Introduction
According to GLOBOCAN 2022, pancreatic cancer (PC)
ranks as the sixth leading cause of cancer-related mortality among
both sexes globally. The 5-year relative survival rate remains
critically low at 5.9%, although this represents a slight
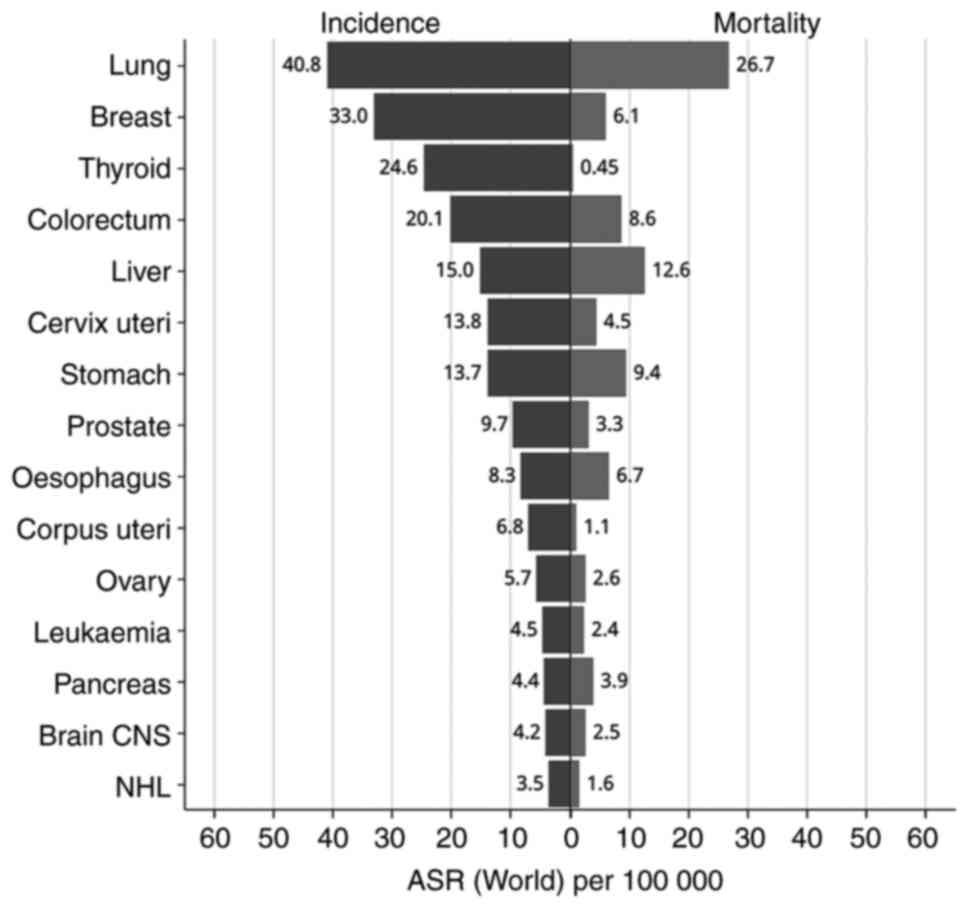
improvement from previous figures. The global age-standardized
incidence rate for PC is 4.7 per 100,000 individuals, with a
corresponding mortality rate of 4.2 per 100,000(1). As illustrated in Fig. 1, the age-standardized incidence
rate of PC in China was 4.4 per 100,000, and the mortality rate was
3.9 per 100,000, as of 2022. In China, PC ranks as the 13th most
prevalent cancer and the eighth leading cause of cancer-related
death among both sexes.
The stroma constitutes a significant component of
the prostate, accounting for over 70% of its mass. This substantial
presence contributes to the insidious onset, challenges in early
detection, and the development of drug resistance (2). Cancer-associated fibroblasts (CAFs)
are critical elements within the tumor stroma and play an essential
role in promoting malignancy. Pancreatic stellate cells (PSCs),
which serve as the primary source of CAFs in PC, stimulate tumor
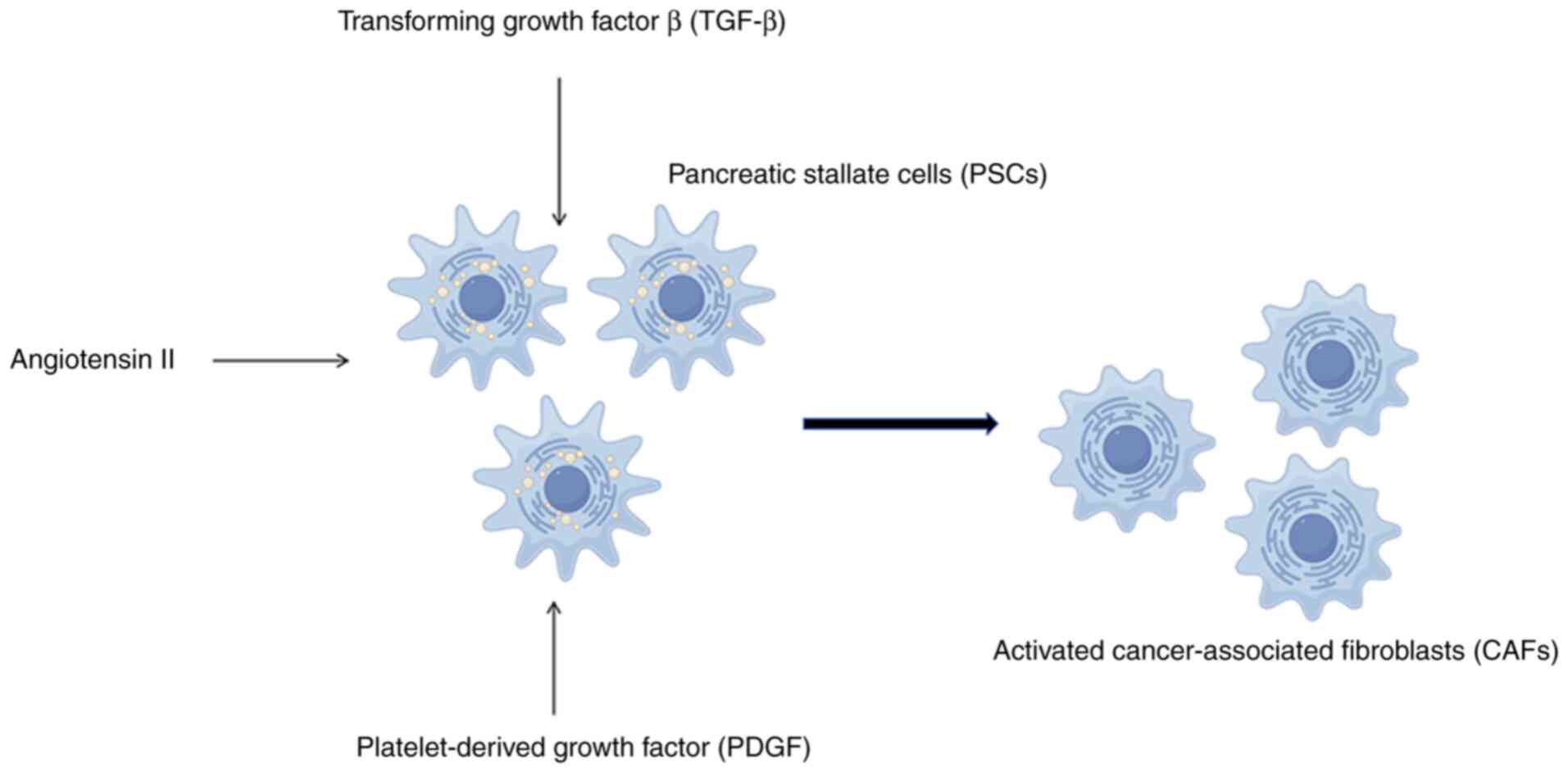
growth and enhance cell survival and metastasis (3). Cytokines (4), including transforming growth factor
β, platelet-derived growth factor and angiotensin II, among others,
secreted by PC cells can promote activation of CAFs (Fig. 2). Through paracrine or autocrine
signaling mechanisms, activated CAFs target EGFR and the downstream
PI3K-AKT-mTOR pathway, leading to both enhanced tumor proliferation
and reduced tumor suppression (5).
Activation of PSCs leads to an increased expression
of ADAM12(6). ADAM12 is a type I
transmembrane multidomain protein that is overexpressed in various
cancers, including glioblastoma, breast cancer, bladder cancer,
prostate cancer, lung cancer, liver cancer and PC (7-13).
As a protease, ADAM12 plays a significant role in tumorigenesis and
metastasis by proteolyzing downstream substrates. Notable
substrates include HB-EGF, Delta-like 1, Ephrin-A1, among others
(14,15). The overexpression of HB-EGF has
been documented in numerous tumors, including PC, hepatocellular
carcinoma, colorectal carcinoma and bladder cancers (16,17).
Its upregulation correlates with PC cell proliferation, metastasis,
chemoresistance and poor outcomes. Furthermore, the interaction
between HB-EGF and EGFR in PC cells promotes tumor development,
proliferation, differentiation and migration (Fig. 3) (18,19).
Additionally, the activation of the EGFR signaling pathway plays a
critical role in EMT with PC (20). EMT has been recognized as a pivotal
