Introduction
The prognosis of patients with pancreatic ductal
adenocarcinoma (PDAC) remains poor, with a 5-year survival rate
less than 10% (1). Outcomes are
even worse in PDAC cases with distant metastases, particularly in
those with liver involvement (2).
These cases are typically considered unresectable. Generally,
patients with PDAC and liver metastases are treated with palliative
chemotherapy rather than surgery, as systemic disease progression
is common and surgical intervention has traditionally not been
recommended.
However, recent advances in chemotherapy regimens,
such as combination therapy with modified 5-fluorouracil,
leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) and
gemcitabine-based combinations, have led to improved response
rates, raising the possibility of conversion surgery in selected
cases (3). Such progress has
sparked growing interest in expanding surgical indications and
reevaluating treatment strategies for advanced PDAC.
Despite these encouraging findings, the clinical
criteria for selecting candidates for such aggressive multimodal
treatment remain unclear, and evidence is still limited.
Furthermore, in most reported cases of successful treatment for
liver metastases from PDAC, conversion surgery was performed
following long-term chemotherapy (4-7).
Reports describing an alternative approach, initial resection of
liver metastases followed by chemotherapy and subsequent radical
resection of the primary tumor, are extremely limited (8).
Here, we report a patient with PDAC in which the
liver metastasis was resected first, followed by conversion surgery
of the primary tumor, and who has remained disease-free for >4
years.
Case report
In May 2020, a 72-year-old man with a history of
diabetes and hypertension presented to his physician with decreased
appetite and weight loss. Blood tests revealed liver dysfunction,
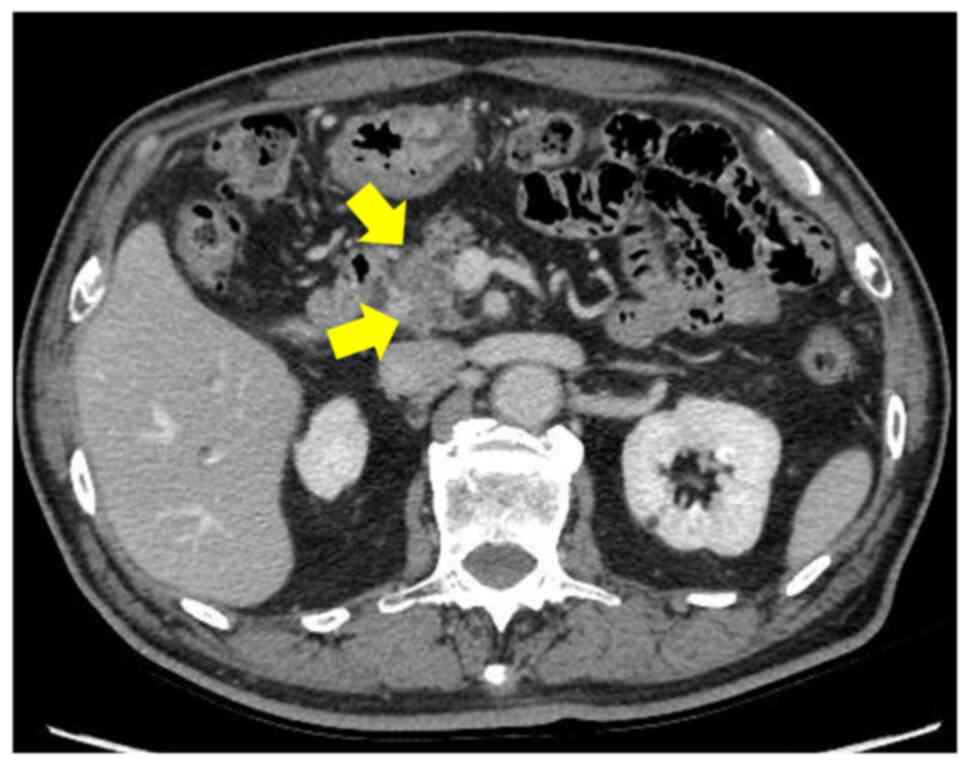
and computed tomography (CT) showed a 2 cm tumor in the pancreatic
