
Opioids are commonly administered for the management
of severe pain, because they can primarily target the µ, κ, δ and
nociception (NOP) receptors (13).
Amongst these, the µ receptor, with its two subtypes µ1 and µ2, is
predominantly responsible for the key analgesic effects of opioids
(14,15). In addition, they have also been
implicated in opioid addiction (16). The µ1 receptor associated with
central analgesia, euphoria and opioid use dependence (17). The µ2 receptor primarily mediates
physiological processes, such as respiratory depression and
inhibition of gastrointestinal motility (18). By contrast, the κ receptor and its
three subtypes (κ1, κ2 and κ3), which are also involved in
analgesia and sedation, exhibit relatively milder respiratory
depression effects compared with these exerted by the µ receptor
(19). The δ receptor is
associated with anti-anxiety and antidepressant effects, whilst the
NOP receptor is involved in regulating various responses, including
reward and depression (20).
Introduction to the impact of opioids
on the tumor immune microenvironment
Opioid receptors, including µ, δ, κ and NOP, are
involved in regulating a number of physiological activities, such
as analgesia, memory modulation, respiratory control and
angiogenesis (21,22). A number of studies have suggested
that opioids may contribute to the oxidative stress response within
the breast cancer and human neuroblastoma tumor microenvironment
(23-25),
promoting the production of reactive oxygen species (ROS) (26,27).
As a major mutagen, ROS can increase the risk of tumor development
in opioid users (23,28). Additionally, opioids may exacerbate
the oxidative imbalance, particularly in patients with cancer who
frequently exhibit heightened oxidative stress due to concurrent
inflammation (29). This suggests
that opioids could cause greater harm to patients with cancer
compared with healthy individuals. Table I presents a functional overview of
opioid receptors in the tumor immune microenvironment.
 | Table IA summary of the functions of opioid
receptors in the tumor immune microenvironment. |
Table I
A summary of the functions of opioid
receptors in the tumor immune microenvironment.
| Opioid
receptor | Main function | Impact on Tumor
Immune Microenvironment | Key
studies/findings | (Refs.) |
|---|
| µ | Central analgesia,
respiratory depression | Promotes tumor
growth by enhancing angiogenesis and suppressing immune cell
activity (such as natural killer cells and T cells) | Morphine binding to
µ receptors increases tumor invasiveness by modulating macrophage
activity and promoting M2 polarization | (12,34,37) |
| κ | Analgesia, mild
respiratory depression | Inhibits
angiogenesis by reducing VEGFR2 and neurophilin 1 expression,
thereby potentially suppressing tumor growth | κ receptor agonists
exhibit anti-angiogenic properties, suggesting potential
therapeutic applications in cancer treatment | (51-53) |
| δ | Anti-anxiety,
antidepressant effects | May promote the
proliferation and metastasis of cancer cells through pathways, such
as Janus kinase/STAT, in specific cancer types | δ receptor
activation can stimulate the metastasis and progression of breast
cancer cells | (50) |
| Nociception
receptors | Regulates reward,
depression, anxiety | Modulates various
physiological responses, including immune suppression, though its
direct impact on tumors is less studied | Involved in complex
signaling pathways that may indirectly influence tumor immune
microenvironment | (18) |
Opioids can modulate immune functions through both
direct and indirect pathways, influencing the body's response to
tumor cells (9,30). Direct effects occur when opioids
bind to opioid receptors on immune cells, directly regulating their
physiological activity, which is primarily mediated through µ
receptors. By contrast, indirect effects are mediated through the
inhibitory effects of opioids on the central nervous system,
affecting various receptors within the sympathetic nervous system.
They can involve D1 receptors, ultimately affecting peripheral Y1
receptors to inhibit spleen NK cell cytotoxicity (9,30).
Previous animal studies and studies on human CD3+ T cell and
leukemic T-cell lines have demonstrated that µ receptor activation
can suppress the activity of NK cells and inhibit the mitogenic
responses of T cells (31-33).
In addition, it can also suppress the mitogen responses of B cells
in mice following an in vivo administration (34).
Existing evidence suggests that opioids may have
dual effects on tumor progression, depending on the concentrations
applied and the tumor cell heterogeneity profile (35,36).
Previous studies on morphine have indicated that µ receptor
expression in Lewis lung carcinoma cells may be crucial to these
mechanisms, influencing tumor growth and metastasis by regulating
apoptosis and VEGF signaling. As a result, opioid antagonists may
represent a novel therapeutic option for cancer treatment (9,12,34).
The following sections discuss the mechanisms of various opioid
receptors and their potential effects on the immune system.
µ receptor
µ receptor agonists are considered immunosuppressive
agents that can hinder the body's ability to combat cancer cells,
particularly by promoting angiogenesis (37). Morphine, as a µ receptor agonist,
also shows an agonistic effect on δ and κ receptors. However, it
shows a low drug selectivity on δ and κ receptors compared with µ
receptors (38). Therefore,
morphine is primarily the predominant µ receptor agonist. Previous
studies have shown that activation of morphine-induced µ receptors
can promote breast cancer cell proliferation both in vivo
and in vitro (37,39). Morphine can also regulate the
polarization of macrophages toward an M1 or M2 (alternative)
activation state in vitro. A previous study on murine bone
marrow cells documented that morphine can inhibit macrophage M1
polarization in vitro (40). In addition, morphine was found to
inhibit M2 macrophage (alternative) activation in vitro,
including the processes of antigen uptake and caused morphological
changes, such as decrease in perimeter, area and aspect ratio,
increase in roundness, circularity and solidity (40). An experimental study on breast
cancer indicated that morphine can decrease macrophage M2
polarization through blocking the activation of IL-4, which may
lead to breast cancer progression (41). However, other studies on morphine
have indicated that it can inhibit tumor invasiveness by modulating
the production of macrophage proteases in the tumor
microenvironment. Specifically, morphine affects the production of
certain proteins such as inhibiting the production of MMP-9 and
MMP-2 by inhibiting the nitric oxide (NO)/NO synthase (NOS) system]
in MCF-7 breast cancer cells and pancreatic cancer (42,43).
The NO/NOS system serves an important role in tumor progression,
NO, as an important bioregulatory mediator in the human body, is
produced in the body by the action of NOS, exhibiting the function
of inducing extracellular matrix degradation (44,45).
MMP-9 breaks down the extracellular matrix (ECM) and the basement
membrane, which are structural components that provide physical
barriers to multiple tumor cells, including giant cell tumor of
bone (GCTB), non-small cell small lung cancer (NSCLC) and breast
cancer. By degrading these barriers, MMP-9 enables tumor cells to
invade surrounding tissues and spread to other parts of the body,
increasing tumor invasiveness and metastasis (46,47).
Therefore, by inhibiting the production of MMP-9 by inhibiting the
NO/NOS system, morphine may exhibit an inhibitory function against
tumor metastasis and invasiveness.
