1. Introduction
The current pneumonia pandemic outbreak caused by
severe acute respiratory syndrome (SARS) coronavirus 2
(SARS-CoV-2), originated from Wuhan (China) in December, 2019 and
since then, has spread worldwide, with a basic reproduction number
(R0) of 2-2.5 (1-3).
The majority (64%) of cases have been between the ages of 25-64
years and as regards sex, ~47% of cases have been female (4,5).
According to these current statistics, >50% of patients with
COVID-19 belong to the reproductive age group. However, limited
data are available for pregnant women. Moreover, there are a number
of unanswered questions regarding the impact of COVID-19 on
pregnancy, such as its association with complications during
pregnancy, the management of infected pregnant women, vertical
maternal-fetal transmission and the effects of COVID-9 postpartum
(6,7). The present review summarizes the
current data related to these prevailing questions, and also
provides information on pregnancy outcomes associated with related
and highly pathogenic coronaviruses, namely SARS and Middle East
respiratory syndrome coronavirus (MERS-CoV)]. The authors elected
to perform a narrative review approach as opposed to systematic
one, as this was considered more appropriate regarding the
relatively recent pandemic outbreak. The authors also present their
own experience from the University General Hospital of
Alexandroupolis.
2. Comparison of SARS-CoV-2, SARS-CoV and
MERS-CoV in terms of genome, transmission and incubation
period
Coronaviruses belong to the Coronoviridae
family of the order Nidovirales and constitute the
Orthocoronavirinae subfamily. They are divided into four
genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus and
Deltacoronavirus. SARS-CoV, MERS-CoV and SARS-CoV-2 all belong to
the Betacoronavirus genera (8).
Coronaviruses are enveloped, spherical viruses with
a crown-like appearance under an electron microscope, as a result
of the spike glycoproteins on the virion surface. Their genome is
organized in a positive-sense, single-stranded RNA of ~30 kb in
size (9,10). Unlike the majority of eukaryotic
mRNAs, coronavirus genomes are very large and contain multiple open
reading frames (ORFs), a 5' cap structure and a 3' poly (A) tail.
At the 5' end, there is a leading sequence followed by the 5'
untranslated region (5'UTR) and the initiation codon for ORF1,
which encodes a number of non-structural proteins important for
replication, and accounts for the two-thirds of the whole genome.
The main structural proteins, spike (S), envelope (E), membrane (M)
and nucleocapsid (N), as well as the accessory proteins, are
encoded within the one-third of the genome near the 3'end (11,12).
As regards the structural proteins, S,E,M and N, all
are involved in the formation of the viral particle, but also seem
to play a role in other aspects of the replication cycle. Spike
protein (S) consists of a transmembrane domain (TM), a short
intracellular C-terminal segment and a large N-terminal segment
with two subunits (S1 and S2), which are responsible for receptor
binding and cell-to-cell fusion, respectively (13,14). The
N protein binds the RNA of the virus and seems to play a critical
role in the transcription and translation of the virus (13). The M protein is involved in viral
assembly and, along with the E protein, mediates the envelop
construction and viral budding (13-16).
The genome size of SARS-CoV-2 is ~29.9 kb similar to
the 29.75 and 30.11 kb genomes of SARS-CoV and MERS-CoV,
respectively. Previous studies have confirmed that SARS-CoV-2
shares a 79.5% sequence identity with SARS-CoV, which are both
lineage B Betacoronaviruses, whereas it only has 50% similarity
with MERS-CoV, a lineage C Betacoronavirus, indicating a closer
association between SARS-CoV-2 and SARS-CoV (9-16).
When comparing the genome organization of these two viruses, the
main differences are found in ORF3b, ORF8 and spike S1(16). In detail, there is a major difference
in the length of the ORF3b between the two viruses. SARS-CoV-2 with
a longer ORF3b appears to have a greater ability to suppress
interferon (IFN) activity (17). As
regards ORF8b, an accessory protein which appears to be poorly
conserved among coronaviruses, Shi et al (18) indicated that SARS-CoV ORF8Bb can
activate the NLR family pyrin domain containing 3 (NLRP3)
inflammasome and trigger stress pathways, whereas SARS-CoV-2 ORF8
does not yet contain a known functional domain (16-18).
As already mentioned, Spike protein mediates the entry of the virus
into host cells. The S1 subunit includes the receptor binding
domain (RBD), which is responsible for binding to the host cell
receptor. The host cell receptor for MERS-CoV is dipeptyl peptidase
4 (DPP4 or CD26), while the receptor for both SARS-CoV and
SARS-CoV-2 is angiotensin-converting enzyme 2 (ACE2), a finding
supported by the high homology rate between their S proteins. After
binding to their receptors, S proteins undergo cleavage by
proteases of the host to permit fusion and both SARS-CoV and
SARS-CoV-2 have been shown to mainly use the serine protease,
transmembrane serine protease 2 (TMPRSS2) and the cysteine
proteases, cathepsin B and L (9).
Despite the similarities with SARS-CoV, a distinctive
characteristic of SARS-CoV-2 is the furin cleavage site (motif
RRxR) at the S1-S2 boundary that may contribute to high affinity
binding with the host cell receptor, leading to efficient
infection, overcoming the species barrier and high transmissibility
from human-to-human. Although MERS-CoV has a similar motif (RxxR)
at the S1-S2 boundary, the insertion of the second arginine in
SARS-CoV-2 S1-S2 site appears to enable a more sufficient cleavage
from furin-like enzymes (19-22).
Transmissibility, the ability of a pathogen to
spread, can be measured by the basic reproductive number
(R0), which represents the cases directly generated by
one infected individual in a population where all individuals are
susceptible to infection. The average R0 for SARS-CoV-2
is estimated to be 2.5, higher than the R0 of SARS-CoV
and MERS-CoV. Specifically, the R0 of SARS-CoV was
estimated to be 2-3 before and 1.1 following the introduction of
the public measures in 2003, while for MERS-CoV, the R0
was <1 (9-22).
SARS-CoV-2 mainly causes cluster transmission between members of a
family, while the human-to-human transmission of SARS-CoV and
MERS-CoV mostly occurs through nosocomial transmission and only at
a rate of 22-39 and 13-21% between family members, respectively
(22,23). This may be due to the fact that the
SARS-CoV and MERS-CoV viral load peaks occur after first week of
illness, in contrast to SARS-CoV-2, whose viral load peaks during
the first week (24). SARS-CoV can
be transmitted by direct person-to-person contact through air
droplets and indirect contact through contaminated surfaces, while
previous studies have indicated airborne transmission as well
(9-25).
MERS-CoV can be acquired through contact with both infected
dromedary camels, which serve as a host reservoir for the virus and
occasionally by infected patients (9). In addition, MERS-CoV has been isolated
from environmental objects, mainly in healthcare facilities,
underlying the risk of fomite transmission (26). Evidence ofthe fecal excretion of both
SARS-CoV and MERS-CoV, as long as prolonged viability permits under
various conditions, indicates the fecal-oral transmission as a
possible route for viral transmission (27).
Similarly, the main pathway of transmission of
SARS-CoV-2 is direct human-to-human transmission through contact
routes or air droplets. Direct droplet transmission will occur when
the uninfected individuals closer than 1 m to the infected
individual, as droplets can travel a distance of ~6 ft (28). Furthermore, studies have demonstrated
that SARS-CoV-2 can remain viable for a long period of time in the
environment, thus suggesting other methods of transmission similar
to SARS-CoV, such asairborne transmission or fomite transmission
(29,30). However, the greater affinity of
SARS-CoV-2 compared with that ofSARS-CoV for the ACE receptor, as
aforementioned, not only enables SARS-CoV-2 to be a much more
virulent virus, but justifies its potential spread via the
fecal-oral route, as regards the expression of ACE receptor in the
intestine (23-30).