head with poor contrast enhancement (Fig. 1). In July 2020, the patient was
referred to the gastroenterology department of our hospital. His
blood test results were as follows: carbohydrate antigen 19-9
(CA19-9), 26.7 U/ml (<37.0), and carcinoembryonic antigen, 2.7
ng/ml (<5.0). Endoscopic ultrasound (EUS) revealed a 20 mm tumor
in the pancreatic head. Although imaging suggested possible
pancreatic head cancer, we planned to administer neoadjuvant
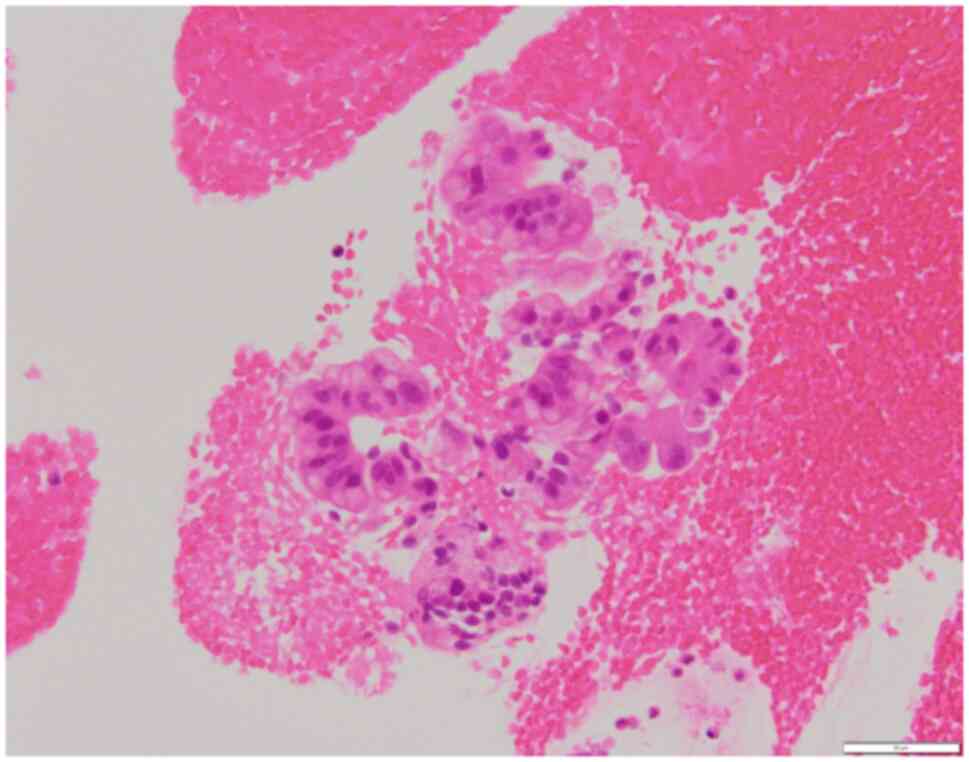
chemotherapy (NAC), and an EUS fine-needle aspiration (EUS-FNA) was
performed to obtain a definitive pathological diagnosis. However,
the results of EUS-FNA did not provide a definitive diagnosis of
cancer (Fig. 2). The patient was
diagnosed with resectable pancreatic head cancer. In August 2020,
the patient was referred to the surgery department. The patient
received NAC combination therapy with gemcitabine and S-1. He was
scheduled to undergo subtotal stomach-preserving
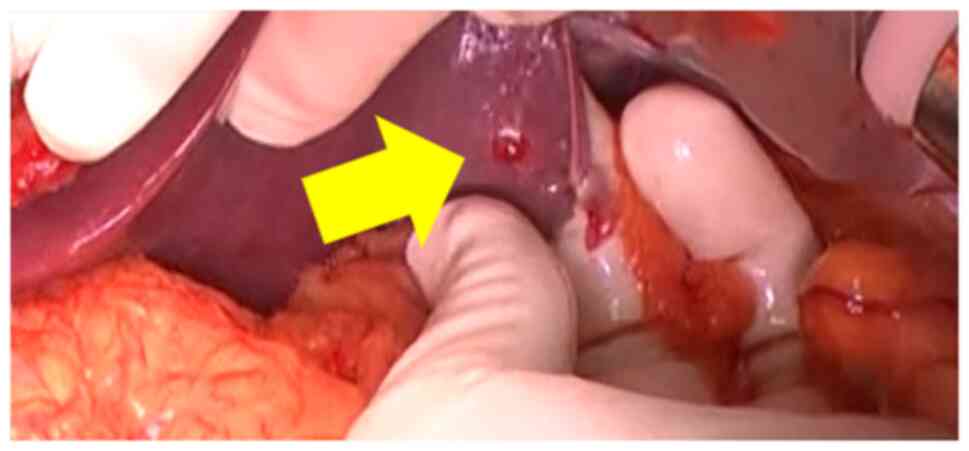
pancreaticoduodenectomy (SSPPD) in October 2020. Intraoperatively,
a 1.5 cm liver nodule was found on the surface of segment (S) 3
(Fig. 3). Peritoneal lavage
cytology was performed and proved negative for malignant cells. No
distant metastatic sites beyond S3 were observed during
intraoperative inspection. To confirm the pathological diagnosis
and allow for the small chance of complete resection in the future,
partial hepatectomy of S3 was performed. Intraoperatively, the
liver nodule was diagnosed as an adenocarcinoma, and the SSPPD was
discontinued. Although the pathological diagnosis of the primary
lesion was not confirmed, the patient was diagnosed with a hepatic
metastasis from the pancreatic head cancer based on the clinical
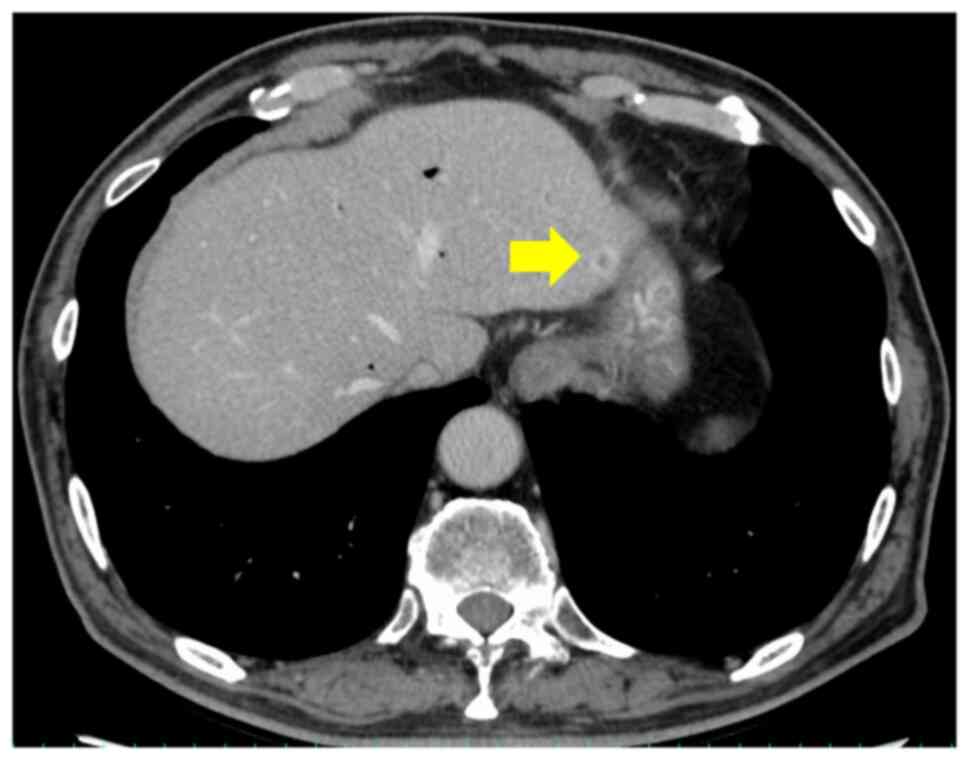
context. Although metastasis was not identified preoperatively, a
retrospective examination revealed a small metastasis in S3 on the