step in the progression and development of drug resistance in
various tumors, including PC (21,22).
The specific contribution of ADAM12 to regulating EMT has been
observed across diverse human cancer cell types (23,24).
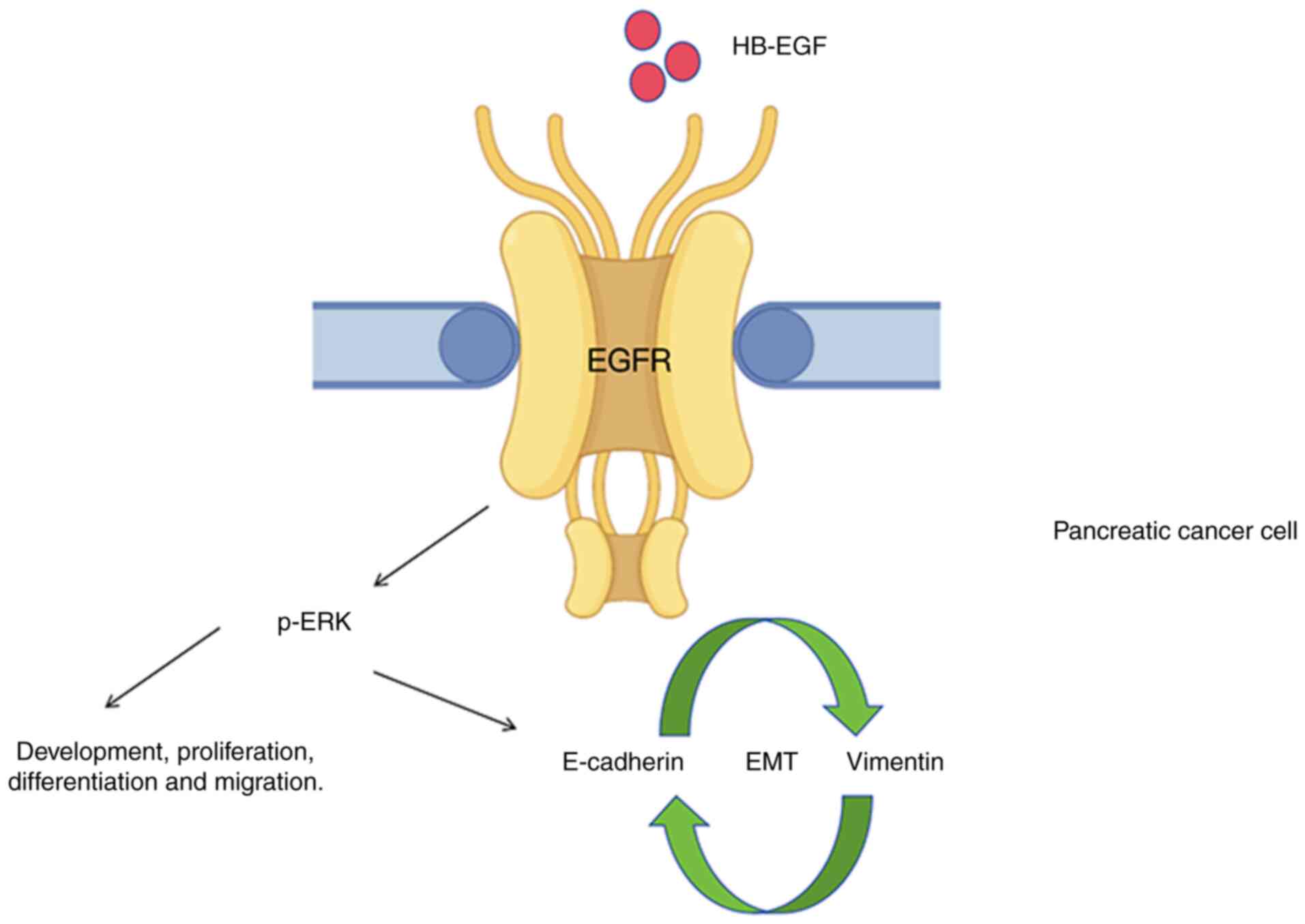 | Figure 3Diagram depicts HB-EGF/EGFR mediated
development and migration in PC cells. The binding of HB-EGF to
EGFR promotes the activation of the EGFR signaling pathway, which
plays a critical role in PC cell proliferation, metastasis,
chemoresistance, poor prognosis and EMT through p-ERK. However, the
specific mechanisms remain unclear. HB-EGF, heparin-binding
epidermal growth factor; EGFR, EGF receptor; PC, pancreatic cancer;
EMT, epithelial-mesenchymal transition; p-ERK, phosphorylated
extracellular signal-regulated kinase. |
Clinically, remarkably poor chemotherapeutic
efficacy was observed in PC, which is primarily attributed to the
abundant presence of stromal cells within tumor tissues.
Furthermore, extensive studies have highlighted the pivotal role of
EMT in pancreatic carcinogenesis. Motivated by these findings, it
was hypothesized that stromal components within pancreatic tumors
may drive its pathogenesis and progression, and it was aimed to
elucidate their functional mechanisms while identifying potential
therapeutic targets. According to the report, circulating levels of
ADAM12 may serve as a prognostic indicator for patients with PC. As
previously mentioned, ADAM12 is likely to promote EMT through the
HB-EGF/EGFR signaling pathway, contributing to poor outcomes and
chemoresistance in PC. The present study systematically examined
the expression levels of ADAM12, HB-EGF, EGFR and EMT markers in PC
using immunohistochemical (IHC) analysis, followed by an assessment
of survival outcomes in patients with PC. The primary objective is
to elucidate how ADAM12 influences the progression of EMT in PC by
modulating the HB-EGF/EGFR signaling pathway.
Materials and methods
Patients and specimens
The present study was approved by the academic
committee at Lihuili Hospital of Ningbo Medical Center (approval
no. KYSB2021SL023-01; Ningbo, China). All experiments were
conducted in accordance with relevant guidelines and regulations.
Informed consent was obtained from each patient, and the research
was conducted in accordance with the Declaration of Helsinki
(2013). From January 2017 to December 2020, a total of 62 patients
with pancreatic masses were recruited from Sun Yat-Sen Memorial
Hospital, Sun Yat-Sen University (Guangzhou, China), and Lihuili
Hospital of Ningbo Medical Center (Ningbo, China). Initially, it
was aimed to collect pancreatic tissue via endoscopic
ultrasound-guided fine-needle aspiration; however, it became
evident that the specimen volume was insufficient for multiple IHC