Previous studies have demonstrated that morphine can
exert a dual effect on the proliferation of pancreatic cancer
cells. In murine experiments, the tumor volume was found to be
significantly increased in the low-dose morphine group (at doses of
0.5 mg/kg), whereas it was significantly smaller in the high-dose
morphine group (at doses of 5 mg/kg) compared with that in the
control group. The underlying mechanism was proposed to be mediated
through the p38/JNK pathway. Specifically, low concentrations of
morphine in this concentration led to an increase in phosphorylated
JNK, whilst the phosphorylation of p38 was reduced. By contrast,
higher concentrations of morphine reversed these aforementioned
processes, resulting in increased p38 activation and decreased JNK
activation (48). Another previous
in vitro study reported that in renal cell carcinoma, when
using morphine at a dose of 2 nmol to 3.5 µmol in serum
concentrations, converted to clinical dosage at 10-2,450 mg/day, it
exerted no significant proliferative effects. However, after
increasing the dose to 50 µmol in serum concentrations, which
equated to a commonly used clinical dose of 35 mg, morphine
promoted the migratory ability of renal cell carcinoma cells
(49). Therefore, it is essential
to carefully consider the pharmacokinetic and tolerance effects of
morphine during its administration. A clinical trial on patients
with cervical cancer have been previously performed assessing the
effects of morphine combined with ketamine (morphine, 1 mg/kg/day;
ketamine, 1 mg/kg/day) and morphine alone (1 mg/kg/day) for pain
management, where both groups yielded a decrease in CD4+ and
CD4+/CD8+ ratios. This was proposed to be achieved by modulating T
cell activation and cytokine expression, specifically by inhibiting
the secretion of IL-2, IFN-γ and IL-17, through the Janus kinase
3/STAT5 pathway (50).
Animal models of NSCLC have shown that morphine (0.1
µg/µl) can promote the proliferation, migration and invasion of
H460 cell-induced NSCLC by activating the Src/PI3K/AKT/mTOR
signaling pathway, inhibiting the G2 phase of the cell
cycle and suppressing apoptosis (51). Furthermore, another in vitro
and in vivo study has demonstrated that, morphine (1.0
µmol/l) pre-treatment under hypoxic conditions can inhibit the
production of the pro-angiogenic factor VEGF in Lewis lung
carcinoma cells in vitro. In in vivo experiments, morphine
exhibited an inhibitory effect on angiogenesis. This effect was
mediated by morphine's interaction with hypoxia-inducible factor 1,
which binds to its hypoxia response element, resulting in the
suppression of VEGF production by µ receptor. Another murine study
previously reported that morphine (plasma levels maintained between
250-400 ng/ml) can significantly reduce the blood vessel density,
branching and length compared with those in the placebo group
(52). This suggests that the MOR
receptor-mediated processes can inhibit angiogenesis within the
tumor microenvironment under certain conditions, thereby
suppressing tumor growth. Morphine exhibit a dual effect on promote
or inhibit tumor progression in different cancer models, including
lung cancer model and breast cancer model, which may be due to the
different downstream proteins activated by µ receptors on the
surface of different cancer cells, the mechanism underlying the
differences in performance among each cancer cell type warrants
further exploration.
δ receptor
A previous study has addressed the δ receptor's role
in breast cancer progression, with the majority primarily focusing
on breast cancer. A mouse study previously indicated that δ
receptor agonist (D-Ala2,D-Leu5)-Enkephalin
treatment promoted lung metastasis by breast cancer cells, through
the migratory Janus kinase 1/2-STAT3 axis and by enhancing
epithelial-mesenchymal transition (53). Subsequent studies on human breast
cancer cells have shown that expression of the δ receptor was
increased in breast cancer cells, where the inhibition of the δ
receptor was found to suppress cell proliferation, suggesting its
role in the proliferation process (53,54).
In addition, δ receptor was found to underly the progression of
breast cancer by activating the protein kinase C/ERK signaling
pathway. Therefore, the δ receptor inhibitors were proposed to
serve as an adjunctive treatment for patients with breast cancer
(53).
κ receptors
The role of the κ receptor in the tumor
microenvironment primarily involved inhibiting angiogenesis,
particularly the transformation of vascular progenitor cells into
vascular endothelial cells. κ receptor agonists were found to
inhibit VEGFR2 expression, thereby suppressing endothelial cell
migration and angiogenesis in Lewis lung carcinoma and breast
cancer (22,52,53).
Previous studies have shown that the κ receptor is
highly expressed in endothelial progenitor cells and endothelial
cells, where its activation can reduce the expression of VEGFR2 and
neuropilin 1, which are necessary for angiogenesis, by decreasing
cAMP production through inhibiting the cAMP/protein kinase A (PKA)
pathway (22,52,53).
In murine tumor models, κ receptor knockout mice exhibited enhanced
tumor proliferation and angiogenesis following lung cancer cell
transplantation compared with those in wild-type mice.
Consequently, κ receptor agonists may inhibit tumor growth by
suppressing angiogenesis (22,52,53).
The anti-angiogenic properties of κ receptor hold
potential therapeutic value in cancer treatment. However, κ
receptor agonists, such as butorphanol, nalbuphine and dynorphins,
pose a risk of drug dependence and tolerance (55,56),
necessitating careful consideration in their development and
application, particularly for the combined treatment of cancer pain
and anti-angiogenesis.
Additionally, another previous study on breast
cancer cells found that the expression of κ receptor was
significantly increased in breast cancer cells compared with that
in non-tumor cells (normal human mammary epithelial cells). The
inhibition of PI3K/AKT signaling by using inhibitors Recilisib and
Buparlisib, or knockdown of the κ receptor, was found to reduce the
viability and migration of breast cancer cells. These findings
suggested that κ receptor may serve as a potential breast cancer
growth antagonist (57).
4. Impact of opioids on the immune
system
Previous studies have indicated that opioids can
suppress the immune system, where in vitro studies have
shown that opioids can damage immune cells, including macrophages
and T cells (12,30). Epidemiological studies have also
suggested that long-term opioid use may increase the risk of
infections (30,34,58).