CT performed after NAC completion (Fig. 4). The absence of hepatic metastases
prior to NAC, and the lack of elevated tumor markers led to the
assumption that the likelihood of distant metastasis was low. These
assumptions, along with the difficulty of identifying small lesions
at the hepatic margin, contributed to the oversight of the hepatic
metastatic lesions.
On postoperative day 19 after hepatic resection, the
patient started a 6-month course of 13 cycles of modified
FOLFIRINOX. Fluorouracil (2,400 mg/m2), leucovorin (200
mg/m2), irinotecan (150 mg/m2), and
oxaliplatin (85 mg/m2) were administered in a 14-day
cycle for PDAC liver metastasis. The primary tumor showed no
changes and no new metastatic lesions were found on dynamic CT,
positron emission tomography-CT, or
gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid
(Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI).
After obtaining informed consent, we decided to
perform the SSPPD as a conversion surgery in May 2021. There was no
evidence of metastatic lesions during the operation. Owing to
surgical adhesions, the operation lasted 657 minutes, with a blood
loss of 2,806 ml. The patient required 4 units of red blood cells
and 4 units of fresh frozen plasma. A small intra-abdominal abscess
and wound infection were observed; however, these improved with
antibiotic treatment, and the patient was discharged on the 18th
postoperative day.
Histopathological findings revealed that, according
to the 8th edition of the Union for International Cancer Control
guidelines, the diagnosis was T3N0M1 well-differentiated
adenocarcinoma. The estimated residual cancer cell rate following
chemotherapy is between 50 and 90%, corresponding to Evans grades
IIa-IIb (9). Hematoxylin and eosin
(H&E) staining of the pancreatic specimen revealed that
atypical cells with enlarged round nuclei and strong staining
proliferated invasively while forming large irregularly shaped
glandular ducts (Fig. 5A).