staining procedures. Consequently, all pancreatic tissue specimens
were ultimately acquired through surgical resections. There were 43
PC tissues.
Furthermore, the remaining 19 benign pancreatic
tissues were all derived from patients with surgically resected
chronic pancreatitis, benign pancreatic mucinous tumors, autoimmune
pancreatitis, and other conditions. Patients diagnosed with PC were
offered follow-up for at least 2 years and subjected to the
following inclusion conditions: i) complete follow-up data, ii)
histologically confirmed PC cases, iii) no history of another
malignant tumor, iv) no history of any antitumor treatment and v)
no death within 30 days of surgery caused by postoperative
complications. Relevant data on ADAM12 and HB-EGF were retrieved
from the GEPIA database (http://gepia.cancer-pku.cn/) as supplementary
controls.
IHC
Paraffin-embedded samples were sectioned at a
thickness of 4 µm. Specimens were incubated with antibodies
specific for ADAM12 (1:100; rabbit anti-human; Abcam), HB-EGF
(1:100; rabbit anti-human; Abcam), EGFR (1:100; rabbit anti-human;
Abcam), vimentin (1:100; rabbit anti-human; Abcam), or E-cadherin
(1:100; mouse anti-human; Abcam) overnight at 4˚C according to the
manufacturer's instructions. Two experienced pathologists
independently evaluated 63 samples. The scores were recorded, each
value corresponding to a range of the positive percentage: The
score of 0 is negative, the score of 1 is 1-25%, the score of 2 is
26-50%, the score of 3 is 51-75%, and the score of 4 is ≥76%. Lower
expression was considered as a score ≤2, while higher expression
was considered as a score of 4.
Statistical analysis
SPSS version 19.0 (IBM Corp.) was used for
statistical analyses. The Mann-Whitney U test was applied for the
analysis of group differences, and Pearson's chi-square test and
Spearman's rank correlation were used to evaluate the results of
IHC. Survival curves were plotted by the Kaplan-Meier method and
evaluated by the log-rank test. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological features
Of the 62 participants involved in the present
study, 69.4% (n=43) were diagnosed with PC, and their clinical
characteristics and outcomes are detailed in Table I. The median age of the entire
cohort was 69 years (range 42-83), with a predominance of male
patients, comprising 27 individuals (62.8%). Consistent with
previous literature (25), tumors
were predominantly located in the pancreatic head, accounting for
29 cases (67.4%), while those situated in the body/tail comprised
14 cases (32.6%). At diagnosis, a significant proportion of
patients presented with larger tumor sizes (>3.0 cm) at a rate
of 69.8%, and histologic grade II was observed in 74.4% of cases.
