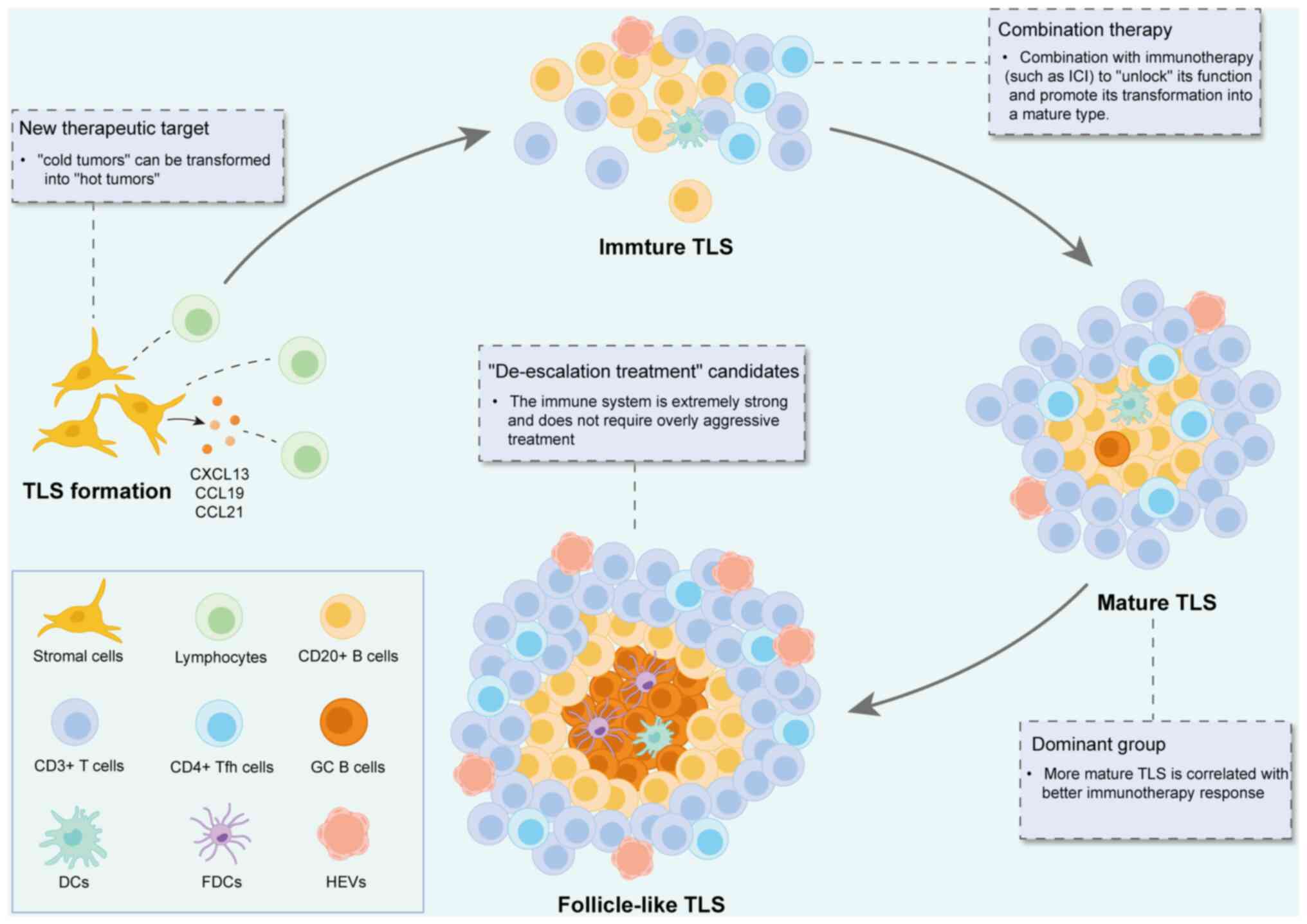
Tertiary lymphoid structure (TLS) refers to an
assembly of immune cells with a definite organized structure that
is acquired in non-lymphoid tissues out of the normal physiological
conditions (1). It is a kind of
ectopic lymphoid structure which is similar to secondary lymphoid
organ (SLO). TLS mainly consists of B cells, T cells, dendritic
cells (DCs), macrophages, fibroblast reticular cells, germinal
centers (GCs) and high endothelial venules (2-4).
TLS usually forms in the chronic inflammatory microenvironment
derived of autoimmune diseases, chronic infections and tumors. TLS
can be located in the stroma, intra-tumoral and peritumoral
areas.
TLS initially forms in perivascular regions, which
are rich in extracellular matrix, microvessels, lymphatic vessels
and neurons (Fig. 1). These
regions are relatively conserved in various organs of the organism,
providing a unique niche for resident immune cells in the tissue.
At first, TLS appears as small T/B cell aggregates, which then
gradually expands and matures to form complex structures containing
different B and T cell partitions (5,6).
During the immature phase of TLS, antigen presentation and T cell-B
cell interactions jointly drive lymphocyte activation and TLS
maturation. Accompanied by this process, the previously resident
fibroblasts undergo differentiation and switch to multiple
phenotypes to facilitate the overall development of TLS (7). The core region of TLS is composed of
CD20+ B cells, which are closely surrounded by
CD3+ T cells, forming a lymphoid follicle structure
similar to that in the SLO (8).