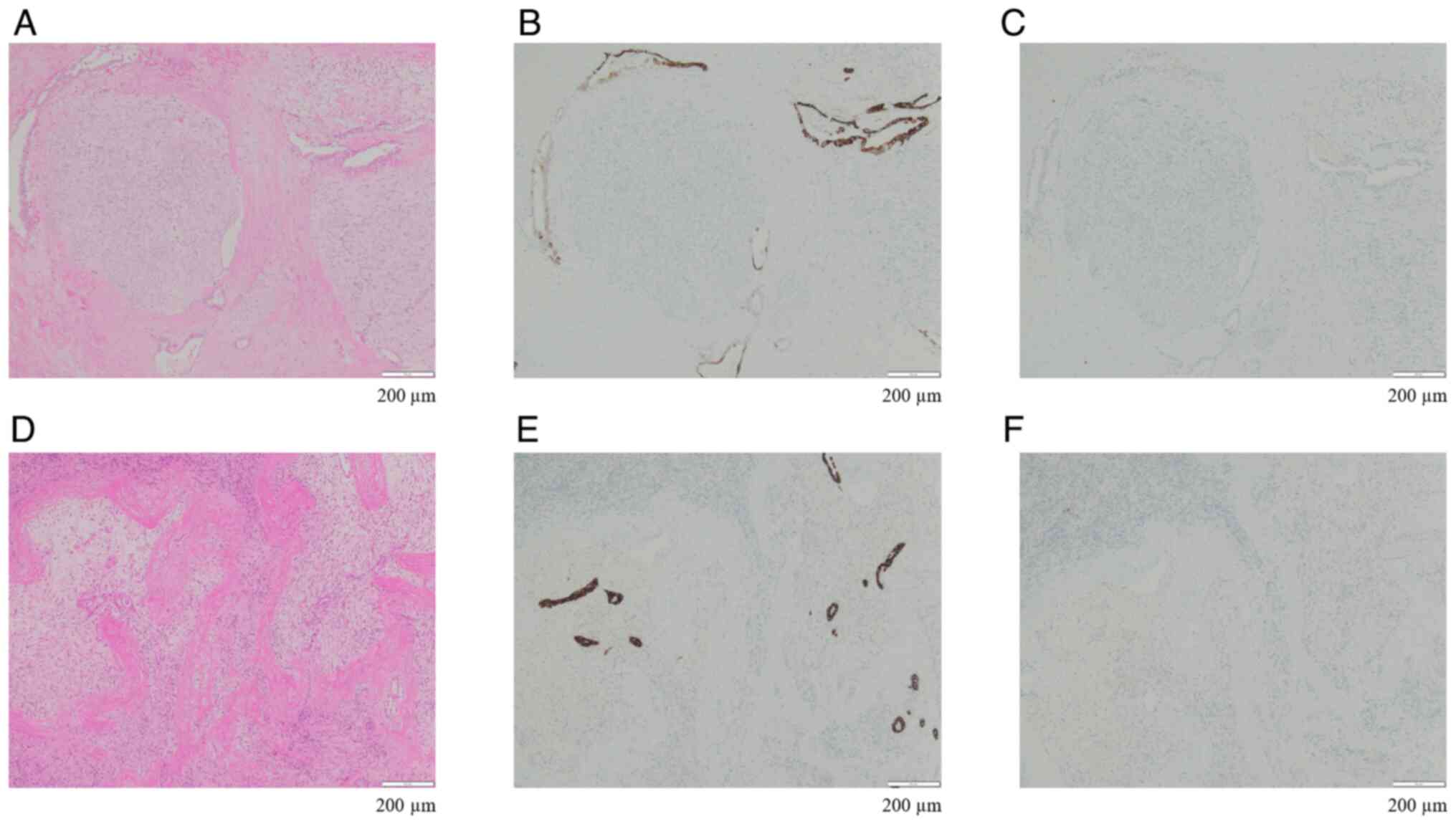
Immunohistochemical staining revealed positivity for both CK7
(Fig. 5B) and CK20 (Fig. 5C). H&E staining of the liver
specimens from the initial procedure revealed similar findings
(Fig. 5D), and immunohistochemical
staining was positive for both CK7 (Fig. 5E) and CK20 (Fig. 5F). Based on the above findings, the
liver tumor resected during the initial procedure was diagnosed as
a PDAC liver metastasis.
Postoperatively, the possibility of readministering
modified FOLFIRINOX, which had been administered preoperatively,
was considered; however, considering the patient's tolerance, S-1
was selected instead. Approximately 1 year after the initiation of
adjuvant chemotherapy, symptoms including muscle weakness and
ptosis appeared, leading to the discontinuation of adjuvant
chemotherapy. He was followed up at the outpatient clinic every 3
months. At the time of this case study, 5 years after the start of
treatment and 4 years and 3 months after the last operation, the
patient is still alive without any recurrence.
We investigated the favorable clinical course of
this patient by examining the types of genetic mutations and
characteristics of the immune microenvironment. The presence of
specific genetic mutations has been linked to a more favorable
prognosis in PDAC. For example, patients with tumors with high
microsatellite instability (MSI) have been reported to achieve a
5-year survival rate of 77% (10).