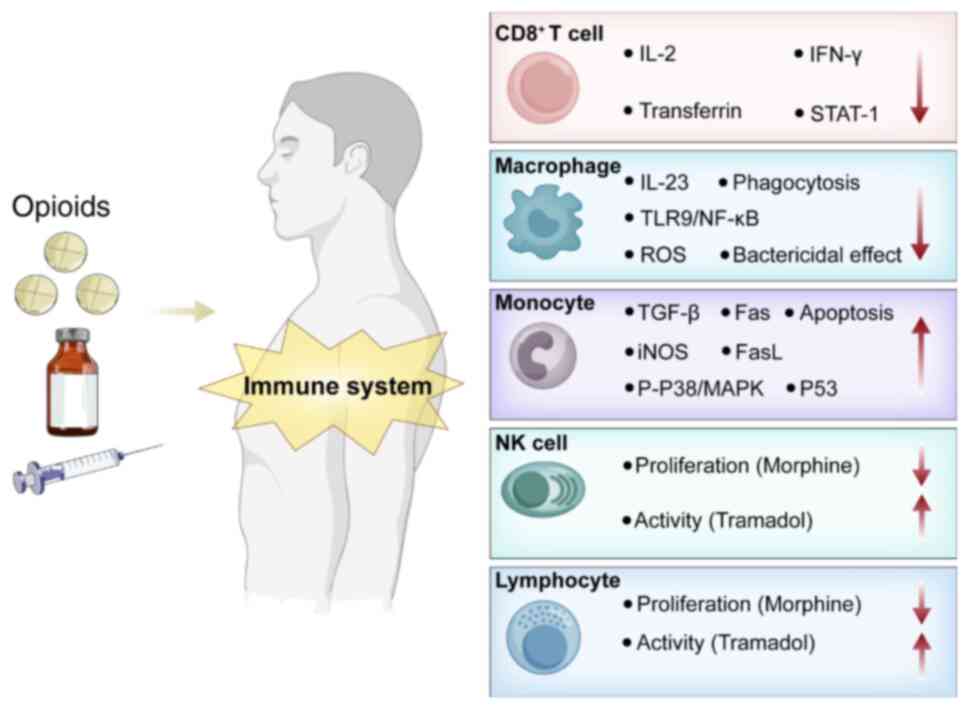
The effects of opioids on immune cells are summarized in Fig. 1 and Table II.
 | Table IIA summary of the effects of opioids
on different types of lymphocytes within the tumor immune
microenvironment. |
Table II
A summary of the effects of opioids
on different types of lymphocytes within the tumor immune
microenvironment.
| Opioid | Lymphocyte | Effect |
Mechanism/Outcome | (Refs.) |
|---|
| Morphine | T Cells | Decrease | Inhibits IL-2
production and receptor expression; reduces T cell activity and
immune response | (100,101) |
| | CD8+ T Cells | Decrease | Suppresses IFN-γ
production, inhibits STAT-1 pathway activation and leads to
impaired antiviral response | (102,103) |
| | NK cells | Decrease | Suppresses the
activity of NK cells through central opioid receptors | (102,103) |
| | B Cells | Decrease | Suppresses
mitogen-induced responses and reduces antibody production | (78,104) |
| Fentanyl | NK Cells | Decrease | Prolonged
post-operative suppression, particularly in cancer surgery | (30) |
| Buprenorphine | T Cells | No Effect/reversed
suppression | Shows no effect or
reverses postoperative suppressive effects | (9) |
| Tramadol | NK Cells | Increase/reversed
Suppression | Increases the
activity of NK cells or reverses the suppressive effects of
surgery | (30) |
Impact on T cells
Animal and cell studies have demonstrated that
morphine can reduce the activity of T cells in mice by inhibiting
the lymphocyte IL-2 production and IL-2 receptor expression in the
spleen, which in turn reduces T cell activity and impairs immune
function (32,59). In addition, cell experiments have
demonstrated that morphine can reduce lactate dehydrogenase (LDH)
release and the number of CD8+ T cells, whilst increasing the ratio
of CD4+/CD8+ T cells (32,59). Another previous study on the
effects of morphine on the anti-human immunodeficiency virus
properties of CD8+ T cells has indicated that morphine can inhibit
the activity of CD8+ T cells by directly binding to µ receptors,
which can downregulate CD8+ T cell activity. In addition, morphine
can reduce the secretion of IFN-γ by CD8+ T cells, thereby
impairing their function. Morphine was also found to reduce the
production of IFN-γ by CD8+ T cells and inhibits CD8 T cell-induced
expression of the STAT1, further impairing IFN-γ signaling to
compromise CD8 T cell activity, resulting in immune
dysfunction.
Impact on monocytes and
macrophages
Morphine can inhibit the macrophage-mediated
clearance of pathogens (such as Streptococcus pneumoniae) by
suppressing the toll-like receptor (TLR)9/NFκB signaling pathway,
which increases the risk of infections (60). It can also reduce the production of
IL-23 by dendritic cells and macrophages by inhibiting the TLR3 and
nucleotide-binding oligomerization domain-containing protein 2
signaling pathways, weakening the body's defense mechanisms
(60,61). In addition, recent studies suggest
that morphine can significantly increase the expression of TLR4,
p65, NF-κB and cytokines (IL-6 and TNF-α) in microglia and
macrophages (62,63).
Numerous studies have demonstrated that morphine, as
an opioid receptor agonist, can directly downregulate various
phagocytic activity and enhance cell apoptosis in a number of cell
types, including mouse peritoneal macrophages, bone marrowderived
macrophage (BMDM) and peripheral blood monocytes (64,65).
In particular, in BMDM, morphine can enhance LPS-induced macrophage
apoptosis through a peroxisome proliferator-activated receptor
γ-dependent mechanism (65). This
interaction inhibits the production of ROS, including superoxide
and peroxide, resulting in decreased superoxide anion production.
Since these intermediates are involved in the bactericidal
mechanism of phagocytes, morphine treatment resulted in decreased
phagocytic efficacy and reduced macrophage bactericidal activity
(40). Morphine has also been
found to inhibit the formation of macrophage colonies in soft agar
from cultured mouse bone marrow progenitors (34).
Impact on NK cells
In rat models, the proliferation of NK cells was
reported to be significantly suppressed in mice treated with
morphine compared with that in controls. Administration of a
nucleus accumbens shell blocker reversed the suppressive effects of
morphine on the proliferation of NK cells in mice, suggesting that
the nucleus accumbens serves a critical role in regulating
peripheral immune responses. This process is likely mediated by the
activation of sympathetic pathways and dopaminergic neurohumoral
immunomodulation, with morphine indirectly affecting NK cells
(34,66,67).
A previous study demonstrated that morphine can inhibit lymphocyte
proliferation and NK cell activity in vitro (68), whereas tramadol exhibits the
opposite effects (67,68). This effect of tramadol was due to
the (+)-enantiomer of tramadol inhibiting serotonin (5-HT)
reuptake, increase extracellular 5-HT concentrations in the
synaptic cleft, prolonging its combining time with 5-HT receptors
and amplifying 5-HT signaling, which can promote immune activation,
increase NK cell activity and IL-2 production (67). These findings suggest that
different opioids may have distinct impact on the immune
system.
Contradictory research findings
Opioids are recognized for their inhibitory effects
on immune cell function (12,34),
including the suppression of lymphocytes, T cells and monocytes,
which may in turn adversely affect the immune system and promote
tumor growth. However, the literature also presents significant
contradictions regarding these effects. These conflicting findings
highlight the dual effects of opioids.