Although the specific cellular composition of different TLS may
vary, CD4+ T follicular helper (Tfh) cells usually
dominate the T cell area, and CD8+ cytotoxic T cells,
CD4+ T helper 1 (Th1) cells and regulatory T cells
(Tregs) can also be observed (9).
In addition to the B and T cell populations that constitute the
major portion of TLS immune cells, TLS also contains a diverse
subset of DCs, such as CD21+ follicular DCs derived from
the mesenchyme, which are primarily localized in the follicular
region and are essential for TLS function (10). In addition, the follicles are
interspersed with CD68+ macrophages responsible for the
clearance of apoptotic cells, a function similar to their role in
SLO. According to the maturity of TLS, TLS can be at least divided
into three different states: Lymphoid aggregate, immature TLS and
mature TLS (Table I). Immature TLS
could also be further divided into two subtypes: Conforming TLS and
deviating TLS (11,12).
In the present review, an overview of the biological
characteristics (such as components and classifications) was
provided. The latest advances of the close correlation between the
location, density and maturity of TLS and tumor prognosis and
response to immunotherapy were reviewed. The predictive,
therapeutic and prognostic values of TLS for head and neck squamous
cell carcinoma (HNSCC) were also stated. Promising therapeutic
strategies targeting TLS for HNSCC and potential methods to enhance
the efficacy of current treatment were also summarized.
As an important site of tumor-specific immune
response, TLS significantly affects the progression and outcome of
tumors by enhancing local immune response (1,7,8). In
the tumor microenvironment (TME), the formation and maintenance of
TLS is the joint consequence of multiple immune cells and
chemokines. B cells, T cells, DCs and other immune cells aggregate
to form a complex cell network, thereby promoting the local
reaction of immune cells and tumor-specific antigens (9). Since TLS has multiple potential roles
in antitumor immunity, further study on its biological
characteristics and application potential in tumor immunotherapy
will provide new ideas and strategies for improving the efficacy of
antitumor immunity. Previous studies revealed that continuous
antigen presentation and recognition were observed within TLS. In
addition, there is also a correlation between the formation of TLS
and antigen-specific T cell responses. Presence of TLS and its
higher degree of maturity are generally associated with an improved
prognosis in malignancies and infection (13,14).
In autoimmune diseases and organ transplantation, TLS often
aggravates local immune reactivity. It has been reported that TLS
is closely related to improved prognosis and higher response rate
to the immunotherapy (15). TLS
also exhibits predictive value for immune checkpoint blockade (ICB)
treatment. Therefore, accurate assessment such as the location,
density and maturity of TLS can provide great value for predicting
the prognosis of patients and developing novel targeting therapies
for malignancies.
The location of TLS shows significant influence on
tumor prognosis. TLS is distributed both within the tumor and in
the peritumoral regions. TLS in different locations shows certain
differences in immune response and tumor prognosis. In most
malignancies, stronger antitumor immune response and improved
prognosis was found in patients with larger quantity of
intra-tumoral TLS. By contrast, TLS that have an adverse effect on
disease prognosis are more commonly found in the peritumoral
region. There was a strong link between the intra-tumoral TLS
formation and maintenance and improved tumor prognosis in oral
cancers (16). It was reported
that TLS within the tumor was correlated with more infiltrating B
cells and CD4+ T cells (17). TLS in the intra-tumoral areas was
characterized with more memory B cells and macrophages. On
contrary, TLS in the peritumoral areas tended to exhibit higher
epithelial-mesenchymal transition activity and facilitated tumor
progression and immune escape. TLS located in the intra-tumoral
region was more likely correlated with lower TNM stage while TLS
found in the peritumoral regions exhibited the opposite outcome
(18,19). In a study on recurrent/metastatic
HNSCC, patients with TLS which located close to tumor cells
intended to have improved prognosis (15). Studies on breast cancer (20), melanoma (21), non-small cell lung cancer (NSCLC)
(18) and colorectal cancer
(22,23) have shown that intra-tumoral TLS was
associated with an improved prognosis, while peritumoral TLS not.
This indicated that the spatial distribution of immune cells in TLS
had a great significance on tumor prognosis. Notably, TLS in the
peritumoral regions was not always associated with poor prognosis
(24,25). It was reported that TLS in the
peritumoral regions had a close correlation with improved prognosis
in hepatocellular carcinoma (HCC), while TLS in the intra-tumoral
regions was associated with poorer prognosis and higher tumor
invasiveness (26,27). The relationship between the spatial
location of TLS and prognosis in different tumors shows significant
heterogeneity. It remains unclear why TLS plays different roles in
distinct tumors. It may be related to TLS maturity, cell
composition in TLS, cytokines and immunogenicity. Because of this,
it is crucial to analyze the impact of TLS on prognosis in
combination with the specific type of tumor.
The density of TLS in the TME is closely related to
the prognosis of tumor patients. More immune cells are contained in
the TLS with higher density. Immune cells in TLS can recognize and
fight tumor cells and enhance the immune response, thereby leading
to improved survival benefits. TLS has been proved to be an
independent prognostic marker, which could predict the response
rate of immunotherapy in different tumors (22,28-33).
Improved overall survival (OS) and lower recurrent rate were found
in patients with high-density TLS (24,34,35).