There is evidence demonstrating detectable RNA levels in both feces
and blood, increasing the possibility of fecal-oral and blood
transmission (31). Additionally,
there is evidence of the transplacental transmission of SARS-CoV-2
during the last weeks of pregnancy, causing placental inflammation;
however, further evidence needs tobe provided on how the virus can
be transmitted from the mother to the fetus (32). Transmission via ocular surfaces
should not be ignored, since there is evidence of SARS-CoV
predominantly being transmitted through contact with mucous
membranes and incidents of patients exhibiting conjunctivitis prior
to the onset of COVID-19 (31-33).
3. Incubation period
A previous systematic review of acute respiratory
viral infections demonstrated that the median incubation period of
SARS-associated coronaviruses was 4 days [95% confidence interval
(CI), 3.6-4.4] (34). As regards the
median incubation period of MERS-CoV, Memish et al (35) indicated that although this was 5.2
days (95% CI, 1.9-14.7), a longer incubation time is likely to be
observed among immunocompromised patients. Although the incubation
period of SARS-CoV-2 has not yet been established, it has been
indicated that there is no significant difference between the
incubation times of the three coronaviruses (36). In a previous study, from an analysis
that included data from 181 cases with confirmed COVID-19 infection
outside Hubei province, it was estimated that the median incubation
time was 5.1 days (95% CI, 4.5-5.8 days) (37). Another meta-analysis examining
published results between January 24 and April 2, 2020 revealed
that the average incubation time was 5.08 days (95% CI, 4.77-5.39)
(38). An additional meta-analysis
on 22,595 patients indicated that the overall pooled average
incubation period was 5.7 days (95% CI, 5.1-6.4), which is slightly
higher than that of SARS-CoV and MERS-CoV. The same analysis
estimated a longer incubation period of up to 6.1 days (95% CI,
5.34-6.94) of SARS-CoV-2 in China compared with other countries
(39). The indicative
epidemiological characteristics of each coronavirus are shown in
Table I.
 | Table IEpidemiological characteristics of
SARS-CoV-2, SARS-CoV and MERS-CoV. |
Table I
Epidemiological characteristics of
SARS-CoV-2, SARS-CoV and MERS-CoV.
| Characteristic | SARS-CoV-2 | SARS-CoV | MERS-CoV |
|---|
| Genome size
(kb) | 29.9 | 29.75 | 30.11 |
| Family |
Coronaviridae |
Coronaviridae |
Coronaviridae |
| Genus |
Betacoronavirus |
Betacoronavirus |
Betacoronavirus |
| Lineage | B | B | C |
| Host cell
receptor | ACE2 | ACE2 | DPP4 |
| Furin cleavage
site | Yes (RRxR) | No | Yes (RxxR) |
| Viral load
peak | At the first week
of illness | After the first
week of illness | After the first
week of illness |
| R0 | 2.5 | 2-3 | <1 |
| Transmission
scenario | Mainly cluster
transmission | Mainly nosocomial
transmission | Mainly nosocomial
transmission |
| Human-to-human
transmission | Through air
droplets, contact routes, fomites and possible through airborne and
maternal-fetal transmission | Through air
droplets, fomites, airborne and fecal-oral transmission | Occasionally
through air droplets, contact routes and fomites |
| Median incubation
period | Current estimates
indicate5-6 days | 4 days (95% CI,
3.6-4.4)a | 5.2 days (95% CI,
1.9-14.7)a |
4. Diagnostic tests
Sensitivity, specificity and accuracy determine the
reliability of a diagnostic test (40). There are two main categories of
diagnostic tests for COVID-19; the nucleotide acid-based methods,
which include reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and antibody-based methods, such as