Given that most participants in our cohort had resectable or
borderline resectable PC, 31 patients were categorized (72.0%) as
early stage (I/II) and the remaining 12 patients (28.0%) as
advanced stage (III/IV). Furthermore, the incidence of positive
lymph nodes was relatively low at 39.5% and only two patients
(4.7%) exhibited distant metastases. Among 43 patients with PC,
treatment modalities included chemotherapy for 21 individuals
(51.2%), while there was a total of 24 deaths recorded with this
population; specifically, these fatalities included 15 male and 9
female patients.
 | Table IClinicopathological characteristics
and outcomes of patients with PC. |
Table I
Clinicopathological characteristics
and outcomes of patients with PC.
| Characteristics | Total number
(percentage of all PC cases) |
|---|
| Sex | |
|
Male | 27 (62.8%) |
|
Female | 16 (37.2%) |
| Tumor site | |
|
Head | 29 (67.4%) |
|
Body/Tail | 14 (32.6%) |
| Tumor size, cm | |
|
≤3.0 | 13 (30.2%) |
|
>3.0 | 30 (69.8%) |
| Histopathologic
grade | |
|
G1 | 4 (9.3%) |
|
G2 | 32 (74.4%) |
|
G3 | 7 (16.3%) |
| Lymph node
status | |
|
Positive | 17 (39.5%) |
|
Negative | 26 (60.5%) |
| Metastases | |
|
Positive | 2 (4.7%) |
|
Negative | 41 (95.3%) |
| TNM staging | |
|
I | 15 (34.9%) |
|
II | 16 (37.2%) |
|
III | 10 (23.3%) |
|
IV | 2 (4.7%) |
| Chemotherapy | |
|
Yes | 21 (51.2%) |
|
No | 22 (48.8%) |
| Outcomes | |
|
Death | 24 (55.8%) |
|
Survive | 19 (44.2%) |
Expression of ADAM12, HB-EGF, EGFR and
EMT markers in PC
The IHC examination revealed that the expression
levels of ADAM12, as well as HB-EGF, EGFR and vimentin were
significantly upregulated in 43 PC tissues compared with to 19
benign pancreatic mass (P<0.0001, Fig. 4). Conversely, E-cadherin was found
to be significantly downregulated in PC tissues (P<0.0001,
Fig. 4). Furthermore, a
significant correlation was observed between high ADAM12 expression
and lymph node metastasis (P<0.0001) and T stage (P=0.024),
along with E-cadherin (P=0.035, P=0.010) and vimentin (P=0.021,
P=0.005). The elevated expression levels of HB-EGF and EGFR were
positively correlated with lymph node metastasis (P=0.004 and
P=0.032), but not with T stage (P=0.097 and P=0.243). Additionally,
high ADAM12 expression was associated with increased levels of
HB-EGF (P=0.025), EGFR (P=0.012), and vimentin (P=0.001), while it
correlated negatively with E-cadherin levels (P=0.012).
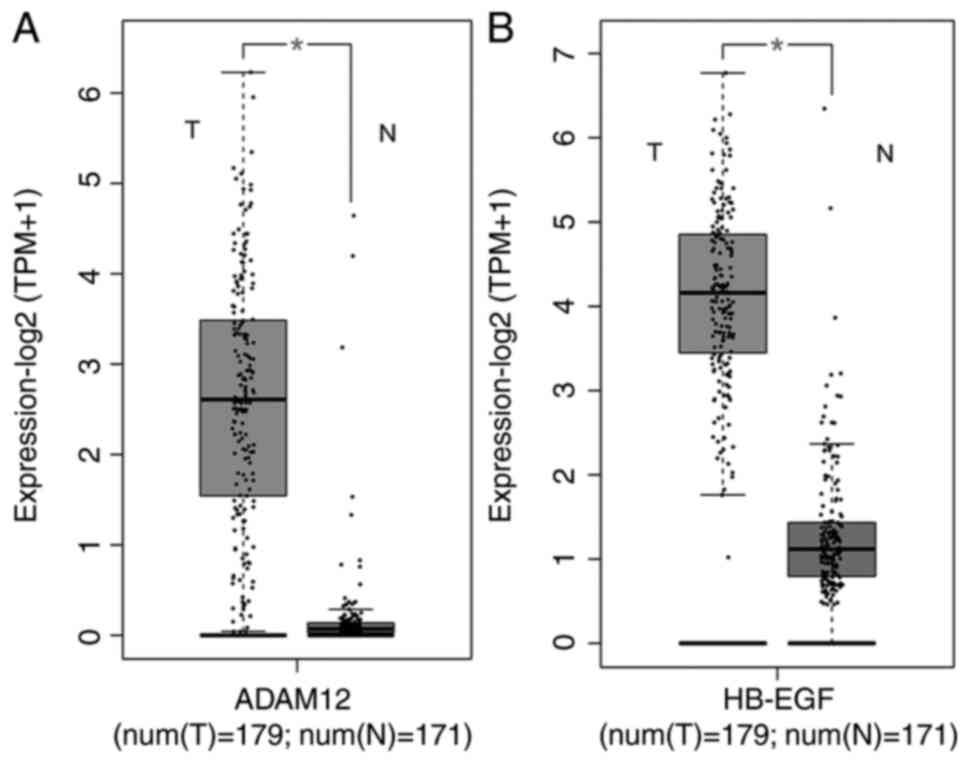
Consistently, results from the GEPIA database indicated that both
ADAM12 and HB-EGF are upregulated in PC tissues (Fig. 5A and B). In the current study cohort, there
were 17 cases of lymph node metastasis vs. 26 cases without
metastasis; higher expression levels of ADAM12 (P<0.0001),
HB-EGF (P=0.020), EGFR (P=0.024) and vimentin (P=0.029) were
significantly associated with advanced TNM stage (III and IV
stage). It is noteworthy that most PCs collected for the present
study were classified as stage II, with only a small proportion
categorized into other stages. Thus, the sample size for
correlation analysis is insufficient. That was the reason for
conducting differential analyses solely between the I/II stage
group and the III/IV stage group.