In a single-armed, phase II trial (ChiCTR2200066119) on advanced
oral squamous cell carcinoma, neoadjuvant immunochemotherapy
(treatment included PD-1 monoclonal antibody camrelizumab,
nab-paclitaxel and cisplatin) was proved to significantly increase
the density of TLS, along with bringing a promising response rate
and prognosis (36). In a study on
recurrent/metastatic HNSCC, the density of TLS was proved to
predict the response to immunotherapy with 80% accuracy (37). Therefore, TLS density could be
regarded as an independent predictive biomarker for patients with
HNSCC which accepted ICB. In spite of this, there is no direct
evidence to prove TLS to be an independent prognostic biomarker,
and more preclinical studies and clinical trials should be carried
out to link the density of TLS and prognosis of HNSCC.
The maturity of TLS may also play an important role
in predicting the prognosis of tumor patients. Mature TLS is more
likely to contain functional GCs which promote the production of
high-affinity antibodies and induce immune responses against tumor
antigens (13,14,38).
By contrast, immature TLS may not be able to sufficiently activate
immune cells, which in turn makes it difficult to recruit enough
immune cells to eliminate the tumor cells. This may explain why
immature TLS is associated with poorer prognosis. More mature TLS
was proved to be related to improved prognosis of patients with
HNSCC (39). In addition, higher
GC activity was proved to have a correlation with improved OS of
patients. This also illustrates that mature TLS could be regarded
as a prognostic biomarker. In non-metastatic colorectal carcinoma,
high density of TLS was proved to be correlated with high tumor
mutation burden and low recurrent rate (22,40).
A total of 3 different TLS was identified according to the
maturity: early TLS, primary follicle-like TLS and secondary
follicle-like TLS in laryngeal squamous cell carcinoma (LSCC)
(41). In addition, follicle-like
TLS could serve as an independent positive prognostic biomarker for
LSCC, as more infiltrating immune cells and higher response rates
were found in patients with follicle-like TLS. Studies on NSCLC
(42) and pancreatic cancer
(43) have also shown that the
maturity of TLSs played an important role in influencing the immune
response of patients. In a study on pancreatic ductal
adenocarcinoma (PDAC), B cells within mature TLS were proved to
exhibit an immunomodulatory efficacy and could activate the antigen
presentation process (44). On the
contrary, immature TLSs lack clear tissue structure, which does not
significantly improve the prognosis of patients. Therefore, mature
TLSs predict a stronger immune response and improved prognosis,
thereby contributing to improve OS of patients.
Since the composing proportions of diverse cells
within TLS have been reported to vary in different types of tumors,
the roles of TLS in distinct tumors can also be diverse. Different
degrees of maturation and cell proportions of TLS have been
demonstrated to determine the heterogeneity of TLS in distinct
tumors (45). In a pan-cancer
study, TLS was demonstrated to have a close relationship with gene
mutations regarding tumor suppressor, immune regulation, DNA repair
(46). Interestingly, increasing
TLS was also proved to be linked to the Epstein-Barr virus
infection in gastric cancer and human papillomavirus (HPV)
infection in HNSCC (46-49).
Patients with more TLS also had the characteristics of more T
lymphocyte infiltration, higher TCR/BCR activation and more potent
antigen presentation (50).
PD-L1 Combined Positive Score (CPS) is currently a
clinically standard biomarker approved by the FDA for the selection
of anti-PD-1/PD-L1-based immunotherapy (36). However, not all tumors with high
PD-L1 expression rely on the PD-1/PD-L1 pathway for immune escape.
This leads to a nonnegligible high rate of false positives for CPS.
TP53 mutations impair DNA repair and lead to abnormal cell
proliferation (51). Although TP53
mutation was recognized to be related to tumorigenesis of HNSCC,
there is no evidence to identify TP53 as an independent predictive
biomarker for HNSCC (52). EGFR
overexpression facilitates tumor growth, invasion and therapy
resistance. High EGFR expression was reported to be a negative
prognostic factor in HNSCC. However, EGFR gene copy number and EGFR
mutations just showed no prognostic value in HNSCC. EGFR appears to
be unstable to be a predictive biomarker in HNSCC and need more
acknowledge into its underlying mechanisms (53). The predictive value of
tumor-infiltrating lymphocytes (TILs) may be compromised depending
on the functional status. There may be a large number of exhausted
T cells in the tumor, which are present but have lost their
eliminating ability (37). Mature
TLS comprises PD-L1+ cells, CD8+ T cells,
CD4+ Tfh cells, B cells and DCs. TLS integrates the
information of PD-L1 and TILs and shows that there are effective
interactions occurring among these components. This is more
meaningful than measuring a single indicator alone. The existence
of TLS suggests an active and ongoing antitumor immune response,
rather than just the static presence of immune cells. The
antibodies produced by B cells can form immune complexes, which
further activate DCs and macrophages, amplifying the immune
response (22).
Although TLS formation in HNSCC has a close
correlation with the prognosis and response to immunotherapy, how
to directly induce TLS to develop into an antitumor phenotype stays
an obstacle currently. The formation of TLS is a highly ordered
process involving multiple cells (lymphocytes, stromal cells),
cytokines (such as CXCL13, CCL19, CCL21 and LTαβ) and chemokines.
Currently, there is no single ‘universal drug’ that can perfectly
simulate this complex process. The use of a single agonist (such as
LTβR agonists) may only induce structurally incomplete and
functionally immature TLS (merely lymphocyte aggregation) but fail
to form functional TLS with GCs. This would result in a significant
reduction in therapeutic efficacy. In spite of this, combination
with ICR, multiple induction and local delivery system can be
beneficial to TLS-based therapy. Therefore, TLS-targeted therapy
depends on in-depth understanding of its complex biology and
precise clinical manipulation capabilities.