enzyme-linked immunosorbent assay (ELISA), colloidal gold
immunochromatographic assay (GICA) and chemiluminescence
immunoassay (CLIA) (41).
RT-qPCR remains the gold standard for the diagnosis
of COVID-19 using mainly nasopharyngeal swabs and sputum, as well
as other upper respiratory tract specimens, such as oropharyngeal
swabs and saliva (42). The need for
the rapid diagnosis of COVID-19 led to the development of other
promising technologies for DNA amplification, such as reverse
transcription loop-mediated isothermal amplification (RT-LAMP) and
clustered regularly interspaced short palindromic repeats
(CRISPR)-based methods which are faster, cost-effective
technologies and appear to have a high sensitivity and specificity
as well (40-49).
The limitations in nucleotide acid-based methods, such as the long
turnaround time and the specific equipment needed, as well as the
number of false-negative results, led to the supporting role of
antibody-based methods for the detection of SARS-CoV-2. The
efficiency and simplicity of ELISA and CLIA render these methods
suitable for first-line screening. The accuracy of various tests
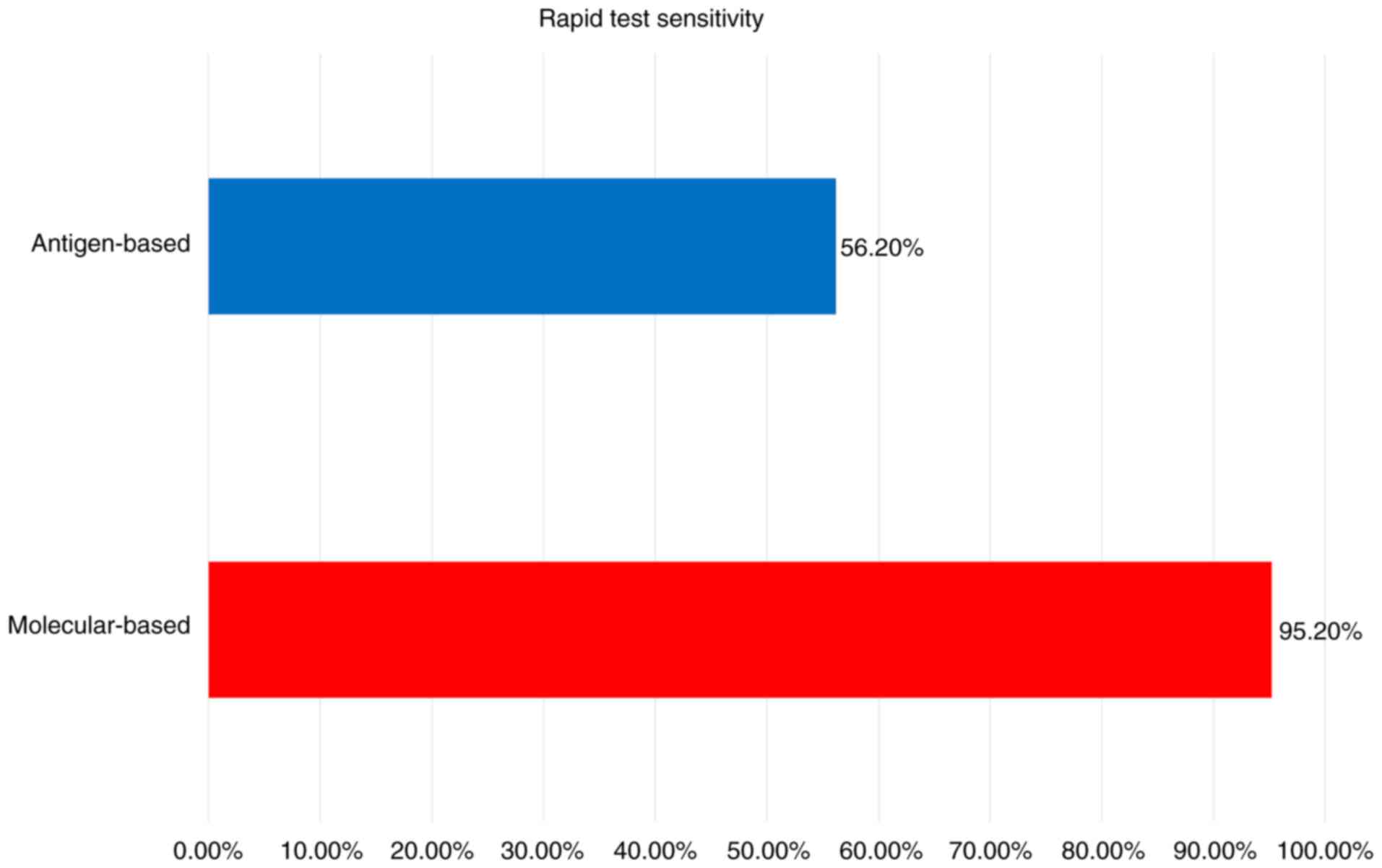
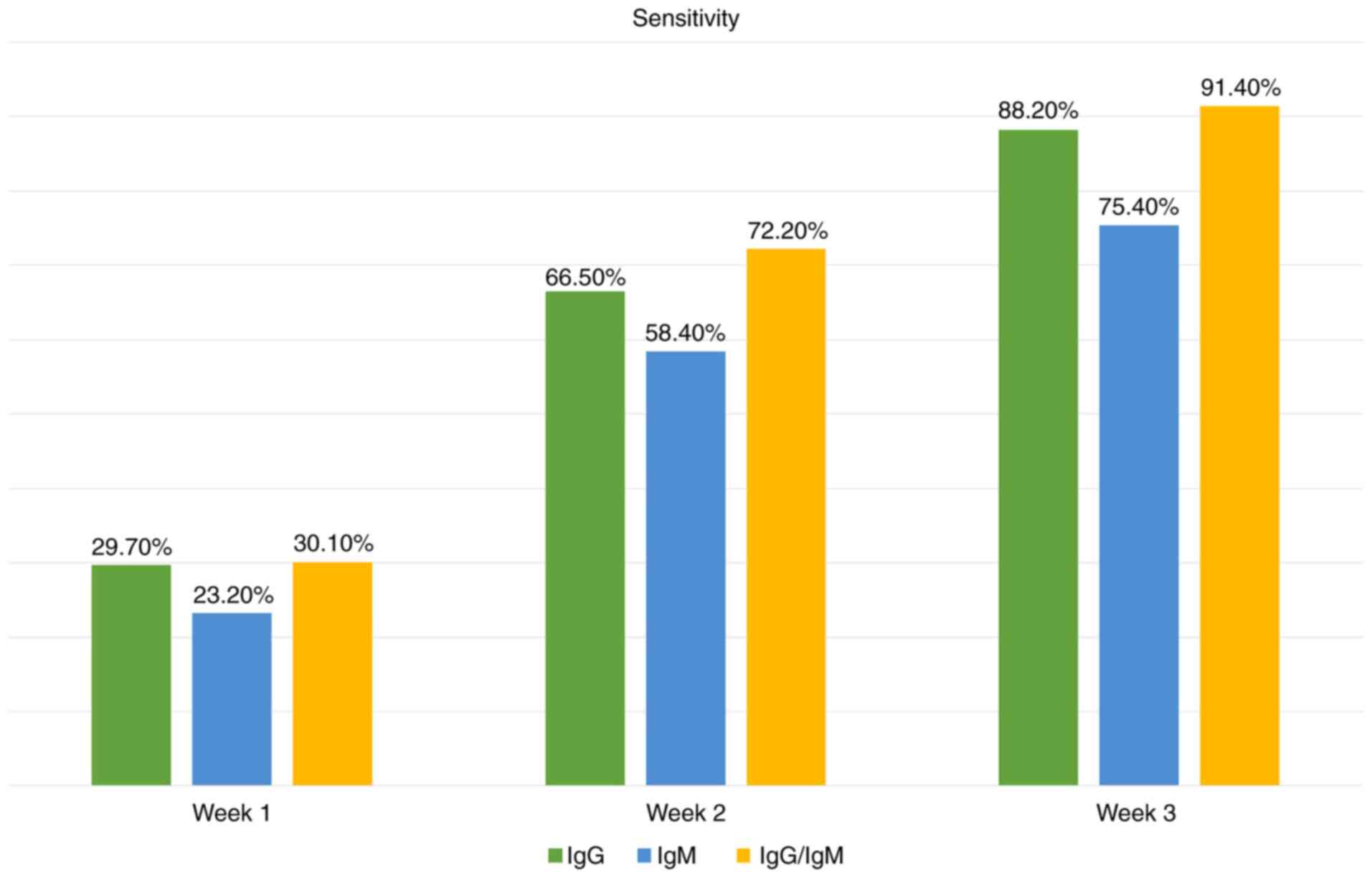
for the detection of SARS-CoV-2 is presented in Figs. 1 and 2, and Table
II (40-49).
 | Table IIAccuracy of diagnostic techniques for
the diagnosis of SARS-CoV-2 infection. |
Table II
Accuracy of diagnostic techniques for
the diagnosis of SARS-CoV-2 infection.
| Diagnostic test
(Refs.) Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) (46) | Sensitivity (Refs.)
73.3-97.2% (respiratory tract samples) 0-24.1% (other specimens)
60.2-97.9% (PCR protocol) (44) | Specificity (Refs.)
90-100% (depending on sample type) (44) |
|---|
| Antibody tests
(126) | 1st week | 2nd week | 3rd week | |
| IgG | 29.7% (95% CI,
22.1-38.6%) | 66.5% (95% CI,
57.9-74.2%) | 88.2% (95% CI,
83.5-91.8%) | 99.1% (95% CI,
98.3-99.6%) |
| IgM | 23.2% (95% CI,
14.9-34.2%) | 58.4% (95% CI,
45.5-70.3%) | 75.4% (95% CI,
64.3-83.8%) | 98.7% (95% CI,
97.4-99.3%) |
| IgG/IgM | 30.1% (95% CI,
21.4-40.7%) | 72.2% (95% CI,
63.5-79.5) | 91.4% (95% CI,
87-94.4%) | 98.7% (95% CI,
97.2-99.4%) |
| Rapid tests
(125) | | | | |
| Molecular-based
tests (126) | 95.2% (95% CI,
86.7-98.3%) | 98.9% (95% CI,
97.3-99.5%) |
| Antigen-based tests
(126) | 56.2% (95% CI,
29.5-79.8%) | 99.5% (95% CI,
98.1-99.9%) |
5. Immunology of pregnancy and COVID-19
Pregnant women during this pandemic represent a
susceptible group due to their altered physiological physiology,
immunology and pathology mechanisms, not only pre-but also
post-partum. Pregnancy alters the immunological environment,
initially to a Th1 phenotype during the first trimester, and then
to Th2 by the second trimester, and finally again polarizes toward
to a Th1 phenotype at the end of third trimester. Concomitant with
the initiation of parturition, cytokines produced by Th1
lymphocytes are pro-inflammatory and microbicidal, and consist by
interferon-γ and certain interleukin (IL) proteins (IL-1a, IL-1b,
IL-6 and IL-12). By contrast, Th2 cytokines are anti-inflammatory
and include IL-4, IL-10 and IL-13, and transforming growth factor
(TGF)-b (50). On account of this
normal shift to a Th2-dominant immune system which protects the
fetus, the debility of Th1 renders the mother vulnerable to viral
infections, which are more effectively contained by the Th1 system
(50). The contributions of the
immune system to pregnancy and fetal development provide important
insight into the pathogenesis underlying the infection of pregnant
women with COVID-19, as well as into possible targets for therapy.