Dual role of µ receptors. µ receptor agonists
may promote tumor growth under certain conditions. The activation
of morphine-induced µ receptors can promote breast cancer cell
proliferation both in vivo and in vitro (37,39).
In addition, low concentrations of morphine in pancreatic cancer
led to an increase in phosphorylated JNK, whilst the
phosphorylation of p38 was reduced, resulting in an increment in
tumor volume (48). However,
morphine pre-treatment under hypoxic conditions inhibits VEGF
production, which can suppress tumor growth (51,69).
By inhibiting angiogenesis and production of M2-polarized
macrophages, whilst inhibiting the production of MMP9 by
macrophages, morphine can also suppress tumor growth (42,43).
Dual effect of κ receptors. Although κ
receptor agonists can inhibit angiogenesis and tumor growth through
the cAMP/PKA pathway, they may promote metastasis of breast cancer
when activated in breast cancer cells (57,70).
The expression and functional profiles of different
receptors, including µ and κ receptors, may vary in different types
of tumors, leading to differences in anti-tumor or pro-tumor
effects. The existing contradictory information are summarized in
Table III.
 | Table IIIA summary of the existing conflict
effects of opioids. |
Table III
A summary of the existing conflict
effects of opioids.
| Conflicts
existing | Possible
explanations | (Refs.) |
|---|
| µ receptors may
promote or inhibit tumor growth | The receptor
activation effect is dose-dependent; Anoxic conditions also
influence the effect of µ receptor agonists | (48) |
| κ receptors can
reduce angiogenesis to reduce tumor growth, but they are highly
expressed in breast cancer cells | κ receptor agonists
can reduce VEGF expression and inhibit angiogenesis in endothelial
cells; but in breast cancer, κ receptors can promote tumor growth
through PI3K/AKT pathway | (30,57) |
5. Opioids in different cancer types
Effects of opioids on breast
cancer
Previous studies of opioid use in breast cancer have
shown that the use of fentanyl, a δ receptor agonist, as
anesthetics can reduce NK cell cytotoxicity compared with
sevoflurane, during surgery. The use of δ receptor agonists in
breast cancer can also promote the metastasis of breast cancer
cells (53,71). However, activation of κ receptor
has been documented to exhibit an inhibitory effect on estrogen
receptor (ER)-positive breast cancer through the κ
receptor/ER/X-box binding protein 1 pathway (72).
Effects of opioids on lung cancer
Opioids can inhibit lung cancer angiogenesis through
different pathways. In Lewis lung carcinoma murine experiments, κ
receptor knockout mice showed increased proliferation and enhanced
tumor angiogenesis compared with those in wild-type mice, through
the VEGF signaling pathway, indicating that κ receptor may inhibit
the blood supply and growth of lung cancer (70). However, in lung cancer cell
experiments, morphine may suppress the immune function by
upregulating maelstrom spermatogenic transposon silencer (MAEL)
expression, increasing the levels of Programmed death-ligand
(PD-L)1, TGF-β and IL-10, whilst decreasing IL-2 levels (59).
Effects of opioids on liver
cancer
Morphine, as a µ receptor agonist, can suppress the
metastasis and growth of liver cancer cells by upregulating the
expression of opioid growth factor receptor and downregulation of
µ-opioid receptor and MMP-9 expression (73). Murine experiments have previously
reported that tramadol can suppress the growth of orthotopic liver
tumors by promoting M1 macrophage polarization in the tumor
microenvironment (74).
6. Interactions between opioids and common
analgesics
Anesthesia during cancer surgery rarely involves a
single anesthetic, such that cancer pain management typically
requires the application of multiple drugs (75). There are complex pharmacokinetic
interactions between various anesthetics, which significantly
affect the patient's postoperative recovery. Opioids can inhibit
the proliferation of T cells and hinder the function of B cells
through µ receptors and by upregulating the production of
proinflammatory cytokines, such as IL-1β and IL-6. By promoting the
production of cAMP, µ receptor agonists can inhibit T cell receptor
signaling through the PKA/Csk-binding phosphoprotein associated
with glycosphingolipid-enriched microdomains/C-terminal Src
kinase-leukocyte-specific protein tyrosine kinase pathway,
resulting in a decrease in IL-2, which can inhibit the
proliferation of T cells (76,77).
In addition, they can inhibit the activity of NK cells through
opioid receptors in the central nervous system (78). They can also stimulate the
metabolism of benzodiazepines, potentially leading to respiratory
depression when combined with benzodiazepines (79). When co-administered with certain
general anesthetics, such as isoflurane and propofol, opioids may
enhance respiratory depression, which causes severe respiratory
depression and apnea (80). By
contrast, co-administration with NSAIDs may affect drug metabolism
through the liver, increasing the risk of gastrointestinal injury
and bleeding (81). Combining
opioids with alcohol can increase central nervous system
depression, potentially leading to confusion and respiratory
depression (82). Since patients
with cancer may also take antidepressants, co-administration with
opioids can result in respiratory depression, confusion, mood
instability, drowsiness and other mental symptoms, such as anxiety,
depression and hallucinations (specifically false sensory
perceptions in the absence of external stimuli) (83). Combining opioids with certain
sedatives, including benzodiazepines, may cause hypotension and
bradycardia, increasing the cardiovascular burden, especially in
elderly patients or those with a history of cardiovascular disease
(84). Therefore, careful
monitoring of cardiovascular function and the timely adjustment of
medication may prevent the aforementioned complications (85).
Clinical scenarios for the combined
use of opioids
As common analgesics in clinical settings, opioids
have the potential to be combined with non-traditional cancer
therapies. Emerging non-traditional cancer therapies, such as
immunotherapy, primarily involve immune checkpoint inhibitors,
adoptive cell therapy and cancer vaccines (86-88).
Cancer immunotherapy is fundamentally based on the inhibition of
immune escape mechanisms utilized by tumor cells. Currently, cancer
immunotherapies primarily focus on immune checkpoint inhibitors
targeting programmed cell death protein 1 (PD-1) and cytotoxic
T-lymphocyte associated protein 4 (CTLA-4) (89). The application of PD-1 or its
ligands, PD-L1 and PD-L2 monoclonal antibodies, selectively blocks
the interaction between tumor cells and T cells. This restores
antitumor immunity and enhances cytotoxic T cell-mediated tumor
destruction, which ultimately causes tumor eradication (90). CTLA-4 monoclonal antibodies inhibit
the co-stimulatory interaction between CTLA-4 molecules on
regulatory T cells and Fc, which induces the death of regulatory T
cells, reduces negative T cell regulation and enhances T
cell-mediated immune responses (91). Immune checkpoint inhibitor therapy
allows T cells to effectively infiltrate into tumor sites and form
long-lasting immune responses by activating the body's T-cell
immunity (87,92,93).