We requested MSI testing for this case from SRL Inc., and the
result was negative. MSI testing was performed using
formalin-fixed, paraffin-embedded tumor tissue. The specimen was
fixed in 10% neutral buffered formalin for 60 h and sectioned at 5
µm thickness. Macrodissection was applied to enrich tumor areas and
tumor cellularity was confirmed to be about 20%. DNA was extracted
from unstained slides and subjected to multiplex PCR amplification
targeting five mononucleotide repeat markers: BAT-25, BAT-26,
NR-21, NR-24, and MONO-27. Fragment analysis was conducted via
capillary electrophoresis, and peak profiles were analyzed using
dedicated software provided by SRL Inc. MSI status was determined
based on the presence of instability in multiple markers. Samples
showing instability in two or more markers were classified as
MSI-High, while those with no instability were considered
microsatellite stable. The assay was performed under room
temperature conditions.
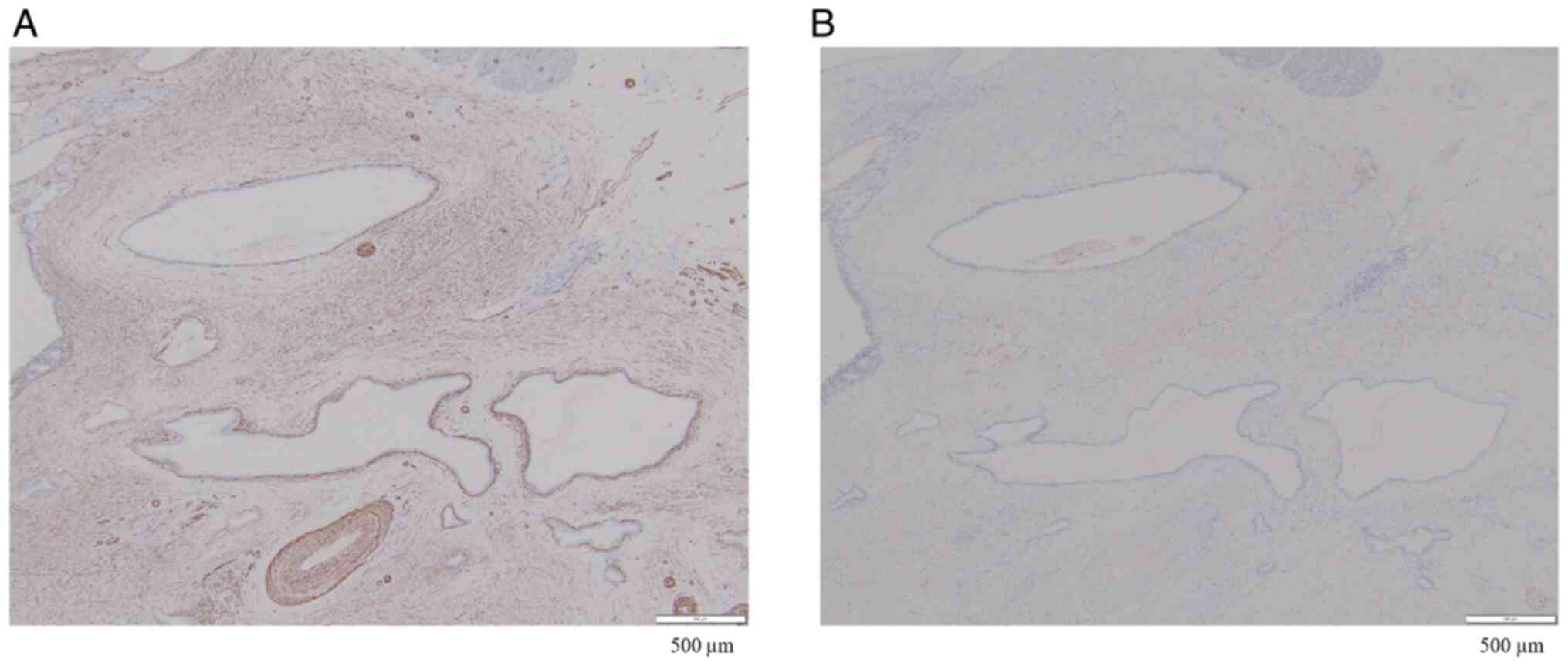
High expression of α-smooth muscle actin (α-SMA) in
PDAC has been reported to be associated with poor prognosis
(11). In our patient, although
α-SMA was diffusely expressed in the peritumoral stroma, no areas
of strong staining were observed (Fig.
7A). Furthermore, although CD10-positive pancreatic stellate
cells have been reported to promote PDAC progression (12), CD10-positive stromal cells were not
detected (Fig. 7B). Although MSI
was low, weak α-SMA positivity and negative CD10 expression suggest
that the malignancy of the PDAC cells was low, which may be related
to the favorable prognosis in this patient.
The histopathology techniques were as follows:
Tissue samples were fixed in 10% neutral buffered formalin solution
at room temperature for 60 h, paraffin embedded, cut to 3 µm thick,
and dewaxed as per standard procedures (13). H&E staining was performed at
room temperature for 10 min for Hematoxylin and 4 min for Eosin.