In non-pregnant patients infected with SARS-Cov-2, the activation
of both Th1 and Th2 immunity has been observed, culminating in the
presence of IFN-γ and IL-1b, in addition to IL-4 and IL-10
(44,45). In addition, in plasma from
COVID-19-positive patients, elevated levels of IL-6 (a
predominantly Th1 response cytokine) are associated with an
increased risk of mortality and severe pneumonia (44). Thus, this physical shift to a
Th2-dominant immunology environment and the lack of Th1, which
affects the period from week 13 to 27 of pregnancy, suggests that
these early adaptive immune responses may be predictive of milder
disease severity in pregnant women. Indeed, the intense
inflammatory response has been reported as the cause of severe
COVID-19 infection (7,44). Therefore, the relative
immunosuppression in pregnancy may be the reason as to why numerous
pregnant women do not develop severe respiratory syndrome. Contrary
to this theory, the American College of Obstetricians and
Gynecologists (5,6) made a comparison between pregnant and
non-pregnant women with COVID-19 and concluded that pregnant women
are at an increased risk of developing severe symptomatology. Thus,
they have a higher possibility of being admitted to the intensive
care unit (ICU; 1.5 vs. 0.9%) and may also have a greater need for
mechanical ventilation (0.5 vs. 0.3%). The vulnerability of
pregnant women to COVID-19 has also been supported by Dashraath
et al, who also reported similar findings (45). Namely, they observed symptoms similar
to non-pregnant patients; the predominant symptoms of COVID-19 in
pregnant patients were fever (prevailing symptom), cough, dyspnea
and lymphopenia and they also reported of other cohort studies in
patients with other infections where no increased risks of
congenital anomalies have been shown. However, the reported
miscarriage, intrauterine growth restriction and pre-term birth as
fetal complications associated with COVID-19, appear to be less
severe compared with those associated with SARS and MERS (46-49,51-59).
6. First trimester: Early pregnancy
Placental development during the 1st trimester is
crucial, due to vulnerability to a number of pre- and post-partum
complications (46,60-67).
This observation has been extensively investigated by a notable
number of research groups, as immunological disturbances are
crucial in mediating a successful pregnancy, from implantation to
parturition. One such example of the importance of the first
trimester, is maternal pyrexia, a disorder that is related to
hyperthermic injury to fetal neurons (67). There is a theoretical risk of
complications, similar to that observed in SARS, as the ACE2
receptor is widely expressed in the placenta (45-47)
with a similar receptor-binding domain structure between SARS-CoV
and SARS-CoV-2. This also sets the hypothesis of vertical
transmission (68). In line with
previous research (69), SARS-CoV
and SARS-CoV-2 share a common binding structure genotype. Crucial
data on maternal and fetal outcomes could be raised by the
histopathological examination of the placenta in order to confirm
COVID-19 binding to placental ACE-2 receptors. Samples of placentas
positive for SARS-CoV-2 have exhibited anincreased deposition of
immune complexes, such as fibrin and lymphocytes. In particular, in
the subchorial space,the infiltration of monocytes and neutrophils
has been identified (48). Placental
and umbilical cord infiltration was also identified with
virological findings in nine cases of women infected with
SARS-CoV-2 during the first trimester of gestation, where severe
hypertension, pre-eclampsia and coagulopathy was reported (52). Evidence of placental hyperfusion
defects in maternal vessels and oxygenation in the intervillous
space has also been observed, affecting perinatal outcomes. All
these thus far suggest pre-eclampsia, where typically, vascular
villous lesions including fibrin deposition within and around the
villi and infarcts have been reported. The aforementioned placental
pathology associated with SARS-CoV-2 infection, has also been
reported in placentas from SARS-CoV-2 and MERS-positive patients,
where placental infiltration has been shown to lead to acute and
chronic placental insufficiency associated with intrauterine growth
restriction or the miscarriage of affected cases during pregnancy
and pre-term delivery (67,70). Therefore, in order to predict the
possible COVID-19-related complications during the first trimester,
examining the placental virological findings is of utmost
importance. Taking into consideration that SARS affects 4/7
pregnant women in first trimester, leading to miscarriage, and at
the same time the common genotypes between SARS-CoV and SARS-CoV-2,
the need for the provision of fetal monitoring, including a serial
ultrasound examination, of women with COVID-19 may be of utmost
significance (45).
7. Second and third trimester: Late
pregnancy
As observed from the SARS data and from the
available SARS-CoV-2 data, there is an increased risk of pregnant
women being infected with COVID-19 (55-57,64).
Early pregnancy data and matched control data on affected pregnant
women are required to estimate the course of infection over the
following trimesters. An infected placenta, as supported by
virological findings from women (52) with ongoing pregnancy, leads to an
acute and chronic pathology, particularly in the placenta and
umbilical cord, and aggravates the respiratory system due to
increased maternal oxygen demands from the heightened metabolism;
thus, COVID-19 infection could lead to physiological dyspnea.
Dyspnea and shortness of breath accompanied by fever are the
commonest symptoms in pregnant women (gestational age, >25
weeks). Oftentimes, it is challenging to differentiate between
physiological dyspnea in pregnancy from COVID-19-related symptoms
(70-74).
As with non-pregnant women, pregnant women infected with SARS-CoV-2
present an extended range of symptoms, from mild to severe, upon
admission to the hospital; these include pneumonia with or without
acute respiratory distress syndrome (ARDS), renal failure and
multi-organ dysfunction. A significant rate of asymptomatic and
moderately-infected pregnant woman with COVID-19 has also been
reported, while 16% of pregnant women have been found to have no
symptoms at all (64). Notably,
asymptomatic pregnant women are not at a high risk of developing
severe morbidity and mortality from COVID-19 infection. However,
changes to the cardiorespiratory and immune systems during
pregnancy increase the susceptibility of a woman to severe
infection and hypoxic compromise. Indeed, some studies have
reported clinical cases that confirm this outcome and concern that
pregnant women may be more susceptible to COVID-19 infection. The
state of compensated respiratory alkalosis with metabolic acidosis
renders women vulnerable to respiratory diseases, such as COVID-19.
Ronnje et al (65) reported
the case of a 26-year-old woman who was 32 weeks pregnant, who
presented with a nine-day history of typical COVID-19 symptoms,
such as fever, shortness of breath, dry cough, myalgia and
abdominal pain. Her clinical condition escalated rapidly, leading
to liver and coagulation malfunction. Recovery was observed
following a cesarean section, which was performed as an emergency
in order to improve the clinical status of the woman. The study by
Shanes et al (66) reported
that the placental pathological findings from 16
SARS-CoV-2-positive pregnant women revealed a hypercoagulable state
and intense systemic inflammation. In another systemic review, in
which data from 1,316 pregnant women were included, it was
concluded that pneumonia was the most common COVID-19 disease
manifestation, with bilateral infiltration and ground-glass opacity
as the main findings in the computed tomography scan (69). These are also the main radiological
findings according to Wu et al (58). The clinical status of pneumonia in
women infected with SARS-COV-2 is followed by pre-term birth,
miscarriage, fetal growth restriction and pre-eclampsia, while 1/3
women are admitted to the ICU (51).
COVID-19 associated with respiratory insufficiency in late
pregnancies certainly creates a complex clinical scenario.