However, the modulation of immune activity,
upregulation of T cell activity and reduction of Treg cell-mediated
immunosuppressive activity can increase the risk of autoimmune
complications and inflammation, such as checkpoint inhibitor
pneumonitis induced by anti-PD-1/PDL-1 immune checkpoint inhibitors
(94). To improve survival rates
and quality of life, it is common to co-administer opioids and
immunosuppressants to patients who commonly experience abdominal
and lesion pain (94). Opioids can
damage T cells and macrophages, where retrospective analyses of
PD-1/PD-L1 concomitant medications have shown that patients with
melanoma and NSCLC who receive opioids along with immunotherapy
exhibit significantly lower overall survival rates, objective
response rates and progression-free survival rates compared with
patients who receive immunotherapy without opioids. This
combination carries a higher risk of disease progression, gut
microbiota dysbiosis and gut barrier dysfunction (95). Therefore, it is necessary to
closely monitor changes in gastrointestinal function and
inflammation markers when the treatment plan involves a combination
of opioid administration and immunotherapy. Alternative treatments
should be promptly adopted in the case of impaired gastrointestinal
function.
Opioids can damage immune components, such as
macrophages and T lymphocytes. However, considering the
dose-dependent effects of opioids on the immune system, further
studies are needed to explore how opioids can affect the tumor
immune microenvironment, which differs from the normal immune
composition (96). The long-term
effects of opioids on the immune system and the complex immune
system within the tumor microenvironment require further
investigation.
7. Therapeutic strategies and future
directions
Opioids are used in cancer treatment to alleviate
patients' pain and improve their quality of life. However, the
efficiency of opioids depends on multiple factors, such as the type
applied and severity of pain, the patient's overall health status,
potential side effects and drug interactions (97). Clinical treatment typically follows
the pain management guidelines published by the World Health
Organization (WHO) (97). It is
necessary to conduct comprehensive pain assessments and select
appropriate dosages of analgesics based on the patient's condition
and response. The choice of analgesics can be guided by the WHO
analgesic ladder. If a single drug is insufficient to control pain,
it is recommended to use a combination of multiple drugs along with
non-pharmacological treatments, such as physical therapy,
psychological support and alternative therapies such as traditional
Chinese medicine, to alleviate the patient’s pain. Opioids may
cause various side effects, such as constipation, vomiting,
drowsiness and respiratory depression (98). Therefore, it is necessary to
regularly monitor the patient's pain status, drug efficacy and side
effects during the clinical treatment regimen. It is important to
take the necessary measures to control these side effects and if
necessary, to change the medications or increase their dosage to
improve the patient's quality of life. Additionally, since cancer
patients may take multiple drugs simultaneously, drug interactions
must be carefully monitored.
The study of opioids represents a dynamic and
advancing area of research. Researchers are actively pursuing novel
drug targets to create innovative non-opioid analgesics and
mitigate the adverse effects associated with the administration of
opioids. To mitigate the risk of drug abuse and potential
addiction, studies are investigating alternative therapies to
overcome challenges, such as opioid abuse and dependence (99). In addition, it is crucial to
develop selective opioid receptor antagonists to inhibit the
potential immunosuppressive effects of opioids. κ receptor
activation can exert anti-tumor effects by reducing VEGF production
and inhibiting tumor angiogenesis (70). However, the use of receptor
antagonists to block κ receptors on breast cancer cells can hinder
breast cancer progression and reduce the risk of metastasis
(70).
Additionally, advances in drug delivery systems,
such as the use of nanotechnology or targeted delivery, can
increase local drug concentration, reduce systemic side effects and
enhance efficacy. Studies on genetic variations in analgesic
responses may facilitate the development of personalized pain
management strategies. Before clinical application, the efficacy,
safety, tolerance and dependence of analgesics should be
extensively investigated to prevent and mitigate their possible
side effects.
8. Conclusion
Although opioids are essential for managing
cancer-related pain, they serve a complex and dual role within the
tumor immune microenvironment. The suppression of immune cell
activity and promotion of angiogenesis is of particular importance
in cancer, since they may undermine the effectiveness of antitumor
therapies. Therefore, a deeper understanding of these mechanisms is
crucial for optimizing the use of opioids in cancer treatment to
ensure that pain management does not inadvertently compromise
treatment outcomes. The following strategies and future research
keynotes should be considered to achieve a balance between
effective analgesia and optimal cancer pain management.
Personalized pain management
plans
It is necessary to develop individualized analgesic
plans under the WHO pain management guidelines to minimize opioid
dosages whilst ensuring effective pain relief. This requires
further development and integration of non-opioid analgesics,
adjuvant therapies and non-pharmacological interventions to reduce
the dependence on opioids.
Regular monitoring and assessment
Regular assessments of pain intensity, functional
capacity and possible opioid-associated side effects are essential,
where treatment strategies must be modified as needed to achieve
effective pain management whilst minimizing immune function
disruption.
Opioid-immune system interactions
The specific pathways through which different opioid
receptors influence immune cell function, angiogenesis and tumor
progression need to be investigated to inform safer analgesic
choices.
Opioid use in conjunction with
immunotherapies
The interaction of opioids with immune checkpoint
inhibitors and other immunotherapies should be examined to
determine their impact on treatment efficacy and patient
outcomes.
Development of novel analgesics
It is necessary to explore and develop novel pain
management agents that provide effective analgesia with minimal
immunosuppressive effects to preserve the integrity of cancer
therapies.
Acknowledgements
Not applicable.
Funding
Funding: The present review was supported by Shandong Natural
Science Foundation Youth Program (grant nos. ZR2022QH227 and
YXH2024YS029) and Research Project of Shandong Medical Association
(grant no. YXH2024YS029).
Availability of data and materials
Not applicable.
Authors' contributions
YZ and WL were involved in the conception and design
of the present review, collection and assembly of data. XH and CM
contributed substantially to the conception and design of the
study. CM also performed data analysis and data visualization. YC
contributed to the refinement of the manuscript's narrative and
logic critically reviewed the content. All authors were involved in
drafting and revising the manuscript. All authors have reviewed and
approved the final version of the submitted manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trescot AM: Review of the role of opioids
in cancer pain. J Natl Compr Canc Netw. 8:1087–1094.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Paice JA, Bohlke K, Barton D, Craig DS,
El-Jawahri A, Hershman DL, Kong LR, Kurita GP, LeBlanc TW,
Mercadante S, et al: Use of opioids for adults with pain from
cancer or cancer treatment: ASCO guideline. J Clin Oncol.
41:914–930. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fallon M, Giusti R, Aielli F, Hoskin P,
Rolke R, Sharma M and Ripamonti CI: ESMO Guidelines Committee.