The cellular component and biological
characteristics of TLS were reviewed. The correlation and clinical
meaning of the location, density and maturity of TLS in distinct
tumors was stated. TLS with high density and maturity which was
within the intra-tumoral regions exhibited a positive correlation
with improved prognosis and higher response rates to immunotherapy
in most malignancies. In several tumors (such as LSCC, NSCLC,
breast cancer and melanoma), TLS was also regarded as an
independent biomarker for prognosis or response to treatment.
Predictive, prognostic and therapeutic values of TLS were also
found in HNSCC. Studies and clinical trials have proved that TLS
formation and activation significantly improved the efficacy of
other treatments in HNSCC. Other therapeutic strategies such as
neoadjuvant ICB were proved to successfully induce TLS in HNSCC.
More studies are required to realize the precise regulation of TLS
for HNSCC treatment in the future. To sum up, TLS could be a
promising predictive, prognostic and therapeutic biomarker for
HNSCC, and TLS-targeting therapeutic strategies could be a
promising alternative treatment regime for patients with HNSCC in
the future.
Not applicable.
Funding: No funding was received.
Not applicable.
JS approved the final list of included studies and
revised the manuscript and performed the literature search and
wrote the manuscript. XY performed the literature review. Data
authentication is not applicable. Both authors read and approved
the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Schumacher TN and Thommen DS: Tertiary
lymphoid structures in cancer. Science.
375(eabf9419)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhao L, Jin S, Wang S, Zhang Z, Wang X,
Chen Z, Wang X, Huang S, Zhang D and Wu H: Tertiary lymphoid
structures in diseases: Immune mechanisms and therapeutic advances.
Signal Transduct Tar. 9(225)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gao J, Navai N, Alhalabi O, Siefker-Radtke
A, Campbell MT, Tidwell RS, Guo CC, Kamat AM, Matin SF, Araujo JC,
et al: Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with
cisplatin-ineligible operable high-risk urothelial carcinoma. Nat
Med. 26:1845–1851. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu Y, Ye SY, He S, Chi DM, Wang XZ, Wen
YF, Ma D, Nie RC, Xiang P, Zhou Y, et al: Single-cell and spatial
transcriptome analyses reveal tertiary lymphoid structures linked
to tumour progression and immunotherapy response in nasopharyngeal
carcinoma. Nat Commun. 15(7713)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xie M, Lin X, Bao X, Liang Y, Deng H, Song
J, Ma X, Zhang X, Yao J, Pan L and Xue X: Tertiary lymphoid
structure in tumor microenvironment and immunotherapy of lung
cancer. Arch Bronconeumo. 60 (Suppl 2):S77–S85. 2024.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
6
|
Zhao R, Zhang J, Ma J, Qu Y, Yang Z, Yin
Z, Li F, Dong Z, Sun Q, Zhu S, et al: cGAS-activated endothelial
cell-T cell cross-talk initiates tertiary lymphoid structure
formation. Sci Immunol. 9(eadk2612)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Meylan M, Petitprez F, Becht E, Bougoüin
A, Pupier G, Calvez A, Giglioli I, Verkarre V, Lacroix G, Verneau
J, et al: Tertiary lymphoid structures generate and propagate
antitumor antibody-producing plasma cells in renal cell cancer.
Immunity. 55:527–541.e5. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fridman WH, Meylan M, Petitprez F, Sun CM,
Italiano A and Sautes-Fridman C: B cells and tertiary lymphoid
structures as determinants of tumour immune contexture and clinical
outcome. Nat Rev Clin Oncol. 19:441–457. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sato Y, Silina K, van den Broek M,
Hirahara K and Yanagita M: The roles of tertiary lymphoid
structures in chronic diseases. Nat Rev Nephrol. 19:525–537.
2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Teillaud JL, Houel A, Panouillot M,
Riffard C and Dieu-Nosjean MC: Tertiary lymphoid structures in
anticancer immunity. Nat Rev Cancer. 24:629–646. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu Y, Zhou Z, Du X, Lin X, Liang ZM, Chen
S, Sun Y, Wang Y, Na Z, Wu Z, et al: Cancer cell-derived arginine
fuels polyamine biosynthesis in tumor-associated macrophages to
promote immune evasion. Cancer Cell. 43:1045–1060.e7.
2025.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tang Z, Bai Y, Fang Q, Yuan Y, Zeng Q,
Chen S, Xu T, Chen J, Tan L, Wang C, et al: Spatial transcriptomics
reveals tryptophan metabolism restricting maturation of
intratumoral tertiary lymphoid structures. Cancer Cell.
43:1025–1044.e14. 2025.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vanhersecke L, Brunet M, Guégan JP, Rey C,
Bougouin A, Cousin S, Moulec SL, Besse B, Loriot Y, Larroquette M,
et al: Mature tertiary lymphoid structures predict immune
checkpoint inhibitor efficacy in solid tumors independently of
PD-L1 expression. Nat Cancer. 2:794–802. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu W, You W, Lan Z, Ren Y, Gao S, Li S,
Chen WW, Huang C, Zeng Y, Xiao N, et al: An immune cell map of
human lung adenocarcinoma development reveals an anti-tumoral role
of the Tfh-dependent tertiary lymphoid structure. Cell Rep Med.
5(101448)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sadeghirad H, Monkman J, Tan CW, Liu N,
Yunis J, Donovan ML, Moradi A, Jhaveri N, Perry C, Adams MN, et al:
Spatial dynamics of tertiary lymphoid aggregates in head and neck
cancer: Insights into immunotherapy response. J Transl Med.