The microscope was an Olympus BX53 (light microscope). The
following primary antibodies were used in immunohistochemical
staining: smooth muscle actin (SMA) (1:4; clone1A4; Cat. No.:
IR61161-2; Dako), and CD10 (Ready to use, clone 56C6; Cat. No.:
413261; Nichirei). CC1 buffer (Cat. No. : 950-124; Roche) was used
for antigen retrieval. The antigen retrieval step was performed at
95˚C for 64 min. Primary antibody incubation was performed at 36˚C
for 32 min. Secondary antibody (ultraView Universal DAB Detection
Kit; Cat. No.; 951-124; Roche) incubation was performed at 36˚C for
20 min.
Discussion
Despite advances in multidisciplinary treatment
options, the 5-year survival rate of patients with PDAC remains
below 10% (1). In particular, for
PDAC with distant metastasis or recurrence, surgical intervention
is not recommended except in patients with remnant pancreatic
recurrence (14,15). In recent years, even in select
patients with lung metastasis, improved prognoses have been
reported after surgical resection (16). Similarly, for other types of
metastatic recurrence, there has been an increase in reports
showing favorable outcomes for oligometastases treated using
multidisciplinary approaches, including surgery. Although liver
metastases from PDAC are associated with poor prognosis (2), some reports have demonstrated the
utility of resection in such patients (3). Yamada et al (17) reported that achieving long-term
survival through resection alone for liver metastases is difficult,
emphasizing the importance of developing new treatment modalities.
However, there are some reports of achieving long-term survival
through metastatic lesion resection for PDAC liver metastasis with
comprehensive treatment (18-20).
Sakaguchi et al (4)
reported that the median overall survival in patients with
synchronous liver metastases who underwent conversion surgery
following a favorable response to initial chemotherapy was 27 or 34
months. Frigerio et al (5)
reported that local resectability, good nutritional status, and low
inflammatory scores could be useful indicators for predicting the
benefits of chemotherapy and surgical resection. Furthermore, Lu
et al (6) recommended the
resection of metastatic lesions only for patients where (I) R0 can
be achieved, (II) the primary tumor has responded to neoadjuvant
chemotherapy, (III) oligometastasis is resectable, and (IV) the
patient is in good health with few comorbidities. Changes in CA19-9
levels and the RECIST criteria appear to be important
considerations for conversion surgery.
A systematic review by Clements et al
demonstrated the efficacy of surgical resection for PDAC with liver
metastases (7). They identified
the following 3 factors as important: i) response to induction
chemotherapy, ii) ability to achieve R0 resection, and iii)
minimally invasive approaches, which remain critical for optimal
patient selection.
In this case, the patient had no vascular invasion,
was in good health, had good nutritional status, and had no
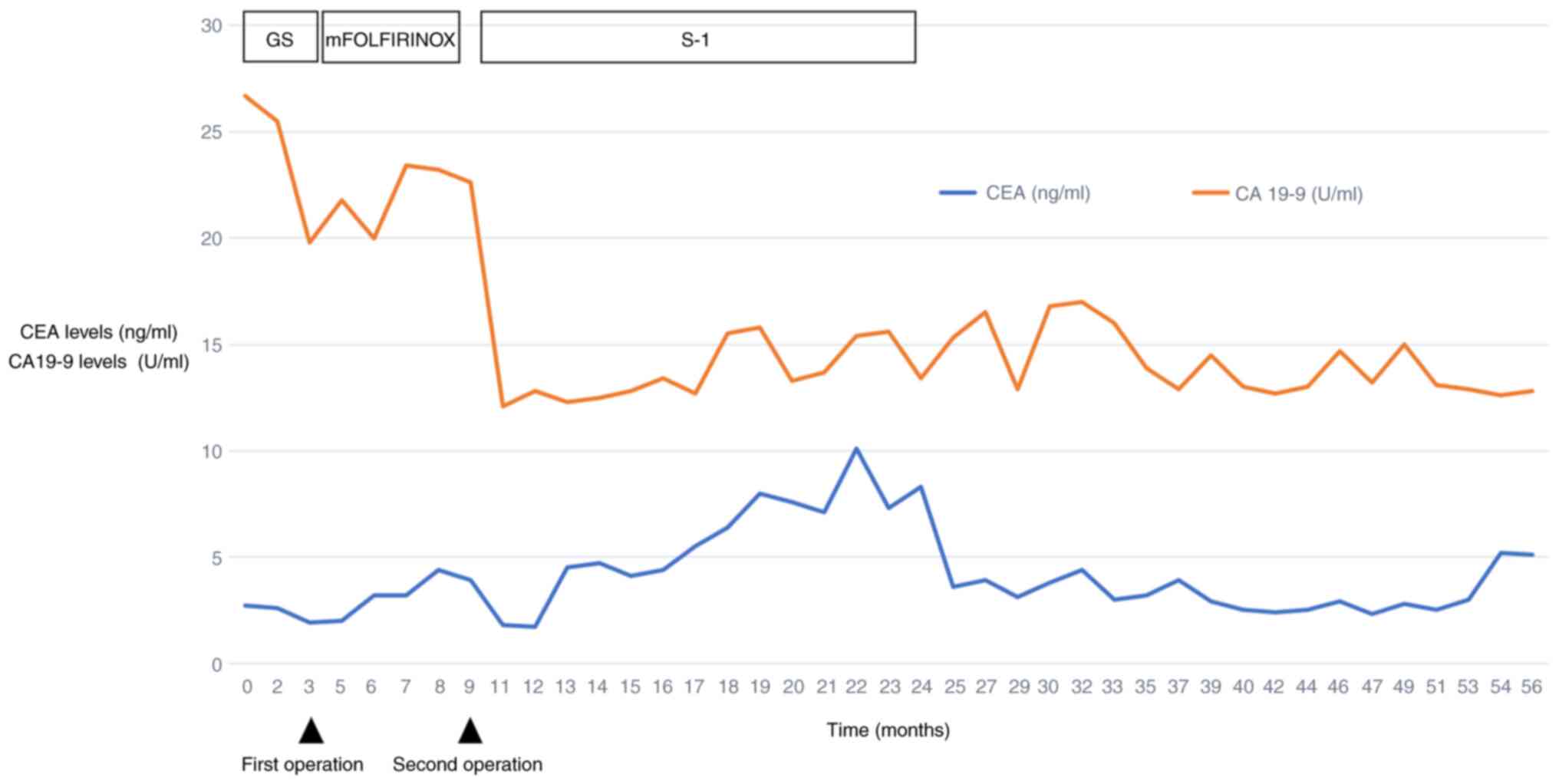
findings suggestive of inflammation. The CA19-9 levels remained
consistently within the normal range throughout the study period
(Fig. 6). These findings suggested
that the patient had a favorable long-term prognosis.
One characteristic of this patient was the