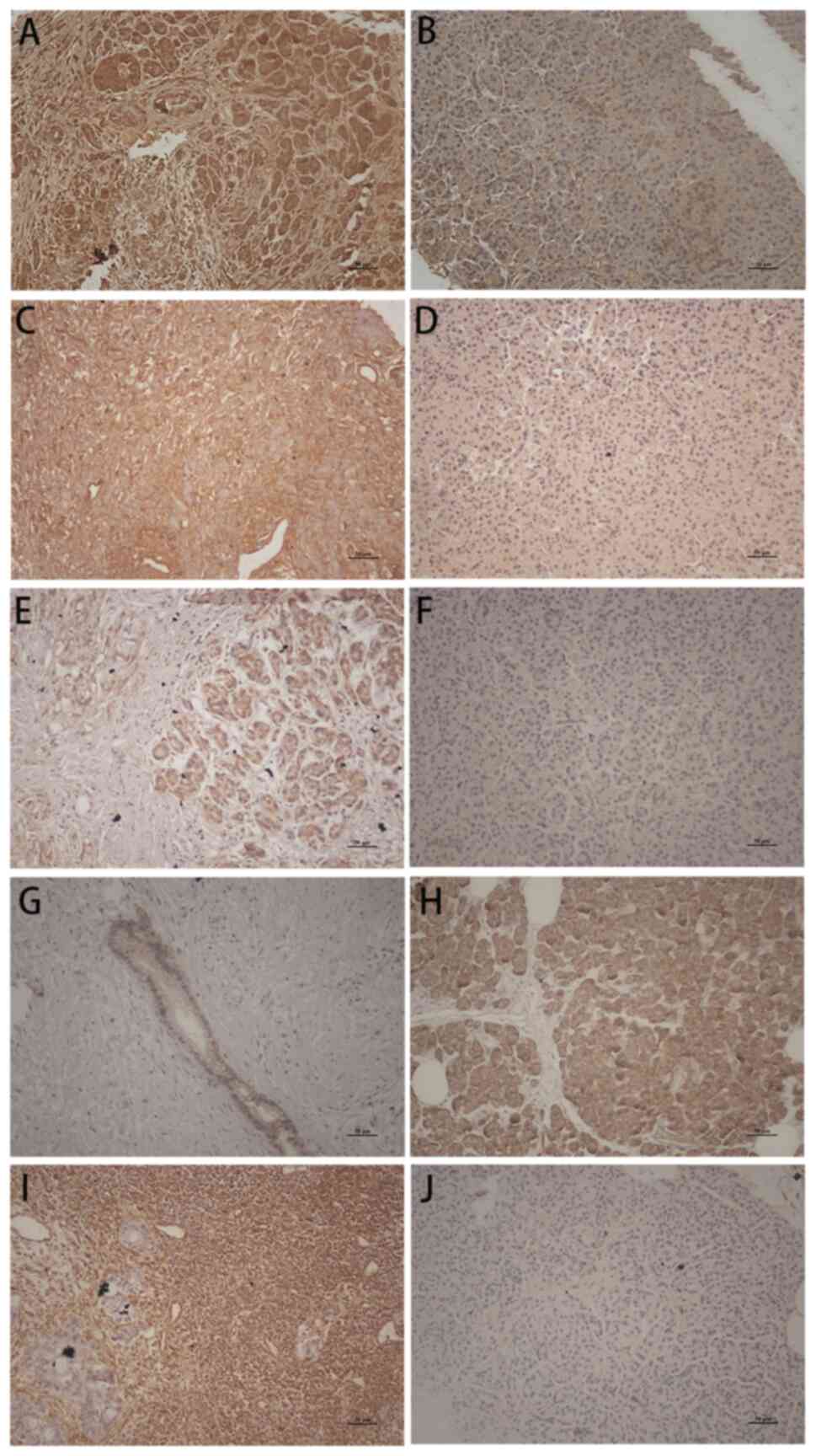 | Figure 4Distinction between PC tissue and
benign pancreatic mass. (A) A disintegrin and metalloproteinase 12,
(C) heparin-binding EGF, (E) EGF receptor and (I) vimentin show
strong staining of the PC tissue, while benign pancreatic mass is
weak (B, D, F and J); by contrast, (G) E-cadherin shows weak
staining of the PC tissue, while benign pancreatic mass is strong
(H). H&E stain, x200 magnification, all images. PC, pancreatic
cancer; EGF, epidermal growth factor. |
Association between ADAM12 and HB-EGF
protein expression with survival
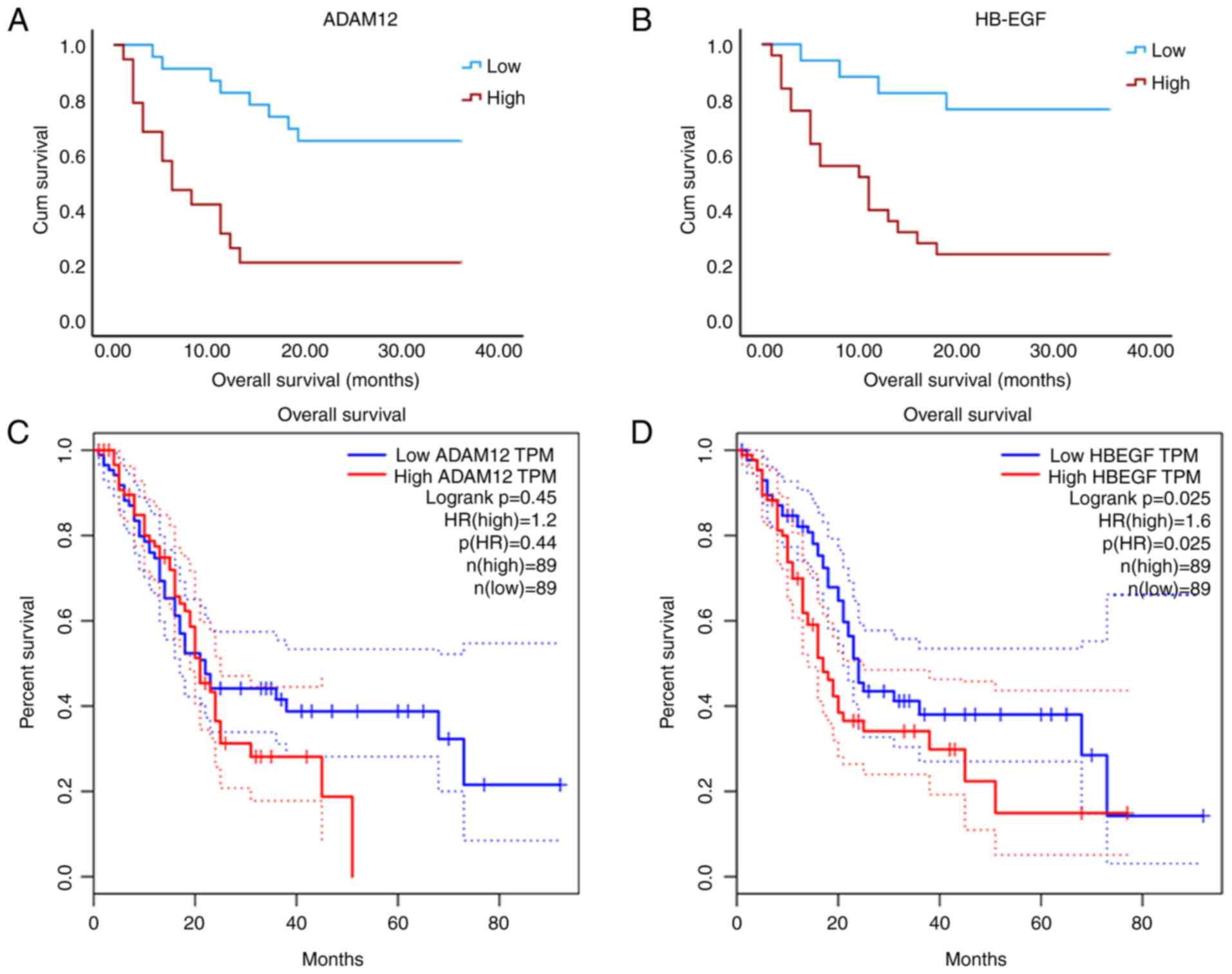
The median overall survival (OS) for the 43 patients
with PC was 17 months. These patients were categorized into two
groups based on their IHC scores: A high-expression group (IHC ≥
score 3) and a low-expression group (IHC ≤ score 2). Specifically,
the median OS was found to be 36 months in the ADAM12
low-expression group compared with just 6 months in the
high-expression group. Similarly, for HB-EGF, the median OS was 36
months in the low-expression group and 11 months in the
high-expression group. Kaplan-Meier survival analysis was conducted
to assess the relationship between protein levels and outcomes.
Analysis of data from 198 patients with PC revealed a significant
correlation with outcomes, which our findings corroborated
(Fig. 6B and D). Conversely, while high expression of
ADAM12 demonstrated a significant association with poor OS among
this cohort (Fig. 6A), it did not
reach statistical significance within the GEPIA database (Fig. 6C). This discrepancy may stem from
differing criteria used for grouping: Patients were classified
based on IHC score levels (high vs. low), whereas stratification in
the GEPIA database utilized median IHC scores.
Discussion
Based on the GLOBOCAN 2022 estimates, PC was the
eighth most diagnosed cancer type in China. There were 124,994 new
cases of PC and 121,853 deaths attributed to this disease. The
observed 5-year survival rate was a mere 6.6%. The incidence rates
were reported as 6.3 per 100,000 men (age-standardized for China)
and 4.2 per 100,000 women; mortality rates stood at 6.0 per 100,000
men and 4.2 per 100,000 women. In the present study, data from 43
patients with PC were analyzed, comprising 27 males and 16 females.
Among these patients, there were a total of 24 deaths (15 males and
nine females), indicating that men exhibit a higher risk and poorer
outcomes for PC compared with women. Consistent with findings from
other studies, it was observed that most tumors (67.4%) were