Pregnancy causes a reduction in pulmonary and end-expiratory
volume, functional residual capacity and residual volume due to
diaphragmatic splinting by the gravid uterus, leading to decreased
total lung capacity at term and an inability for the effective
clearance of pulmonary secretions. As COVID-19 pneumonia escalates
from focal to the diffuse bilateral consolidation of lung
parenchyma, pregnant women are more vulnerable to hypoxemic
respiratory failure, considering the pulmonary changes described
above (75). Furthermore, both
venous and arterial thromboembolism are prevalent through the
second and third trimester in pregnant women diagnosed with
COVID-19, not only due to excessive inflammation and hypoxia, but
also as a result of diffuse intravascular coagulation. Considering
that only 1% of women in the third trimester have normal d-dimer
levels based on conventional thresholds, such pregnant patients may
be at a high risk of developing thrombosis and thromboembolic
disorders when infected with COVID-19 (50,76-79).
Thrombocytopenia and liver function abnormalities (65), both of which are complications of
COVID-19, are also associated with pre-eclampsia with severe
symptoms. Ramlakhan et al (53) also described a rapidly worsening
maternal status with the ultimate diagnosis of cardiomyopathy in
pregnant women with certain risk factors, such as obesity and an
advanced maternal age. The aforementioned studies reported higher
risks of pregnancy-related complications, including pre-eclampsia
and pre-term delivery as the main outcomes, in normal and high-risk
pregnancies. Of note, higher rates of cesarean delivery have been
reported, while as it has been suggested, the cesarean section
improves the clinical status of woman with complications
exacerbated by COVID-19. It would appear that a number of the
cesarean section procedures were performed for the mother's best
interests, due to concern for maternal respiratory function
(61,62).
8. Obstetric and postpartum management
Thus far, pregnant patients with COVID-19 have
almost invariably delivered their babies by cesarean section and
frequently before term gestation. However, authorities and
professional societies, such as the Italian Health Council, the
English Royal College of Obstetricians and Gynaecologists, and the
Society for Maternal-Fetal Medicine, have taken a stance that
COVID-19 is not a contraindication to vaginal delivery. According
to recent data from an Italian study, a vaginal delivery rate of
57.1% was reported (64), while
these high rates of cesarean section do not seem to be
representative of women who have mild to moderate disease.
Obstetric surgery is an indication for pregnant women with
pneumonia due to COVID-19 infection, while it is beneficial to the
rehabilitation of maternal respiratory malfunction (70-73,80-96).
A previous retrospective analysis in Wuhan during the COVID-19
pandemic performed by Liao et al (97) revealed no significant difference in
postpartum hemorrhage and perineal resection rates between
SARS-CoV-2-positive and -negative pregnant women. In the same
study, no evidence of vertical transmission was reported, while all
neonates delivered by pregnant women infected with SARS-Cov-2
tested negative for the infection (97). However, to date, to the best of our
knowledge, there are no data available to support the
recommendation of either vaginal delivery or cesarean section in
order to reduce the risk of transmission to the neonate (61,70).
Diabetic patients are associated with a higher intrapartum risk of
transmission (51-54).
With reference to breastfeeding, in a mother with COVID-19, the
close contact between the mother and the infant appears to be the
main risk factor of transmission through infective airborne
droplets (81-82,94-96).
Breast milk has not been found to exhibit any viral presence
(47-49,59)
and according to current data, the potential risks of transmission
of the virus through breast milk do not outweigh the benefits of
breastfeeding. However, wearing a facemask while breastfeeding and
applying strict hand hygiene before touching the infant is
required. Hitherto, human milk is not considered a vehicle of
COVID-19 transmission; thus, there is no need for its
pasteurization and it can be also given to the baby using a breast
pump, thus avoiding physical contact in the event of the COVID-19
infection of the mother (82,83,94).
Undoubtedly, the separation between mother and neonate combined
with social isolation increases levels of anxiety and depression
(98-109).
To control the risk of developing postpartum depression, healthcare
providers should pay closer attention to maternal mental health by
providing sufficient supporting services (110-112).
SARS-CoV-2 is a highly contagious virus,
particularly the Brazilian and South African mutations. Governments
and the scientific community are facing a challenge as data on
COVID-19 are still accumulating and for specific groups, such as
immunocompromised individuals, the available data remain
insufficient.
9. The authors' experience
According to preliminary results obtained by the
authors, during the period from June, 2020 until January, 2021, 14
cases of pregnant women infected with COVID-19 noted. The women had
mild symptoms and diagnosis was performed based on RT-qPCR tests of
nasopharyngeal swabs during the third trimester. The
clinicopathological data for all patient samples used in the
present study are provided in Table
III. The pregnant women who participated in the study provided
written informed consent in the majority of cases, and oral consent
in emergency cases, indicating that they agreed to the use of their
samples for scientific research. Participants provide a document
proving that patient consent for participation was obtained at the
time of the study. Ethical approval for the study was provided by
the Ethics Committee of the University Hospital in Alexandroupolis,
Democritus University of Thrace (Alexandroupolis, Greece; reference
no. 42398/07/10/21). For the procedure of RT-qPCR, 8 µl from each
RNA extraction sample was used and the master mix buffer was
processed by mixing 10 µl from OneStep 2X RT-qPCR Master Mix buffer
and 2 µl from COVID-19 Primer & Probe Mix oasig™ buffer in
accordance to the Primerdesign coronavirus COVID-19
genesig® Real-Time PCR assay kit (Varelas S.A.). The
oligonucleotide primers and probe for the detection of SARS-CoV-2
were selected from the orf1 ab genome region (the exact sequence of
primers is a proprietary right of the company). As a result, a
total volume of 20 µl was used per reaction and 45 cycles of
RT-qualitative PCR reaction were applied.
 | Table IIIPlacental pathological and clinical
findings in COVID-19-positive mothers. |
Table III
Placental pathological and clinical
findings in COVID-19-positive mothers.
| No. | Pathological
findings | Case
no.a | Clinical
findings | Labor modus |
|---|
| 1 | Maternal vascular
malperfusion | 9/14 | Moderate fever,
mild cough | Spontaneous
delivery |
| 2 | Decidual
arteriopathy/atherosis | 8/14 | Moderate fever,
mild cough | Cesarean
section |
| 3 | Fibrinoid
necrosis | 11/14 | Moderate fever,
mild cough | Cesarean
section |
| 4 | Mural hypertrophy
of the arterioles of the membranes | 8/14 | Moderate fever,
mild cough | Spontaneous
delivery |
| 5 |
Oligohydramnios | 5/14 | Moderate fever,
mild cough | Cesarean
section |
| 6 | Massive chronic
histolytic intervillositis | 6/14 | Moderate fever,
mild cough | Cesarean
section |
| 7 | Deciduitis | 14/14 | Moderate fever,
mild cough | Spontaneous
delivery |
| 8 | Delayed chronic
villous maturation | 10/14 | Moderate fever,
mild cough | Cesarean
section |
| 9 | Vascular
thrombosis | 12/14 | Moderate fever,
mild cough | Cesarean
section |
| 10 | Acute
chorioamnionitis | 13/14 | Moderate fever,
mild cough | Cesarean
section |
| 11 | Acute
funisitis | 12/14 | Moderate fever,
mild cough | Cesarean
section |
| 12 | Massive fibrin
deposition | 13/14 | Moderate fever,
mild cough | Cesarean
section |
| 13 | Intramural fibrin
deposition | 12/14 | Moderate fever,
mild cough | Spontaneous
delivery |
| 14 | Avascular
villi | 8/14 | Moderate fever,
mild cough | Cesarean
section |
The RT-qPCR method is based on TaqMan technology by
detecting two fluorophores the FAM and HEX fluorophore. The FAM
marked probe is designed for a specific region on Sars-CoV 2 RNA
and the HEX marked probe is designed for the IC, an artificial gene
template. The probes are labeled with fluorescent reporter and
quencher dyes so as their hybridization on the sequence of interest
report us the existence of the desired sequence.