sequential resection of only the metastatic liver lesion, followed
by resection of the primary lesion. There is only one case report
of PDAC with synchronous liver metastasis in which, after prolonged
chemotherapy, the liver metastasis was resected and a pathological
complete response was confirmed before proceeding to curative
resection (8). All other reports
either administered chemotherapy until the liver lesions
disappeared and then performed pancreatectomy alone or carried out
simultaneous resection of both the pancreatic primary and liver
metastases.
By performing hepatectomy alone rather than radical
resection upon identification of the oligometastasis in S3 of the
liver, we were able not only to confirm the diagnosis but also to
initiate intensive chemotherapy promptly postoperatively and fully
observe its excellent chemosensitivity.
The implications of this case are as follows: If a
liver metastasis that was not identified preoperatively is
discovered incidentally intraoperatively, and it is determined that
the lesion may be controllable, it should be resected for diagnosis
and future curative treatment. Subsequently, chemotherapy should be
administered while assessing the disease status through various
modalities for PDAC with distant metastasis. Surgical intervention
may be useful if the disease is controlled and deemed curable.
In conclusion, in patients with PDAC with liver
metastases, chemotherapy is typically administered first, and
resection is considered if disease control is satisfactory.
However, as demonstrated in the present case, even when hepatic
metastasis is initially resected, a favorable response to
chemotherapy can permit subsequent curative resection, leading to
an excellent prognosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TT contributed to the conception and design of the
study, acquisition of data, and analysis and interpretation of the
findings, in addition to drafting the manuscript. MT revised the
manuscript. NM performed EUS and other diagnostic procedures,
leading to the diagnosis of PDAC in the patient. MT, KF and YM
performed the procedures. THi and THa were responsible for
postoperative management of the patient and contributed to
determining the postoperative treatment strategy. JA made the
diagnosis based on imaging findings. HO determined the pathological
diagnoses. TT and MT confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kneuertz PJ, Cunningham SC, Cameron JL,
Torrez S, Tapazoglou N, Herman JM, Makary MA, Eckhauser F, Wang J,
Hirose K, et al: Palliative surgical management of patients with
unresectable pancreatic adenocarcinoma: Trends and lessons learned
from a large, single institution experience. J Gastrointest Surg.
15:1917–1927. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wright GP, Poruk KE, Zenati MS, Steve J,
Bahary N, Hogg ME, Zuriekat AH, Wolfgang CL, Zeh HJ III and Weiss
MJ: Primary tumor resection following favorable response to
systemic chemotherapy in stage IV pancreatic adenocarcinoma with
synchronous metastases: A Bi-institutional analysis. J Gastrointest
Surg. 20:1830–1835. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sakaguchi T, Valente R, Tanaka K, Satoi S
and Del Chiaro M: Surgical treatment of metastatic pancreatic
ductal adenocarcinoma: A review of current literature.