located in the head of the pancreas. Due to the insidious onset of
PC, it is frequently diagnosed at an advanced stage; indeed, in the
present study cohort, it was found that tumor sizes exceeded 3 cm
in 30 cases (69.8%). However, few patients included in our analysis
presented with lymph node or distant metastasis. This phenomenon
can be attributed to our tissue samples being obtained from
surgical resections, where most operable patients with PC do not
exhibit lymphatic or distant spread. In alignment with previous
research findings, it was identified that ADAM12 was highly
expressed in PC tissues but not present in benign pancreatic
tissues.
ADAM12 has been identified as essential for myotube
formation, playing significant roles in proteolysis, cell adhesion,
fusion, apoptosis and signal transduction. Its diagnostic and
prognostic value has been established across various diseases,
including Alzheimer's disease, arthritis, cardiac hypertrophy and
various cancers (26). The ADAM12
gene, located on chromosome 10q26, encodes two distinct isoforms: A
long transmembrane isoform (ADAM12L) and a short, secreted variant
(ADAM12S). Both isoforms can activate or inhibit pathways involved
in cell proliferation and invasion by proteolytically processing
substrates. In PC, an upregulation of ADAM12 has been observed;
however, its functional contributions remain unexplored (13). Consistent with this finding, the
present study demonstrated a marked upregulation of ADAM12 in PC
tissues compared with benign pancreatic tissues. This finding
suggests that ADAM12 may play a critical role in the progression of
PC. ADAM12 interacts with numerous substrates, including
insulin-like growth factor binding proteins 3 and 5 (IGFBP-3 and
-5), HB-EGF and others (27). The
shedding of HE-EGF, mediated by ADAM12, promotes cell proliferation
and contributes to cardiac hypertrophy through its interaction with
EGFR (28). In breast cancer
studies, it was shown that the extracellular domain of HB-EGF
released by ADAM12 enhances cellular migration and invasion
capabilities (29).
Furthermore, research on pituitary adenoma revealed
that the shedding of HB-EGF by ADAM12 plays a pivotal role in
facilitating cell migration and invadopodia formation through EGFR
activation (23). In the current
investigation, significantly elevated levels of both HB-EGF and
EGFR expression were observed in PC tissues. Collectively, these
findings suggested that ADAM12 may be implicated in the activation
of the HB-EGF/EGFR signaling pathway during tumorigenesis and
progression. However, the underlying mechanism remains to be
elucidated.