22(677)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li K, Guo Q, Zhang X, Dong X, Liu W, Zhang
A, Li Y, Yan J, Jia G, Zheng Z, et al: Oral cancer-associated
tertiary lymphoid structures: Gene expression profile and
prognostic value. Clin Exp Immunol. 199:172–181. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu S, Han C, Zhou J, Yang D, Dong H, Zhang
Y, Zhao T, Tian Y and Wu Y: Distinct maturity and spatial
distribution of tertiary lymphoid structures in head and neck
squamous cell carcinoma: Implications for tumor immunity and
clinical outcomes. Cancer Immunol Immunother.
74(107)2025.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xin S, Wen S, He P, Zhao Y and Zhao H:
Density of tertiary lymphoid structures and their correlation with
prognosis in non-small cell lung cancer. Front Immunol.
15(1423775)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Berthe J, Poudel P, Segerer FJ, Jennings
EC, Ng F, Surace M, Andoni A, Testori M, Saraiya M, Vuko M, et al:
Exploring the impact of tertiary lymphoid structures maturity in
NSCLC: Insights from TLS scoring. Front Immunol.
15(1422206)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takeshita T, Yamamoto Y, Yamamoto-Ibusuki
M, Tomiguchi M, Sueta A, Murakami K and Iwase H: Clinical
significance of plasma cell-free DNA mutations in PIK3CA, AKT1, and
ESR1 gene according to treatment lines in ER-positive breast
cancer. Mol Cancer. 17(67)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Helmink BA, Reddy SM, Gao J, Zhang S,
Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et
al: B cells and tertiary lymphoid structures promote immunotherapy
response. Nature. 577:549–555. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Posch F, Silina K, Leibl S, Mundlein A,
Moch H, Siebenhüner A, Samaras P, Riedl J, Stotz M, Szkandera J, et
al: Maturation of tertiary lymphoid structures and recurrence of
stage II and III colorectal cancer. Oncoimmunology.
7(e1378844)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Overacre-Delgoffe AE, Bumgarner HJ, Cillo
AR, Burr AHP, Tometich JT, Bhattacharjee A, Bruno TC, Vignali DAA
and Hand TW: Microbiota-specific T follicular helper cells drive
tertiary lymphoid structures and anti-tumor immunity against
colorectal cancer. Immunity. 54:2812–2824.e4. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hayashi Y, Makino T, Sato E, Ohshima K,
Nogi Y, Kanemura T, Honma K, Yamashita K, Saito T, Tanaka K, et al:
Density and maturity of peritumoral tertiary lymphoid structures in
oesophageal squamous cell carcinoma predicts patient survival and
response to immune checkpoint inhibitors. Brit J Cancer.
128:2175–2185. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Q, Shen X, An R, Bai J, Dong J, Cai
H, Zhu H, Zhong W, Chen W, Liu A and Du J: Peritumoral tertiary
lymphoid structure and tumor stroma percentage predict the
prognosis of patients with non-metastatic colorectal cancer. Front
Immunol. 13(962056)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li H, Liu H, Fu H, Li J, Xu L, Wang G and
Wu H: Peritumoral tertiary lymphoid structures correlate with
protective immunity and improved prognosis in patients with
hepatocellular carcinoma. Front Immunol. 12(648812)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Finkin S, Yuan D, Stein I, Taniguchi K,
Weber A, Unger K, Browning JL, Goossens N, Nakagawa S, Gunasekaran
G, et al: Ectopic lymphoid structures function as microniches for
tumor progenitor cells in hepatocellular carcinoma. Nat Immunol.
16:1235–1244. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Cascone T, Leung CH, Weissferdt A, Pataer
A, Carter BW, Godoy MCB, Feldman H, William WN Jr, Xi Y, Basu S, et
al: Neoadjuvant chemotherapy plus nivolumab with or without
ipilimumab in operable non-small cell lung cancer: The phase 2
platform NEOSTAR trial. Nat Med. 29:593–604. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Savas P, Salgado R, Denkert C, Sotiriou C,
Darcy PK, Smyth MJ and Loi S: Clinical relevance of host immunity
in breast cancer: From TILs to the clinic. Nat Rev Clin Oncol.
13:228–241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kinker GS, Vitiello GAF, Diniz AB,
Cabral-Piccin MP, Pereira PHB, Carvalho MLR, Ferreira WAS, Chaves
AS, Rondinelli A, Gusmão AF, et al: Mature tertiary lymphoid
structures are key niches of tumour-specific immune responses in
pancreatic ductal adenocarcinomas. Gut. 72:1927–1941.
2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Horeweg N, Workel HH, Loiero D, Church DN,
Vermij L, Léon-Castillo A, Krog RT, de Boer SM, Nout RA, Powell ME,
et al: Tertiary lymphoid structures critical for prognosis in
endometrial cancer patients. Nat Commun. 13(1373)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ahuja S, Khan AA, Zaheer S and Ranga S:
Tumor-infiltrating lymphocytes in oral cavity squamous cell
carcinoma and its association with clinicopathological parameters.