Pancreatology. 19:672–680. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Frigerio I, Malleo G, de Pastena M, Deiro
G, Surci N, Scopelliti F, Esposito A, Regi P, Giardino A, Allegrini
V, et al: Prognostic factors after pancreatectomy for pancreatic
cancer initially metastatic to the liver. Ann Surg Oncol.
29:8503–8510. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lu F, Poruk KE and Weiss MJ: Surgery for
oligometastasis of pancreatic cancer. Chin J Cancer Res.
27:358–367. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Clements N, Gaskins J and Martin RCG II:
Surgical outcomes in stage IV pancreatic cancer with liver
metastasis current evidence and future directions: A systematic
review and meta-analysis of surgical resection. Cancers (Basel).
17(688)2025.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shimura M, Mizuma M, Hayashi H, Mori A,
Tachibana T, Hata T, Iseki M, Takadate T, Ariake K, Maeda S, et al:
A long-term survival case treated with conversion surgery following
chemotherapy after diagnostic metastasectomy for pancreatic cancer
with synchronous liver metastasis. Surg Case Rep.
3(132)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Evans DB, Rich TA, Byrd DR, Cleary KR,
Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ and Ames FC:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339.
1992.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eikenboom EL, Nasar N, Seier K, Gönen M,
Spaander MCW, O'Reilly EM, Jarnagin WR, Drebin J, D'Angelica MI,
Kingham TP, et al: Survival of patients with resected
microsatellite instability-high, mismatch repair deficient, and
lynch syndrome-associated pancreatic ductal adenocarcinomas. Ann
Surg Oncol. 32:3568–3577. 2025.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sinn M, Denkert C, Striefler JK, Pelzer U,
Stieler JM, Bahra M, Lohneis P, Dörken B, Oettle H, Riess H and
Sinn BV: α-Smooth muscle actin expression and desmoplastic stromal
reaction in pancreatic cancer: Results from the CONKO-001 study. Br
J Cancer. 111:1917–1923. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ikenaga N, Ohuchida K, Mizumoto K, Cui L,
Kayashima T, Morimatsu K, Moriyama T, Nakata K, Fujita H and Tanaka
M: CD10+ pancreatic stellate cells enhance the progression of
pancreatic cancer. Gastroenterology. 139:1041–1051. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sato M, Kojima M, Nagatsuma AK, Nakamura
Y, Saito N and Ochiai A: Optimal fixation for total preanalytic
phase evaluation in pathology laboratories: A comprehensive study
including immunohistochemistry, DNA, and mRNA assays. Pathol Int.
64:209–216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Thomas RM, Truty MJ, Nogueras-Gonzalez GM,
Fleming JB, Vauthey JN, Pisters PW, Lee JE, Rice DC, Hofstetter WL,
Wolff RA, et al: Selective reoperation for locally recurrent or
metastatic pancreatic ductal adenocarcinoma following primary
pancreatic resection. J Gastrointest Surg. 16:1696–1704.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miyazaki M, Yoshitomi H, Shimizu H,
Ohtsuka M, Yoshidome H, Furukawa K, Takayasiki T, Kuboki S, Okamura
D, Suzuki D and Nakajima M: Repeat pancreatectomy for pancreatic
ductal cancer recurrence in the remnant pancreas after initial
pancreatectomy: Is it worthwhile? Surgery. 155:58–66.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Groot VP, Blair AB, Gemenetzis G, Ding D,
Burkhart RA, van Oosten AF, Molenaar IQ, Cameron JL, Weiss MJ, Yang
SC, et al: Isolated pulmonary recurrence after resection of
pancreatic cancer: The effect of patient factors and treatment
modalities on survival. HPB (Oxford). 21:998–1008. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamada H, Hirano S, Tanaka E, Shichinohe T
and Kondo S: Surgical treatment of liver metastases from pancreatic
cancer. HPB (Oxford). 8:85–88. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Imai K, Margonis GA, Wang J, Wolfgang CL,
Baba H and Weiss MJ: Liver metastases from pancreatic ductal
adenocarcinoma: Is there a place for surgery in the modern era? J
Pancreatol. 3:81–85. 2020.
|
|
19
|
Gu J, Xu Z, Ma Y, Chen H, Wang D, Deng X,
Cheng D, Xie J, Jin J, Zhan X, et al: Surgical resection of
metastatic pancreatic cancer: Is it worth it?-a 15-year experience
at a single Chinese center. J Gastrointest Oncol. 11:319–328.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Klein F, Puhl G, Guckelberger O, Pelzer U,
Pullankavumkal JR, Guel S, Neuhaus P and Bahra M: The impact of
simultaneous liver resection for occult liver metastases of
pancreatic adenocarcinoma. Gastroenterol Res Pract.
2012(939350)2012.PubMed/NCBI View Article : Google Scholar
|