According to previous studies on various tumors, it
has been demonstrated that ADAM12 can promote tumor progression and
metastasis by modulating EMT (30), a relationship that remains unclear
in PC. EMT involves the transition from an epithelial phenotype to
a mesenchymal-like phenotype and plays a crucial role in the
initiation of tumor invasion and metastasis (31). Major hallmarks of EMT include the
increased expression of vimentin and decreased expression of
E-cadherin. It is well established that EMT is induced in PC,
facilitating cell migration and metastasis. Consistent with prior
research, the present study observed upregulation of vimentin and
downregulation of E-cadherin expression in PC tissues.
In the present study, 62 pancreatic specimens were
collected through surgical resection from two hospitals. Among
these specimens, 19 served as controls and were derived from
patients with surgically resected chronic pancreatitis, benign
pancreatic mucinous tumors, autoimmune pancreatitis, and other
related conditions. It is acknowledged that some may question the
appropriateness of our control group sample. This concern arises
because most of the pancreas that can be surgically removed
exhibits inflammation; thus, it is impossible to completely excise
normal pancreatic tissue. Therefore, it is contended that this
represents the most suitable non-cancerous pancreatic tissue for
use as a normal control in our research.
Furthermore, positive correlations were found
between lymph node metastasis, TNM staging, and the expression
levels of ADAM12, HB-EGF, EGFR and vimentin. Taken together, the
authors propose that ADAM12 may activate EGFR through the shedding
of HB-EGF, thereby promoting tumor progression and metastasis in
PC. These hypotheses warrant future studies.
Patients with PC and elevated expression levels of
ADAM12 exhibited poorer survival outcomes in both the present study
and the GEPIA database. According to the GEPIA database, a trend
toward worse survival was noted in patients with PC with higher
ADAM12 expression; however, no statistically significant difference
was observed between the two groups (Fig. 6C). A recent investigation indicated
that increased circulating levels of ADAM12 were predictive of
worse survival for patients with PC following surgical resection or
treatment with nab-paclitaxel. In contrast to ADAM12, the
expression of HB-EGF demonstrated a significant association with
patient survival in both our study and the GEPIA database.
In summary, the present study demonstrated that
ADAM12 is highly expressed in PC, and the upregulation of ADAM12
may serve as a potential marker for poor outcomes in PC. However,
the expression level of ADAM12 does not show a significant
association with the survival of patients with PC based on the
GEPIA database. This discrepancy may be attributed to varying
cutoff points, as elaborated in the results section. The impact of
ADAM12 on outcomes remains controversial, necessitating larger
sample sizes for future research. Furthermore, it was found that
ADAM12 expression is significantly associated with the HB-EGF/EGFR
pathway and EMT in PC. Therefore, it was hypothesized that
activation of ADAM12 in PC may lead to EGFR-mediated EMT through
shedding of HB-EGF, which could contribute to metastasis and poor
outcomes among patients with PC. As a malignant tumor characterized
by an abundant stroma, PC exhibits a deleterious propensity for
drug resistance (32,33). It is now widely accepted that
modulation of EMT represents a promising strategy for enhancing
treatment efficacy in patients with PC (34). In the present study, correlations
between ADAM12 and EMT markers were observed, as well as disease
progression in PC, potentially mediated through the HB-EGF/EGFR
signaling pathway. Collectively, these findings suggested that
ADAM12 may emerge as a novel therapeutic for treating PC. Moving
forward, we cell experiments in conjunction with in vitro
analysis will be conducted in the future to elucidate further the
role of this pathway in both the onset and progression of PC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by General Surgery
Clinical Key Specialty Construction Project of Zhejiang (grant no.
2023-SZZ).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
QZ, FX, ZG, XD, YM, HL and KH contributed to the
study conception and design. KH and ZG collected specimens and
data. XD performed data analysis. QZ wrote the first draft of the
manuscript. FX, YM and HL reviewed and edited the manuscript. QZ
and ZG confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
KYSB2021SL023-01) by the academic committee at Lihuili Hospital of
Ningbo Medical Center (Ningbo, China). All experiments were
performed in accordance with relevant guidelines and regulations.
Informed consent was obtained from each patient. The research was
performed in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization: GLOBOCAN 2022.
Graph production: IARC. Available from: http://gco.iarc.fr/today.
|
|
2
|
Feig C, Gopinathan A, Neesse A, Chan DS,
Cook N and Tuveson DA: The pancreas cancer microenvironment. Clin
Cancer Res. 18:4266–4276. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dauer P, Nomura A, Saluja A and Banerjee
S: Microenvironment in determining chemo-resistance in pancreatic
cancer: Neighborhood matters. Pancreatology. 17:7–12.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mews P, Phillips P, Fahmy R, Korsten M,
Pirola R, Wilson J and Apte M: Pancreatic stellate cells respond to
inflammatory cytokines: Potential role in chronic pancreatitis.
Gut. 50:535–541. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Veenstra VL, Damhofer H, Waasdorp C, van
Rijssen LB, van de Vijver MJ, Dijk F, Wilmink HW, Besselink MG,
Busch OR, Chang DK, et al: ADAM12 is a circulating marker for
stromal activation in pancreatic cancer and predicts response to
chemotherapy. Oncogenesis. 7(87)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fröhlich C, Albrechtsen R, Dyrskjøt L,
Rudkjaer L, Ørntoft TF and Wewer UM: Molecular profiling of ADAM12
in human bladder cancer. Clin Cancer Res. 12:7359–7368.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kodama T, Ikeda E, Okada A, Ohtsuka T,
Shimoda M, Shiomi T, Yoshida K, Nakada M, Ohuchi E and Okada Y:
ADAM12 is selectively overexpressed in human glioblastomas and is
associated with glioblastoma cell proliferation and shedding of
heparin-binding epidermal growth factor. Am J Pathol.