Pathol Res Pract. 251(154882)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li S, Zhang N, Zhang H, Yang Z, Cheng Q,
Wei K, Zhou M and Huang C: Deciphering the role of LGALS2: insights
into tertiary lymphoid structure-associated dendritic cell
activation and immunotherapeutic potential in breast cancer
patients. Mol Cancer. 23(216)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cabrita R, Lauss M, Sanna A, Donia M,
Skaarup LM, Mitra S, Johansson I, Phung B, Harbst K,
Vallon-Christersson J, et al: Tertiary lymphoid structures improve
immunotherapy and survival in melanoma. Nature. 577:561–565.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ding GY, Ma JQ, Yun JP, Chen X, Ling Y,
Zhang S, Shi JY, Chang YQ, Ji Y, Wang XY, et al: Distribution and
density of tertiary lymphoid structures predict clinical outcome in
intrahepatic cholangiocarcinoma. J Hepatol. 76:608–618.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xiang Z, Wei X, Zhang Z, Tang Y, Chen L,
Tan C, Zeng Y, Wang J, Zhao G, Dai Z, et al: Efficacy, safety and
single-cell analysis of neoadjuvant immunochemotherapy in locally
advanced oral squamous cell carcinoma: A phase II trial. Nat
Commun. 16(3968)2025.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ruiz-Torres DA, Bryan ME, Hirayama S,
Merkin RD, Luciani E, Roberts TJ, Patel M, Park JC, Wirth LJ, Sadow
PM, et al: Spatial characterization of tertiary lymphoid structures
as predictive biomarkers for immune checkpoint blockade in head and
neck squamous cell carcinoma. Oncoimmunology.
14(2466308)2025.PubMed/NCBI View Article : Google Scholar
|
|
38
|
He M, He Q, Cai X, Liu J, Deng H, Li F,
Zhong R, Lu Y, Peng H, Wu X, et al: Intratumoral tertiary lymphoid
structure (TLS) maturation is influenced by draining lymph nodes of
lung cancer. J Immunother Cancer 11: J Immunother Cancer.
11(e005539)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guo X, Xu L, Nie L, Zhang C, Liu Y, Zhao
R, Cao J, Tian L and Liu M: B cells in head and neck squamous cell
carcinoma: Current opinion and novel therapy. Cancer Cell Int.
24(41)2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
An Y, Sun JX, Xu MY, Xu JZ, Ma SY, Liu CQ,
Liu Z, Wang SG and Xia QD: Tertiary lymphoid structure patterns aid
in identification of tumor microenvironment infiltration and
selection of therapeutic agents in bladder cancer. Front Immunol.
13(1049884)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liang H, Zhang Z, Guan Z, Zheng S, Lou J,
Liu W, Cai Q and Si Y: Follicle-like tertiary lymphoid structures:
A potential biomarker for prognosis and immunotherapy response in
patients with laryngeal squamous cell carcinoma. Front Immunol.
14(1096220)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Patil NS, Nabet BY, Müller S, Koeppen H,
Zou W, Giltnane J, Au-Yeung A, Srivats S, Cheng JH, Takahashi C, et
al: Intratumoral plasma cells predict outcomes to PD-L1 blockade in
non-small cell lung cancer. Cancer Cell. 40:289–300.e4.
2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tanaka T, Masuda A, Inoue J, Hamada T,
Ikegawa T, Toyama H, Sofue K, Shiomi H, Sakai A, Kobayashi T, et
al: Integrated analysis of tertiary lymphoid structures in relation
to tumor-infiltrating lymphocytes and patient survival in
pancreatic ductal adenocarcinoma. J Gastroenterol. 58:277–291.
2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Delvecchio FR, Fincham REA, Spear S, Clear
A, Roy-Luzarraga M, Balkwill FR, Gribben JG, Bombardieri M,
Hodivala-Dilke K, Capasso M and Kocher HM: Pancreatic cancer
chemotherapy is potentiated by induction of tertiary lymphoid
structures in mice. Cell Mol Gastroenter. 12:1543–1565.
2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang Y, Xu M, Ren Y, Ba Y, Liu S, Zuo A,
Xu H, Weng S, Han X and Liu Z: Tertiary lymphoid structural
heterogeneity determines tumour immunity and prospects for clinical
application. Mol Cancer. 23(75)2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lin Z, Huang L, Li S, Gu J, Cui X and Zhou
Y: Pan-cancer analysis of genomic properties and clinical outcome
associated with tumor tertiary lymphoid structure. Sci Rep.
10(21530)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cillo AR, Kurten CHL, Tabib T, Qi Z, Onkar
S, Wang T, Liu A, Duvvuri U, Kim S, Soose RJ, et al: Immune
landscape of viral- and carcinogen-driven head and neck cancer.
Immunity. 52:183–199.e9. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ruffin AT, Cillo AR, Tabib T, Liu A, Onkar
S, Kunning SR, Lampenfeld C, Atiya HI, Abecassis I, Kürten CHL, et
al: B cell signatures and tertiary lymphoid structures contribute
to outcome in head and neck squamous cell carcinoma. Nat Commun.
12(3349)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hu C, You W, Kong D, Huang Y, Lu J, Zhao
M, Jin Y, Peng R, Hua D, Kuang DM and Chen Y: Tertiary lymphoid
structure-associated B cells enhance
CXCL13+CD103+CD8+Trm cell response to PD-1
blockade in gastric cancer. Gastroenterology: Oct 27:
S0016-5085(23)05198-3, 2023 (Epub ahead of print).
|
|
50
|
Liu Z, Meng X, Tang X, Zou W and He Y:
Intratumoral tertiary lymphoid structures promote patient survival
and immunotherapy response in head neck squamous cell carcinoma.