The results at the end of this procedure were
validated as positives, when signal in FAM and HEX channel was
reported. Otherwise, when the signal was detected in HEX channel
only, the results were considered negatives. For the validation of
this procedure a positive marker was used.
Statistical analysis methods (e.g.,
2-ΔΔCq) were not feasible herein (due to the small
sampling of participants). In four cases, spontaneous delivery
occurred and in the remaining cases, cesarean section was
performed. According to the literature, cesarean section should be
reserved for obstetrical indications (113-117).
No case of neonatal infection with SARS-COV-2 has yet been
documented in Greece, at least to the best of our knowledge.
Concerning placental pathology in SARS-CoV-2 infection, the most
common findings observed in the cases herein were an increased rate
of maternal vascular malperfusion, most commonly decidual
arteriopathy, including atherosis and fibrinoid necrosis and mural
hypertrophy of the arterioles of the membranes. In addition,
maternal vascular malperfusion was noted, associated with
restrictions of fetal growth in three infants (five pre-term
infants and one case of stillbirth due to central placental
abruption and oligohydramnios). Gestational hypertension and
pre-eclampsia of the mother, as well as other hypertensive
disorders were the major risk factors for the observed intrauterine
growth restrictions. Other lesions in the pregnant women in the
present study with COVID-19 were massive chronic histolytic
intervillositis, deciduitis with the presence of lymphocytes and
plasma cells, delayed chronic villous maturation, vascular
thrombosis, acute chorioamnionitis and funisitis. The pregnant
women described herein presented with mild symptoms or were
completely asymptomatic with no presence of pneumonia. The authors
consider that by providing data regarding their own experience and
through the present review article, useful information may be
provided for practicing clinicians in order to better understand
the impact of SARS-CoV-2 on pregnancy. The current sample size of
pregnant women was small. Nevertheless, the results regarding the
clinical manifestations are in agreement with those reported in the
literature for the majority of pregnant women; i.e., the majority
of pregnant women are asymptomatic or have mild symptoms, while in
rare cases, a complicated course is observed. One should be aware
that COVID-19 infection in pregnant women can lead to severe
disease (118-122).
Furthermore, the placental pathology findings of the parturient
women in the present study are also in agreement with those
reported in the literature (114).
The authors believe that a strong point of the present narrative
review is that it is succinct, yet informative, while a study
limitation is the small sample size of pregnant women.
10. Conclusion and future perspectives
The elucidation of interactions between pathogen and
host at the molecular level will bring valuable information
regarding the mechanisms causing adverse disease in pregnant women
infected with SARS-CoV-2. In addition, further data are required
regarding the optimal management of pregnant women with
asymptomatic and symptomatic COVID-19 infection. Currently,
corticosteroids (prednisolone or hydrocortisone) and the antiviral
drug, remdesivir, are used in pregnant women when they require
oxygen alongside antithrombotic prophylaxis. As regards the use of
remdesivir, the guidelines suggest not to withhold its use, unless
otherwise indicated (116,117), while others suggest that its fetal
safety profile is largely unknown (116). Preliminary data for vaccination
against SARS-CoV-2 demonstrate no increase in adverse perinatal
outcomes (117-124).
In addition, no assessment can be made at present regarding the
long-term consequences when infection occurs in pregnant women.
Therefore, further larger and high-quality designed studies are
required, as well as transnational studies in order to monitor and
evaluate the postpartum organic and psychological management of
mothers and their children.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PT wrote the manuscript. VK, AG, EK XV, AC, KN, AA
and PP assisted in the literature search. MP performed the
diagnostic tests for the patients. SZ, AB, GI and FG revised the
manuscript. ML performed the histopathological placenta
examinations. SM assisted in the preparation of the tables and
figures. NN and PR were involved in the conception and design of
the study, supervised the study. PT and PR confirm the authenticity
of the raw data. All authors who have participated in the work take
responsibility for the manuscript which they have read and
approved.
Ethics approval and consent to
participate
The pregnant women who participated in the study
provided written informed consent in the majority of cases, and
oral consent in emergency cases, indicating that they agreed to the
use of their samples for scientific research. Participants provide
a document proving that patient consent for participation was
obtained at the time of the study. Ethical approval for the study
was provided by the Ethics Committee of the University Hospital in
Alexandroupolis, Democritus University of Thrace (Alexandroupolis,
Greece; reference no. 42398/07/10/21).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO: Rolling updates on coronavirus
disease (COVID-19), 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
|
|
2
|
WHO: Coronavirus disease (COVID-19)
outbreak situation, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
|
|
3
|
FIGO: Safe motherhood and COVID-19, 2020.
https://www.figo.org/safe-motherhood-and-covid-19;
https://www.figo.org/sites/default/files/2020-04/30.03.20%20-%20FIGO%20Statement%20on%20Safe%20Motherhood%20and%20COVID-19%20EN.pdf.
|
|
4
|
RCOG: Coronavirus (COVID-19) infection in
pregnancy, 2020. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/covid-19-virus-infection-and-pregnancy/.
|
|
5
|
ACOG: Novel coronavirus 2019 (COVID-19),
2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019.
|
|
6
|
ACOG: Outpatient assessment and management
for pregnant women with suspected or confirmed novel coronavirus
(COVID-19), 2020. https://www.acog.org/media/project/acog/acogorg/files/pdfs/clinicalguidance/practice-advisory/covid-19-algorithm.pdf?la=en&hash=2D9E7F62C97F8231561616FFDCA3B1A6.
|
|
7
|
Centers for Disease Control and
Prevention: Breastfeeding and caring for newborns if you have
COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnancy-breastfeeding.html.
Updated Aug 18, 2021.
|
|
8
|
International Committee on Taxonomy of
Viruses Executive Committee. The new scope of virus taxonomy:
Partitioning the virosphere into 15 hierarchical ranks. Nat
Microbiol. 5:668–674. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu Z, Lian X, Su X, Wu W, Marraro GA and
Zeng Y: From SARS and MERS to COVID-19: A brief summary and
comparison of severe acute respiratory infections caused by three
highly pathogenic human coronaviruses. Respir Res.
21(224)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cascella M, Rajnik M, Aleem A, Dulebohn SC
and Di Napoli R: Features, evaluation and treatment coronavirus
(COVID-19). In: StatPearls. StatPearls Publishing, Treasure Island,
FL, 2021.
|
|
11
|
Xiao Y: Structural and functional studies
on coronavirus non-structural proteins 7/8 and 5. Lübeck, 2013.
https://www.zhb.uni-luebeck.de/epubs/ediss1359.pdf.
|
|
12
|
Ashour HM, Elkhatib WF, Rahman MM and
Elshabrawy HA: Insights into the recent 2019 novel coronavirus
(SARS-CoV-2) in light of past human coronavirus outbreaks.
Pathogens. 9(186)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schoeman D and Fielding BC: Coronavirus
envelope protein: Current knowledge. Virol J. 16(69)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang Y, Yang C, Xu XF, Xu W and Liu SW:
Structural and functional properties of SARS-CoV-2 spike protein:
Potential antivirus drug development for COVID-19. Acta Pharmacol
Sin. 41:1141–1149. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McBride R, van Zyl M and Fielding BC: The
coronavirus nucleocapsid is a multifunctional protein. Viruses.
6:2991–3018. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Hu T, Liu Y, Zhao M, Zhuang Q, Xu L and He
Q: A comparison of COVID-19, SARS and MERS. PeerJ.
8(e9725)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nakagawa S and Miyazawa T: Genome
evolution of SARS-CoV-2 and its virological characteristics.
Inflamm Regen. 40(17)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shi CS, Nabar NR, Huang NN and Kehrl JH:
SARS-coronavirus open reading frame-8b triggers intracellular
stress pathways and activates NLRP3 inflammasomes. Cell Death
Discov. 5(101)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Örd M, Faustova I and Loog M: The sequence
at Spike S1/S2 site enables cleavage by furin and
phospho-regulation in SARS-CoV2 but not in SARS-CoV1 or MERS-CoV.