Management of cancer pain in adult patients: ESMO clinical practice
guidelines. Ann Oncol. 29 (Suppl 4):iv166–iv191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Novy DM, Nelson DV, Koyyalagunta D, Cata
JP, Gupta P and Gupta K: Pain, opioid therapy, and survival: A
needed discussion. Pain. 161:496–501. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Satija A, Ahmed SM, Gupta R, Ahmed A, Rana
SP, Singh SP, Mishra S and Bhatnagar S: Breast cancer pain
management-A review of current & novel therapies. Indian J Med
Res. 139:216–225. 2014.PubMed/NCBI
|
|
6
|
Koulouris AI, Banim P and Hart AR: Pain in
patients with pancreatic cancer: Prevalence, mechanisms, management
and future developments. Dig Dis Sci. 62:861–870. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Montazeri A: Quality of life data as
prognostic indicators of survival in cancer patients: an overview
of the literature from 1982 to 2008. Health Qual Life Outcomes.
7(102)2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mestdagh F, Steyaert A and Lavand'homme P:
Cancer pain management: A narrative review of current concepts,
strategies, and techniques. Curr Oncol. 30:6838–6858.
2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bradley A and Boland JW: Effects of
opioids on immune and endocrine function in patients with cancer
pain. Curr Treat Options Oncol. 24:867–879. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li Y, Sun L, Zhou Q, Lee AJ, Wang L, Zhang
R and Wang S: Effects of opioid drugs on immune function in cancer
patients. Biomed Pharmacother. 175(116665)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Malafoglia V, Ilari S, Vitiello L, Tenti
M, Balzani E, Muscoli C, Raffaeli W and Bonci A: The interplay
between chronic pain, opioids, and the immune system.
Neuroscientist. 28:613–627. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Plein LM and Rittner HL: Opioids and the
immune system-friend or foe. Br J Pharmacol. 175:2717–2725.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang Y, Zhuang Y, DiBerto JF, Zhou XE,
Schmitz GP, Yuan Q, Jain MK, Liu W, Melcher K, Jiang Y, et al:
Structures of the entire human opioid receptor family. Cell.
186:413–427.e17. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stein C: Opioid receptors. Annu Rev Med.
67:433–451. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Faouzi A, Varga BR and Majumdar S: Biased
opioid ligands. Molecules. 25(4257)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wassum KM, Ostlund SB, Maidment NT and
Balleine BW: Distinct opioid circuits determine the palatability
and the desirability of rewarding events. Proc Natl Acad Sci USA.
106:12512–12517. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pasternak GW and Pan YX: Mu Opioids and
their receptors: Evolution of a concept. Pharmacol Rev.
65:1257–1317. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Streicher JM and Bilsky EJ: Peripherally
acting µ-opioid receptor antagonists for the treatment of
opioid-related side effects: Mechanism of action and clinical
implications. J Pharm Pract. 31:658–669. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou Q, Zhang Z, Long S, Li W, Wang B and
Liang N: Opioids in cancer: The κopioid receptor (Review). Mol Med
Rep. 25(44)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Peppin JF and Raffa RB: Delta opioid
agonists: A concise update on potential therapeutic applications. J
Clin Pharm Ther. 40:155–166. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dai ZH, Xu X, Chen WQ, Nie LN, Liu Y, Sui
N and Liang J: The role of hippocampus in memory reactivation: An
implication for a therapeutic target against opioid use disorder.
Curr Addict Rep. 9:67–79. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yamamizu K, Hamada Y and Narita M: κ
Opioid receptor ligands regulate angiogenesis in development and in
tumours. Br J Pharmacol. 172:268–276. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jelic MD, Mandic AD, Maricic SM and
Srdjenovic BU: Oxidative stress and its role in cancer. J Cancer
Res Ther. 17:22–28. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rullo L, Caputi FF, Losapio LM, Morosini
C, Posa L, Canistro D, Vivarelli F, Romualdi P and Candeletti S:
Effects of different opioid drugs on oxidative status and
proteasome activity in SH-SY5Y cells. Molecules.
27(8321)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
de Almeida AS, Pereira GC, Brum EDS, Silva
CR, Antoniazzi CTD, Ardisson-Araújo D, Oliveira SM and Trevisan G:
Role of TRPA1 expressed in bone tissue and the antinociceptive
effect of the TRPA1 antagonist repeated administration in a breast
cancer pain model. Life Sci. 276(119469)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Skrabalova J, Drastichova Z and Novotny J:
Morphine as a potential oxidative stress-causing agent. Mini Rev
Org Chem. 10:367–372. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schattauer SS, Bedini A, Summers F,
Reilly-Treat A, Andrews MM, Land BB and Chavkin C: Reactive oxygen
species (ROS) generation is stimulated by κ opioid receptor
activation through phosphorylated c-Jun N-terminal kinase and
inhibited by p38 mitogen-activated protein kinase (MAPK)
activation. J Biol Chem. 294:16884–16896. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Waris G and Ahsan H: Reactive oxygen
species: Role in the development of cancer and various chronic
conditions. J Carcinog. 5(14)2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boland JW and Pockley AG: Influence of
opioids on immune function in patients with cancer pain: From bench
to bedside. Br J Pharmacol. 175:2726–2736. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mizota T, Tsujikawa H, Shoda T and Fukuda
K: Dual modulation of the T-cell receptor-activated signal
transduction pathway by morphine in human T lymphocytes. J Anesth.
27:80–87. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gomez-Flores R and Weber RJ: Inhibition of
interleukin-2 production and downregulation of IL-2 and transferrin
receptors on rat splenic lymphocytes following PAG morphine
administration: A role in natural killer and T cell suppression. J
Interferon Cytokine Res. 19:625–630. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen C, Farooqui M and Gupta K: Morphine
stimulates vascular endothelial growth factor-like signaling in
mouse retinal endothelial cells. Curr Neurovasc Res. 3:171–180.
2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Eisenstein TK: The role of opioid
receptors in immune system function. Front Immunol.
10(2904)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tuerxun H and Cui J: The dual effect of
morphine on tumor development. Clin Transl Oncol. 21:695–701.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang XY, Liang YX, Yan Y, Dai Z and Chu
HC: Morphine: Double-faced roles in the regulation of tumor
development. Clin Transl Oncol. 20:808–814. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang H, Zhou D, Gu J, Qu M, Guo K, Chen W
and Miao C: Targeting the mu-Opioid receptor for cancer treatment.