Cancer Immunol Immun. 72:1505–1521. 2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Muralidharan S, Nikalje M, Subramaniam T,
Koshy JA, Koshy AV and Bangera D: A narrative review on oral
squamous cell carcinoma. J Pharm Bioallied Sci. 17 (Suppl
1):S204–S206. 2025.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hosseini TM, Park SJ and Guo T: The
mutational and microenvironmental landscape of cutaneous squamous
cell carcinoma: A review. Cancers (Basel). 16(2904)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bossi P, Resteghini C, Paielli N, Licitra
L, Pilotti S and Perrone F: Prognostic and predictive value of EGFR
in head and neck squamous cell carcinoma. Oncotarget.
7:74362–74379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Karabajakian A, Bouaoud J, Michon L, Kamal
M, Crozes C, Zrounba P, Auclair-Perossier J, Gadot N, Attignon V,
Le Tourneau C, et al: Longitudinal assessment of PD-L1 expression
and gene expression profiles in patients with head and neck cancer
reveals temporal heterogeneity. Oral Oncol.
119(105368)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Alessandrini L, Franz L, Ottaviano G, Ghi
MG, Lanza C, Blandamura S and Marioni G: Prognostic role of
programmed death ligand 1 (PD-L1) and the immune microenvironment
in laryngeal carcinoma. Oral Oncol. 108(104836)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li H, Zhang MJ, Zhang B, Lin WP, Li SJ,
Xiong D, Wang Q, Wang WD, Yang QC, Huang CF, et al: Mature tertiary
lymphoid structures evoke intra-tumoral T and B cell responses via
progenitor exhausted CD4+ T cells in head and neck
cancer. Nat Commun. 16(4228)2025.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang J, Zeng L, Song G, Peng G, Chen Z,
Yuan Y, Chen T, Zhong T, Chen S, Luo Z, et al: A novel tertiary
lymphoid structure-associated signature accurately predicts patient
prognosis and facilitates the selection of personalized treatment
strategies for HNSCC. Front Immunol. 16(1551844)2025.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lin X, Zhao X, Chen Y, Yang R, Dai Z, Li
W, Lin C and Cao W: CXC ligand 13 orchestrates an immunoactive
microenvironment and enhances immunotherapy response in head and
neck squamous cell carcinoma. Int J Immunopathol Pharmacol.
38(3946320241227312)2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Bao J, Betzler AC, Hess J and Brunner C:
Exploring the dual role of B cells in solid tumors: Implications
for head and neck squamous cell carcinoma. Front Immunol.
14(1233085)2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Xing A, Lv D, Wu C, Zhou K, Zhao T, Zhao
L, Wang H and Feng H: Tertiary lymphoid structures gene signature
predicts prognosis and immune infiltration analysis in head and
neck squamous cell carcinoma. Curr Genomics. 25:88–104.
2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hong S, Wang Q, Yu D, Zheng J, Huang P, Gu
J and Zhang Y: Tertiary lymphoid structures in head and neck
squamous cell carcinoma (Review). Oncol Rep. 54(76)2025.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gavrielatou N, Fortis E, Spathis A,
Anastasiou M, Economopoulou P, Foukas GRP, Lelegiannis IM,
Rusakiewicz S, Vathiotis I, Aung TN, et al: B-cell infiltration is
associated with survival outcomes following programmed cell death
protein 1 inhibition in head and neck squamous cell carcinoma. Ann
Oncol. 35:340–350. 2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wu F, Cao H, Ren S, Wu J, Liu X, Li Q, Xu
Q, Chen J, Wang R, Chen S, et al: Tertiary lymphoid
structure-related score as a predictor for survival prognosis and
immunotherapy response in head and neck squamous cell carcinoma.
Front Immunol. 15(1483497)2024.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Peng Y, Xiao L, Rong H, Ou Z, Cai T, Liu
N, Li B, Zhang L, Wu F, Lan T, et al: Single-cell profiling of
tumor-infiltrating TCF1/TCF7+ T cells reveals a T
lymphocyte subset associated with tertiary lymphoid
structures/organs and a superior prognosis in oral cancer. Oral
Oncol. 119(105348)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lechner A, Schlößer HA, Thelen M, Wennhold
K, Rothschild SI, Gilles R, Quaas A, Siefer OG, Huebbers CU,
Cukuroglu E, et al: Tumor-associated B cells and humoral immune
response in head and neck squamous cell carcinoma. Oncoimmunology.
8(1535293)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Secrier M, McGrath L, Ng F, Gulati S,
Raymond A, Nuttall BRB, Berthe J, Jones EV, Sidders BS, Galon J, et
al: Immune cell abundance and T-cell receptor landscapes suggest
new patient stratification strategies in head and neck squamous
cell carcinoma. Cancer Res Commun. 3:2133–2145. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Almangush A, Bello IO, Elseragy A,
Hagström J, Haglund C, Kowalski LP, Nieminen P, Coletta RD, Mäkitie
AA, Salo T and Leivo I: Tertiary lymphoid structures associate with
improved survival in early oral tongue cancer. BMC Cancer.
22(1108)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wang C, Huang Z, Zhang M, Xiong G, Chen X
and Xie N: Prognostic value of tertiary lymphoid structures in
early clinical stage oral tongue squamous cell carcinoma. J Oral
Pathol Med. 50:776–784. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Zhang L, Ren S, Lan T, Marco V, Liu N, Wei
B, Chen Y, Wu J, Li Q, Wu F, et al: Mature tertiary lymphoid
structures linked to HPV status and anti-PD-1 based
chemoimmunotherapy response in head and neck squamous cell
carcinoma. Oncoimmunology. 14(2528109)2025.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Li H, Zhu SW, Zhou JJ, Chen DR, Liu J, Wu
ZZ, Wang WY, Zhang MJ and Sun ZJ: Tertiary lymphoid structure
raises survival and immunotherapy in HPV-HNSCC. J Dent Res.