Sci Rep. 10(16944)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
V'kovski P, Kratzel A, Steiner S, Stalder
H and Thiel V: Coronavirus biology and replication: Implications
for SARS-CoV-2. Nat Rev Microbiol. 19:155–170. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Petersen E, Koopmans M, Go U, Hamer DH,
Petrosillo N, Castelli F, Storgaard M, Al Khalili S and Simonsen L:
Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet
Infect Dis. 20:e238–e244. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD,
Jin HJ, Tan KS, Wang DY and Yan Y: The origin, transmission and
clinical therapies on coronavirus disease 2019 (COVID-19)
outbreak-an update on the status. Mil Med Res. 7(11)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han Y and Yang H: The transmission and
diagnosis of 2019 novel coronavirus infection disease (COVID-19): A
Chinese perspective. J Med Virol. 92:639–644. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cevik M, Tate M, Lloyd O, Maraolo AE,
Schafers J and Ho A: SARS-CoV-2, SARS-CoV, and MERS-CoV viral load
dynamics, duration of viral shedding, and infectiousness: A
systematic review and meta-analysis. Lancet Microbe. 2:e13–e22.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yu IT, Li Y, Wong TW, Tam W, Chan AT, Lee
JH, Leung DY and Ho T: Evidence of airborne transmission of the
severe acute respiratory syndrome virus. N Engl J Med.
350:1731–1739. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chafekar A and Fielding BC: MERS-CoV:
Understanding the latest human coronavirus threat. Viruses.
10(93)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yeo C, Kaushal S and Yeo D: Enteric
involvement of coronaviruses: Is faecal-oral transmission of
SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 5:335–337.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kumar M, Taki K, Gahlot R, Sharma A and
Dhangar K: A chronicle of SARS-CoV-2: Part-I-epidemiology,
diagnosis, prognosis, transmission and treatment. Sci Total
Environ. 734(139278)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
van Doremalen N, Bushmaker T, Morris DH,
Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL,
Thornburg NJ, Gerber SI, et al: Aerosol and surface stability of
SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med.
382:1564–1567. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Galbadage T, Peterson BM and Gunasekera
RS: Does COVID-19 spread through droplets alone? Front Public
Health. 8(163)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yan Y, Chang L and Wang L: Laboratory
testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): Current
status, challenges, and countermeasures. Rev Med Virol.
30(e2106)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Vivanti AJ, Vauloup-Fellous C, Prevot S,
Zupan V, Suffee C, Do Cao J, Benachi A and De Luca D:
Transplacental transmission of SARS-CoV-2 infection. Nat Commun.
11(3572)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lu CW, Liu XF and Jia ZF: 2019-nCoV
transmission through the ocular surface must not be ignored.
Lancet. 395(e39)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lessler J, Reich NG, Brookmeyer R, Perl
TM, Nelson KE and Cummings DA: Incubation periods of acute
respiratory viral infections: A systematic review. Lancet Infect
Dis. 9:291–300. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Memish ZA, Perlman S, Van Kerkhove MD and
Zumla A: Middle east respiratory syndrome. Lancet. 395:1063–1077.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jiang X, Rayner S and Luo MH: Does
SARS-CoV-2 has a longer incubation period than SARS and MERS? J Med
Virol. 92:476–478. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng
Q, Meredith HR, Azman AS, Reich NG and Lessler J: The incubation
period of coronavirus disease 2019 (COVID-19) from publicly
reported confirmed cases: Estimation and application. Ann Intern
Med. 172:577–582. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
He W, Yi GY and Zhu Y: Estimation of the
basic reproduction number, average incubation time, asymptomatic
infection rate, and case fatality rate for COVID-19: Meta-analysis
and sensitivity analysis. J Med Virol. 92:2543–2550.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wassie GT, Azene AG, Bantie GM, Dessie G
and Aragaw AM: Incubation period of severe acute respiratory
syndrome novel coronavirus 2 that causes coronavirus disease 2019:
A systematic review and meta-analysis. Curr Ther Res Clin Exp.
93(100607)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhu W, Zeng N and Wang N: Sensitivity,
specificity, accuracy, associated confidence interval and ROC
analysis with practical SAS® implementations. Health
Care and Life Sciences, 2010. https://www.lexjansen.com/nesug/nesug10/hl/hl07.pdf.
|
|
41
|
Li C and Ren L: Recent progress on the
diagnosis of 2019 novel coronavirus. Transbound Emerg Dis.
67:1485–1491. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sethuraman N, Jeremiah SS and Ryo A:
Interpreting DIAGNOSTIC Tests for SARS-CoV-2. JAMA. 323:2249–2251.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Böger B, Fachi MM, Vilhena RO, Cobre AF,
Tonin FS and Pontarolo R: Systematic review with meta-analysis of
the accuracy of diagnostic tests for COVID-19. Am J Infect Control.
49:21–29. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fujisaki S, Mori N, Sasaki T and Maeyama
M: Cell-mediated immunity in human pregnancy: Changes in lymphocyte
reactivity during pregnancy and postpartum. Microbiol Immunol.
23:899–907. 1979.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li
S, Biswas A, Choolani M, Mattar C and Su LL: Coronavirus disease
2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol.
222:521–531. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin
X, Liu Y, Xiao J, Liu H, Deng D, et al: Clinical features and
obstetric and neonatal outcomes of pregnant patients with COVID-19
in Wuhan, China: A retrospective, single-centre, descriptive study.
Lancet Infect Dis. 20:559–564. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou
L, Feng L, Xiong G, Sun G, Wang H, et al: Clinical characteristics
of pregnant women with Covid-19 in Wuhan, China. N Engl J Med.
382(e100)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Elshafeey F, Magdi R, Hindi N, Elshebiny
M, Farrag N, Mahdy S, Sabbour M, Gebril S, Nasser M, Kamel M, et
al: A systematic scoping review of COVID-19 during pregnancy and
childbirth. Int J Gynaecol Obstet. 150:47–52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ferrazzi E, Frigerio L, Savasi V, Vergani
P, Prefumo F, Barresi S, Bianchi S, Ciriello E, Facchinetti F,
Gervasi MT, et al: Vaginal delivery in SARS-CoV-2-infected pregnant
women in Northern Italy: A retrospective analysis. BJOG.
127:1116–1121. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
51
|
Oltean I, Tran J, Lawrence S, Ruschkowski
BA, Zeng N, Bardwell C, Nasr Y, de Nanassy J and El Demellawy D:
Impact of SARS-CoV-2 on the clinical outcomes and placental
pathology of pregnant women and their infants: A systematic review.
Heliyon. 7(e06393)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Berghella V and Hughes BL: COVID-19:
Pregnancy issues and antenatal care. Literature review current
through. Lockwood CJ and Barss VA (eds). 2021.
|
|
53
|
Ramlakhan KP, Johnson MR and
Roos-Hesselink JW: Pregnancy and cardiovascular disease. Nat Rev
Cardiol. 17:718–731. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chen H, Guo J, Wang C, Luo F, Yu X, Zhang
W, Li J, Zhao D, Xu D, Gong Q, et al: Clinical characteristics and
intrauterine vertical transmission potential of COVID-19 infection
in nine pregnant women: A retrospective review of medical records.
Lancet. 395:809–815. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ashokka B, Loh MH, Tan CH, Su LL, Young
BE, Lye DC, Biswas A, Illanes SE and Choolani M: Care of the
pregnant woman with coronavirus disease 2019 in labor and delivery:
Anesthesia, emergency cesarean delivery, differential diagnosis in
the acutely ill parturient, care of the newborn, and protection of
the healthcare personnel. Am J Obstet Gynecol. 223:66–74.e3.