165:1743–1753. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Le Pabic H, Bonnier D, Wewer UM, Coutand
A, Musso O, Baffet G, Clément B and Théret N: ADAM12 in human liver
cancers: TGF-beta-regulated expression in stellate cells is
associated with matrix remodeling. Hepatology. 37:1056–1066.
2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peduto L, Reuter VE, Sehara-Fujisawa A,
Shaffer DR, Scher HI and Blobel CP: ADAM12 is highly expressed in
carcinoma-associated stroma and is required for mouse prostate
tumor progression. Oncogene. 25:5462–5466. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rocks N, Paulissen G, Quesada Calvo F,
Polette M, Gueders M, Munaut C, Foidart JM, Noel A, Birembaut P and
Cataldo D: Expression of a disintegrin and metalloprotease (ADAM
and ADAMTS) enzymes in human non-small-cell lung carcinomas
(NSCLC). Br J Cancer. 94:724–730. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Roy R, Wewer UM, Zurakowski D, Pories SE
and Moses MA: ADAM 12 cleaves extracellular matrix proteins and
correlates with cancer status and stage. J Biol Chem.
279:51323–51330. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu J, Walter K, Omura N, Hong SM, Young A,
Li A, Vincent A and Goggins M: Unlike pancreatic cancer cells
pancreatic cancer associated fibroblasts display minimal gene
induction after 5-aza-2'-deoxycytidine. PLoS One.
7(e43456)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dyczynska E, Sun D, Yi H, Sehara-Fujisawa
A, Blobel CP and Zolkiewska A: Proteolytic processing of delta-like
1 by ADAM proteases. J Biol Chem. 282:436–444. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Asakura M, Kitakaze M, Takashima S, Liao
Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro
H, et al: Cardiac hypertrophy is inhibited by antagonism of ADAM12
processing of HB-EGF: Metalloproteinase inhibitors as a new
therapy. Nat Med. 8:35–40. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang F, Sloss C, Zhang X, Lee SW and
Cusack JC: Membrane-bound heparin-binding epidermal growth factor
like growth factor regulates E-cadherin expression in pancreatic
carcinoma cells. Cancer Res. 67:8486–8493. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rahman FB, Kadowaki Y, Ishihara S, Tobita
H, Imaoka H, Fukuhara H, Aziz MM, Furuta K, Amano Y and Kinoshita
Y: Fibroblast-derived HB-EGF promotes Cdx2 expression in esophageal
squamous cells. Lab Invest. 90:1033–1048. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Korc M: Role of growth factors in
pancreatic cancer. Surg Oncol Clin N Am. 7:25–41. 1998.PubMed/NCBI
|
|
19
|
Dharmaraj N, Engel BJ and Carson DD:
Activated EGFR stimulates MUC1 expression in human uterine and
pancreatic cancer cell lines. J Cell Biochem. 114:2314–2322.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kopantzev EP, Kopantseva MR, Grankina EV,
Mikaelyan A, Egorov VI and Sverdlov ED: Activation of IGF/IGF-IR
signaling pathway fails to induce epithelial-mesenchymal transition
in pancreatic cancer cells. Pancreatology. 19:390–396.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yin T, Wang CY, Liu T, Zhao G and Zha YH:
Expression of Snail and E-cadherin in pancreatic carcinoma and
clinical significance thereof. Zhonghua Yi Xue Za Zhi.
86:2821–2825. 2006.PubMed/NCBI(In Chinese).
|
|
22
|
Gordon KJ, Dong M, Chislock EM, Fields TA
and Blobe GC: Loss of type III transforming growth factor beta
receptor expression increases motility and invasiveness associated
with epithelial to mesenchymal transition during pancreatic cancer
progression. Carcinogenesis. 29:252–262. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang J, Zhang Z, Li R, Mao F, Sun W, Chen
J, Zhang H, Bartsch JW, Shu K and Lei T: ADAM12 induces EMT and
promotes cell migration, invasion and proliferation in pituitary
adenomas via EGFR/ERK signaling pathway. Biomed Pharmacother.
97:1066–1077. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang X and Xie X, Liu P, Yang L, Chen B,
Song C, Tang H and Xie X: Adam12 and lnc015192 act as ceRNAs in
breast cancer by regulating miR-34a. Oncogene. 37:6316–6326.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hariharan D, Saied A and Kocher HM:
Analysis of mortality rates for pancreatic cancer across the world.
HPB (Oxford). 10:58–62. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lambrecht BN, Vanderkerken M and Hammad H:
The emerging role of ADAM metalloproteinases in immunity. Nat Rev
Immunol. 18:745–758. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kveiborg M, Albrechtsen R, Couchman JR and
Wewer UM: Cellular roles of ADAM12 in health and disease. Int J
Biochem Cell Biol. 40:1685–1702. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakayama Y and Yoshioka J: ADAM12 controls
a hypertrophic response in the heart through the distinct
descending pathways. Am J Physiol Heart Circ Physiol.
318:H209–H211. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang R, Godet I, Yang Y, Salman S, Lu H,
Lyu Y, Zuo Q, Wang Y, Zhu Y, Chen C, et al: Hypoxia-inducible
factor-dependent ADAM12 expression mediates breast cancer invasion
and metastasis. Proc Natl Acad Sci USA.
118(e2020490118)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xu J, Wang Y, Jiang J, Yin C and Shi B:
ADAM12 promotes clear cell renal cell carcinoma progression and
triggers EMT via EGFR/ERK signaling pathway. J Transl Med.
21(56)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Özdemir BC, Pentcheva-Hoang T, Carstens
JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C,
Novitskiy SV, et al: Depletion of carcinoma-associated fibroblasts
and fibrosis induces immunosuppression and accelerates pancreas
cancer with reduced survival. Cancer Cell. 25:719–734.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Erkan M, Michalski CW, Rieder S,
Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H
and Kleeff J: The activated stroma index is a novel and independent
prognostic marker in pancreatic ductal adenocarcinoma. Clin
Gastroenterol Hepatol. 6:1155–1161. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu
Y, Yao Y and Li D: The epithelial to mesenchymal transition (EMT)
and cancer stem cells: Implication for treatment resistance in
pancreatic cancer. Mol Cancer. 16(52)2017.PubMed/NCBI View Article : Google Scholar
|