Curr Oncol Rep. 23(111)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
McDonald J and Lambert DG: Opioid
receptors. BJA Education. 15:219–224. 2015.
|
|
39
|
Bimonte S, Barbieri A, Rea D, Palma G,
Luciano A, Cuomo A, Arra C and Izzo F: Morphine promotes tumor
angiogenesis and increases breast cancer progression. Biomed Res
Int. 2015(161508)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Malik JA, Affan Khan M, Lamba T, Adeel
Zafar M, Nanda S, Owais M and Agrewala JN: Immunosuppressive
effects of morphine on macrophage polarization and function. Eur J
Pharmacol. 975(176637)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tariq M, Zhang J, Liang G, Ding L, He Q
and Yang B: Macrophage polarization: Anti-cancer strategies to
target tumor-associated macrophage in breast cancer. J Cell
Biochem. 118:2484–2501. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gach K, Szemraj J, Wyrębska A and Janecka
A: The influence of opioids on matrix metalloproteinase-2 and -9
secretion and mRNA levels in MCF-7 breast cancer cell line. Mol
Biol Rep. 38:1231–1236. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Malsy M, Hackl C, Graf B, Bitzinger D and
Bundscherer A: The effects of analgesics on the migration of
pancreatic cancer cells. In Vivo. 36:576–581. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
O'Sullivan S, Medina C, Ledwidge M,
Radomski MW and Gilmer JF: Nitric oxide-matrix metaloproteinase-9
interactions: Biological and pharmacological significance: NO and
MMP-9 interactions. Biochim Biophys Acta. 1843:603–617.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nikitovic D, Corsini E, Kouretas D,
Tsatsakis A and Tzanakakis G: ROS-major mediators of extracellular
matrix remodeling during tumor progression. Food Chem Toxicol.
61:178–186. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18(3249)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mehner C, Hockla A, Miller E, Ran S,
Radisky DC and Radisky ES: Tumor cell-produced matrix
metalloproteinase 9 (MMP-9) drives malignant progression and
metastasis of basal-like triple negative breast cancer. Oncotarget.
5:2736–2749. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ning J, Chen X, Li Q, Yang D, Xie C, Qin S
and Jiang H: Bidirectional effects of morphine on pancreatic cancer
progression via the p38/JNK pathway. Sci Rep.
14(24233)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ma Y, Ren Z, Ma S, Yan W, He M, Wang D and
Ding P: Morphine enhances renal cell carcinoma aggressiveness
through promotes survivin level. Ren Fail. 39:258–264.
2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jiang Y, Li T, Qian Y, Zuo X and Liu J:
Morphine in combination with ketamine improves cervical cancer pain
and suppresses immune function via the JAK3/STAT5 pathway. Pain Res
Manag. 2022(9364365)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Liu X, Yang J, Yang C, Huang X, Han M,
Kang F and Li J: Morphine promotes the malignant biological
behavior of non-small cell lung cancer cells through the
MOR/Src/mTOR pathway. Cancer Cell Int. 21(622)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Koodie L, Ramakrishnan S and Roy S:
Morphine suppresses tumor angiogenesis through a HIF-1α/p38MAPK
pathway. Am J Pathol. 177:984–997. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tripolt S, Neubauer HA, Knab VM, Elmer DP,
Aberger F, Moriggl R and Fux DA: Opioids drive breast cancer
metastasis through the δ-opioid receptor and oncogenic STAT3.
Neoplasia. 23:270–279. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wei YC, Zhang B, Li X, Liu XM, Zhang J,
Lei B, Li B, Zhai R, Chen Q and Li Y: Upregulation and activation
of δopioid receptors promotes the progression of human breast
cancer. Oncol Rep. 36:2579–2586. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wang S: Historical review: Opiate
addiction and opioid receptors. Cell Transplant. 28:233–238.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Leconte C, Mongeau R and Noble F:
Traumatic stress-induced vulnerability to addiction: Critical role
of the dynorphin/kappa opioid receptor system. Front Pharmacol.
13(856672)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li H, Ma Z and Lei Y: The expression of
kappa-opioid receptor promotes the migration of breast cancer cells
in vitro. BMC Anesthesiol. 21(210)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Eisenstein TK: Opioids and the immune
system: What is their mechanism of action? Br J Pharmacol.
164:1826–1828. 2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wang Q, Liu Z, Tang S and Wu Z: Morphine
suppresses the immune function of lung cancer by up-regulating MAEL
expression. BMC Pharmacol Toxicol. 23(92)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang J, Barke RA, Charboneau R,
Schwendener R and Roy S: Morphine induces defects in early response
of alveolar macrophages to streptococcus pneumoniae by modulating
TLR9-NF-kappa B signaling1. J Immunol. 180:3594–3600.
2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang J, Ma J, Charboneau R, Barke R and
Roy S: morphine inhibits murine dendritic cell IL-23 production by
modulating toll-like receptor 2 and Nod2 signaling. J Biol Chem.
286:10225–10232. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Xie F, Kitagawa Y, Ogata H, Yasuhara S,
You Z and Jeevendra Martyn JA: Morphine induces inflammatory
responses via both TLR4 and cGAS-STING signaling pathways.
Cytokine. 183(156737)2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Malik JA and Agrewala JN: Morphine acts
via TLR4 resulting in neuroinflammation and immunosuppression. Med
Hypotheses. 186(111335)2024.
|
|
64
|
Singhal PC, Sharma P, Kapasi AA, Reddy K,
Franki N and Gibbons N: Morphine enhances macrophage apoptosis. J
Immunol. 160:1886–1893. 1998.PubMed/NCBI
|
|
65
|
Lin M, Deng K, Li Y and Wan J: Morphine
enhances LPSinduced macrophage apoptosis through a PPARγdependent
mechanism. Exp Ther Med. 22(714)2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Saurer TB, Carrigan KA, Ijames SG and
Lysle DT: Suppression of natural killer cell activity by morphine
is mediated by the nucleus accumbens shell. J Neuroimmunol.
173:3–11. 2006.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Saeed I, La Caze A, Hollmann MW, Shaw PN
and Parat MO: New Insights on Tramadol and Immunomodulation. Curr
Oncol Rep. 23(123)2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Maher DP, Walia D and Heller NM:
Suppression of human natural killer cells by different classes of
opioids. Anesth Analg. 128:1013–1021. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Khabbazi S, Goumon Y and Parat MO:
Morphine modulates interleukin-4- or breast cancer cell-induced
pro-metastatic activation of macrophages. Sci Rep.
5(11389)2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yamamizu K, Furuta S, Hamada Y, Yamashita
A, Kuzumaki N and Narita M, Doi K, Katayama S, Nagase H, Yamashita
JK and Narita M: к Opioids inhibit tumor angiogenesis by
suppressing VEGF signaling. Sci Rep. 3(3213)2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cho JS, Lee MH, Kim SI, Park S, Park HS,
Oh E, Lee JH and Koo BN: The effects of perioperative anesthesia
and analgesia on immune function in patients undergoing breast
cancer resection: A prospective randomized study. Int J Med Sci.
14:970–976. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shi Y, Zhang Y, Yu S, Luo J, Pan Z, Wang X
and Tian J: Activation of kappa opioid receptor (KOR) inhibits
estrogen receptor (ER)-positive breast cancer through the
KOR-ER-XBP1 pathway. Biomed Pharmacother.