102:678–688. 2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Li Q, Liu X, Wang D, Wang Y, Lu H, Wen S,
Fang J, Cheng B and Wang Z: Prognostic value of tertiary lymphoid
structure and tumour infiltrating lymphocytes in oral squamous cell
carcinoma. Int J Oral Sci. 12(24)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Chang TG, Spathis A, Schäffer AA,
Gavrielatou N, Kuo F, Jia D, Mukherjee S, Sievers C, Economopoulou
P, Anastasiou M, et al: Tumor and blood B-cell abundance
outperforms established immune checkpoint blockade response
prediction signatures in head and neck cancer. Ann Oncol.
36:309–320. 2025.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Ning J, Hao J, Guo F, Hou X, Li L, Wang J,
Wang S, Gao Y, Zheng X and Gao M: ABCB11 accumulated in immature
tertiary lymphoid structures participates in xenobiotic metabolic
process and predicts resistance to PD-1/PD-L1 inhibitors in head
and neck squamous cell carcinoma. Transl Oncol.
36(101747)2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Zhu J, Lu H, Wang K, Liu B and Yan J:
Tertiary lymphoid structures in head and neck squamous cell
carcinoma. Transl Oncol. 44(101949)2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Weed DT, Zilio S, McGee C, Marnissi B,
Sargi Z, Franzmann E, Thomas G, Leibowitz J, Nicolli E, Arnold D,
et al: The tumor immune microenvironment architecture correlates
with risk of recurrence in head and neck squamous cell carcinoma.
Cancer Res. 83:3886–3900. 2023.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Shu DH, Ho WJ, Kagohara LT, Girgis A, Shin
SM, Danilova L, Lee JW, Sidiropoulos DN, Mitchell S, Munjal K, et
al: Immunotherapy response induces divergent tertiary lymphoid
structure morphologies in hepatocellular carcinoma. Nat Immunol.
25:2110–2123. 2024.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Kroeger DR, Milne K and Nelson BH:
Tumor-infiltrating plasma cells are associated with tertiary
lymphoid structures, cytolytic T-cell responses, and superior
prognosis in ovarian cancer. Clin Cancer Res. 22:3005–3015.
2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Wang M, Zhai R, Wang M, Zhu W, Zhang J, Yu
M, Zhang W, Ye J and Liu L: Tertiary lymphoid structures in head
and neck squamous cell carcinoma improve prognosis by recruiting
CD8+ T cells. Mol Oncol. 17:1514–1530. 2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Xian S, Dosset M, Castro A, Carter H and
Zanetti M: Transcriptional analysis links B cells and TERT
expression to favorable prognosis in head and neck cancer. PNAS
Nexus. 2(pgad046)2023.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Shen S, Cui Y, Li M, Yu K, Zhu Q, Zhang X,
Shen W, Li H, Jiang H, Li M, et al: Toll-like receptor agonists
promote the formation of tertiary lymphoid structure and improve
anti-glioma immunity. Neuro Oncol. 27:140–154. 2025.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Chelvanambi M, Fecek RJ, Taylor JL and
Storkus WJ: STING agonist-based treatment promotes vascular
normalization and tertiary lymphoid structure formation in the
therapeutic melanoma microenvironment. J Immunother Cancer.
9(e001906)2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Sweeney KJ, Tetzlaff MT, Vega F,
Gillenwater A, Zuo Z, Gross N, Nagarajan P, Wargo J, Nelson K,
Prieto VG, et al: Tertiary lymphoid structures with overlapping
histopathologic features of cutaneous marginal zone lymphoma during
neoadjuvant cemiplimab therapy are associated with antitumor
response. J Cutan Pathol. 48:674–679. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Wang Q, Sun K, Liu R, Song Y, Lv Y, Bi P,
Yang F, Li S, Zhao J, Li X, et al: Single-cell transcriptome
sequencing of B-cell heterogeneity and tertiary lymphoid structure
predicts breast cancer prognosis and neoadjuvant therapy efficacy.
Clin Transl Med. 13(e1346)2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Fabian KP, Santiago-Sanchez G, Padget MR,
Lassoued W, Allen CT, Battula S, Kaufman H and Hodge JW:
Alum-anchored IL-12 combined with cytotoxic chemotherapy and immune
checkpoint blockade enhanced antitumor immune responses in head and
neck cancer models. J Immunother Cancer. 12(e009712)2024.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Clubb JHA, Kudling TV, Heiniö C, Basnet S,
Pakola S, Cervera Carrascón V, Santos JM, Quixabeira DCA, Havunen
R, Sorsa S, et al: Adenovirus Encoding tumor necrosis factor alpha
and interleukin 2 induces a tertiary lymphoid structure signature
in immune checkpoint inhibitor refractory head and neck cancer.
Front Immunol. 13(794251)2022.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ruiz-Torres DA, Wise JF, Zhao BY,
Oliveira-Costa JP, Cavallaro S, Sadow PM, Fang J, Yilmaz O, Patel
A, Loosbroock C, et al: Dendritic cell effector mechanisms and
tumor immune microenvironment infiltration define TLR8 modulation
and PD-1 blockade. Front Immunol. 15(1440530)2024.PubMed/NCBI View Article : Google Scholar
|