167(115462)2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Zhang HW, Wang F, Zhou YQ, Xu SP, Yu SY
and Zhang ZG: Morphine suppresses liver cancer cell tumor
properties in vitro and in vivo. Front Oncol.
11(666446)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Wang L, Guo W, Guan H, Yan N, Cai X and
Zhu L: Tramadol suppresses growth of orthotopic liver tumors via
promoting M1 macrophage polarization in the tumor microenvironment.
Naunyn-Schmiedebergs Arch Pharmacol. 397:4205–4218. 2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Schwenk ES and Mariano ER: Designing the
ideal perioperative pain management plan starts with multimodal
analgesia. Korean J Anesthesiol. 71:345–352. 2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Morgan EL: Regulation of human B
lymphocyte activation by opioid peptide hormones. Inhibition of IgG
production by opioid receptor class (µ-, kappa-, and delta-)
selective agonists. J Neuroimmunol. 65:21–30. 1996.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Börner C, Warnick B, Smida M, Hartig R,
Lindquist JA, Schraven B, Höllt V and Kraus J: Mechanisms of
opioid-mediated inhibition of human T cell receptor signaling. J
Immunol. 183:882–889. 2009.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Beilin B, Shavit Y, Trabekin E, Mordashev
B, Mayburd E, Zeidel A and Bessler H: The effects of postoperative
pain management on immune response to surgery. Anesth Analg.
93:822–827. 2003.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Dahan A, van Lemmen M, Jansen S, Simons P
and van der Schrier R: Buprenorphine: A treatment and cause of
opioid-induced respiratory depression. Br J Anaesth. 128:402–404.
2022.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kiyatkin EA: Respiratory depression and
brain hypoxia induced by opioid drugs: Morphine, oxycodone, heroin,
and fentanyl. Neuropharmacology. 151:219–226. 2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Abdel Shaheed C, Beardsley J, Day RO and
McLachlan AJ: Immunomodulatory effects of pharmaceutical opioids
and antipyretic analgesics: Mechanisms and relevance to infection.
Br J Clin Pharmacol. 88:3114–3131. 2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Saunders KW, Von Korff M, Campbell CI,
Banta-Green CJ, Sullivan MD, Merrill JO and Weisner C: Concurrent
use of alcohol and sedatives among persons prescribed chronic
opioid therapy: Prevalence and risk factors. J Pain. 13:266–275.
2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Leppert W: Pain management in patients
with cancer: Focus on opioid analgesics. Curr Pain Headache Rep.
15:271–279. 2011.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Boon M, van Dorp E, Broens S and Overdyk
F: Combining opioids and benzodiazepines: Effects on mortality and
severe adverse respiratory events. Ann Palliat Med. 9:542–557.
2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Chen A and Ashburn MA: Cardiac effects of
opioid therapy. Pain Med. 16 (Suppl 1):S27–S31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Li Y, Wang M, Peng X, Yang Y, Chen Q, Liu
J, She Q, Tan J, Lou C, Liao Z and Li X: mRNA vaccine in cancer
therapy: Current advance and future outlook. Clin Transl Med.
13(e1384)2023.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Szeto GL and Finley SD: Integrative
approaches to cancer immunotherapy. Trends Cancer. 5:400–410.
2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wang Z and Cao YJ: Adoptive cell therapy
targeting neoantigens: A frontier for cancer research. Front
Immunol. 11(176)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Topalian SL, Taube JM and Pardoll DM:
Neoadjuvant checkpoint blockade for cancer immunotherapy. Science.
367(eaax0182)2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Wang SJ, Dougan SK and Dougan M: Immune
mechanisms of toxicity from checkpoint inhibitors. Trends Cancer.
9:543–553. 2023.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Zhang H, Dai Z, Wu W, Wang Z, Zhang N,
Zhang L, Zeng WJ, Liu Z and Cheng Q: Regulatory mechanisms of
immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer
Res. 40(184)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Waldman AD, Fritz JM and Lenardo MJ: A
guide to cancer immunotherapy: from T cell basic science to
clinical practice. Nat Rev Immunol. 20:651–668. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Shiravand Y, Khodadadi F, Kashani SMA,
Hosseini-Fard SR, Hosseini S, Sadeghirad H, Ladwa R, O'Byrne K and
Kulasinghe A: Immune checkpoint inhibitors in cancer therapy. Curr
Oncol. 29:3044–3060. 2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Suresh K, Naidoo J, Lin CT and Danoff S:
Immune checkpoint immunotherapy for non-small cell lung cancer:
Benefits and pulmonary toxicities. Chest. 154:1416–1423.
2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Acharya C, Betrapally NS, Gillevet PM,
Sterling RK, Akbarali H, White MB, Ganapathy D, Fagan A, Sikaroodi
M and Bajaj JS: Chronic opioid use is associated with altered gut
microbiota and predicts readmissions in patients with cirrhosis.
Aliment Pharmacol Ther. 45:319–331. 2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Sacerdote P, Bianchi M, Gaspani L,
Manfredi B, Maucione A, Terno G, Ammatuna M and Panerai AE: The
effects of tramadol and morphine on immune responses and pain after
surgery in cancer patients. Anesth Analg. 90:1411–1414.
2000.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Swarm RA, Paice JA, Anghelescu DL, Are M,
Bruce JY, Buga S, Chwistek M, Cleeland C, Craig D, Gafford E, et
al: Adult cancer pain, version 3.2019, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 17:977–1077.
2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Mercadante S: Opioid analgesics adverse
effects: The other side of the coin. Curr Pharm Des. 25:3197–3202.
2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Weir A: Opioid-free anaesthesia. Br J Hosp
Med (Lond). 85:1–2. 2024.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Chen SH, Chen SS, Wang YP and Chen LK:
Effects of systemic and neuraxial morphine on the immune system.
Medicine (Baltimore). 98(e15375)2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Wang J, Barke RA and Roy S:
Transcriptional and epigenetic regulation of interleukin-2 gene in
activated T cells by morphine. J Biol Chem. 282:7164–7171.
2007.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Amodeo G, Bugada D, Franchi S, Moschetti
G, Grimaldi S, Panerai A, Allegri M and Sacerdote P: Immune
function after major surgical interventions: The effect of
postoperative pain treatment. J Pain Res. 11:1297–1305.
2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Wang Y, Wang X, Ye L, Li J, Song L,
Fulambarkar N and Ho W: Morphine suppresses IFN signaling pathway
and enhances AIDS virus infection. PLoS One.
7(e31167)2012.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Vassou D, Bakogeorgou E, Kampa M,
Dimitriou H, Hatzoglou A and Castanas E: Opioids modulate
constitutive B-lymphocyte secretion. Int Immunopharmacol.
8:634–644. 2008.PubMed/NCBI View Article : Google Scholar
|