2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu H, Liu F, Li J, Zhang T, Wang D and
Lan W: Clinical and CT imaging features of the COVID-19 pneumonia:
Focus on pregnant women and children. J Infect. 80:e7–e13.
2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Servante J, Swallow G, Thornton JG, Myers
B, Munireddy S, Malinowski AK, Othman M, Li W, O'Donoghue K and
Walker KF: Haemostatic and thrombo-embolic complications in
pregnant women with COVID-19: A systematic review and critical
analysis. BMC Pregnancy Childbirth. 21(108)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wu X, Sun R, Chen J, Xie Y, Zhang S and
Wang X: Radiological findings and clinical characteristics of
pregnant women with COVID-19 pneumonia. Int J Gynaecol Obstet.
150:58–63. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
RCPI IoOaG: COVID-19 Infection Guidance
for Maternity Services. Version 3.0, 2020. https://www.rcpi.ie/news/releases/theinstitute-of-obstetricians-andgynaecologists-issuesguidance-on-covid-19-and-maternityservices/.
|
|
60
|
Di Mascio D, Khalil A, Saccone G, Rizzo G,
Buca D, Liberati M, Vecchiet J, Nappi L, Scambia G, Berghella V and
D'Antonio F: Outcome of coronavirus spectrum infections (SARS,
MERS, COVID-19) during pregnancy: A systematic review and
meta-analysis. Am J Obstet Gynecol MFM. 2(100107)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zaigham M and Andersson O: Maternal and
perinatal outcomes with COVID-19: A systematic review of 108
pregnancies. Acta Obstet Gynecol Scand. 99:823–829. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Karami P, Naghavi M, Feyzi A,
Aghamohammadi M, Novin MS, Mobaien A, Qorbanisani M, Karami A and
Norooznezhad AH: WITHDRAWN: Mortality of a pregnant patient
diagnosed with COVID-19: A case report with clinical, radiological,
and histopathological findings. Travel Med Infect Dis.
(101665)2020.PubMed/NCBI View Article : Google Scholar : (Online ahead of
print).
|
|
63
|
Hantoushzadeh S, Shamshirsaz AA, Aleyasin
A, Seferovic MD, Aski SK, Arian SE, Pooransari P, Ghotbizadeh F,
Aalipour S, Soleimani Z, et al: Maternal death due to COVID-19. Am
J Obstet Gynecol. 223:109.e1–109.e16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ferrazzi EM, Frigerio L, Cetin I, Vergani
P, Spinillo A, Prefumo F, Pellegrini E and Gargantini G: COVID-19
obstetrics task force, Lombardy, Italy: Executive management
summary and short report of outcome. Int J Gynaecol Obstet.
149:377–378. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ronnje L, Länsberg JK, Vikhareva O,
Hansson SR, Herbst A and Zaigham M: Complicated COVID-19 in
pregnancy: A case report with severe liver and coagulation
dysfunction promptly improved by delivery. BMC Pregnancy
Childbirth. 20(511)2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Shanes ED, Mithal LB, Otero S, Azad HA,
Miller ES and Goldstein JA: Placental pathology in COVID-19. Am J
Clin Pathol. 154:23–32. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Juan J, Gil MM, Rong Z, Zhang Y, Yang H
and Poon LC: Effect of coronavirus disease 2019 (COVID-19) on
maternal, perinatal and neonatal outcome: Systematic review.
Ultrasound Obstet Gynecol. 56:15–27. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Diriba K, Awulachew E and Getu E: The
effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and
SARS-CoV) during pregnancy and the possibility of vertical
maternal-fetal transmission: A systematic review and meta-analysis.
Eur J Med Res. 25(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kaur N, Singh R, Dar Z, Bijarnia RK,
Dhingra N and Kaur T: Genetic comparison among various coronavirus
strains for the identification of potential vaccine targets of
SARS-CoV2. Infect Genet Evol. 89(104490)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
RCOG: Coronavirus (COVID-19) infection in
pregnancy information for healthcare professionals. Version 8,
2020. https://www.rcog.org.uk/globalassets/documents/guidelines/2020-04-17-coronavirus-covid-19-infection-in-pregnancy.pdf.
|
|
71
|
Baud D, Greub G, Favre G, Gengler C, Jaton
K, Dubruc E and Pomar L: Second-trimester miscarriage in a pregnant
woman with SARS-CoV-2 infection. JAMA. 323:2198–2200.
2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Aliji N and Aliu F: Oligohydramnion in
COVID19. Eur J Obstet Gynecol Reprod Biol. 249(102)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Khan S, Peng L, Siddique R, Nabi G,
Nawsherwan Xue M, Liu J and Han G: Impact of COVID-19 infection on
pregnancy outcomes and the risk of maternal-to-neonatal intrapartum
transmission of COVID-19 during natural birth. Infect Control Hosp
Epidemiol. 41:748–750. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Dennis AT, Hardy L and Leeton L: The prone
position in healthy pregnant women and in women with preeclampsia-a
pilot study. BMC Pregnancy Childbirth. 18(445)2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Akatsuka M, Tatsumi H, Yama N and Masuda
Y: Therapeutic evaluation of computed tomography findings for
efficacy of prone ventilation in acute respiratory distress
syndrome patients with abdominal surgery. J Crit Care Med (Targu
Mures). 6:32–40. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Klok FA, Kruip MJHA, van der Meer NJM,
Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J,
Stals MAM, Huisman MV and Endeman H: Incidence of thrombotic
complications in critically ill ICU patients with COVID-19. Thromb
Res. 191:145–147. 2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Cui S, Chen S, Li X, Liu S and Wang F:
Prevalence of venous thromboembolism in patients with severe novel
coronavirus pneumonia. J Thromb Haemost. 18:1421–1424.
2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Tang N, Bai H, Chen X, Gong J, Li D and
Sun Z: Anticoagulant treatment is associated with decreased
mortality in severe coronavirus disease 2019 patients with
coagulopathy. J Thromb Haemost. 18:1094–1099. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Di Renzo GC and Giardina I: Coronavirus
disease 2019 in pregnancy: Consider thromboembolic disorders and
thromboprophylaxis. Am J Obstet Gynecol. 223(135)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
McIntosh JJ: Corticosteroid guidance for
pregnancy during COVID-19 pandemic. Am J Perinatol. 37:809–812.
2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
India TFoOGSo: Pregnancy with COVID-19
infection. FOGSI GCPR good clinical practice recommendation.
Version 2, 2020. https://www.fogsi.org/the-draft-version1-fogsi_gcpr_on_pregnancy_with_covid_19_infection/.
|
|
82
|
Davanzo R, Moro G, Sandri F, Agosti M,
Moretti C and Mosca F: Breastfeeding and coronavirus disease-2019:
Ad interim indications of the Italian society of neonatology
endorsed by the Union of European Neonatal and Perinatal Societies.
Matern Child Nutr. 16(e13010)2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Pascarella G, Strumia A, Piliego C, Bruno
F, Del Buono R, Costa F, Scarlata S and Agrò FE: COVID-19 diagnosis
and management: A comprehensive review. J Intern Med. 288:192–206.
2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Gursel M and Gursel I: Is global BCG
vaccination-induced trained immunity relevant to the progression of
SARS-CoV2 pandemic? Allergy. 75:1815–1819. 2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu
M, Shi Z, Hu Z, Zhong W and Xiao G: Remdesivir and chloroquine
effectively inhibit the recently emerged novel coronavirus
(2019-nCoV) in vitro. Cell Res. 30:269–271. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Gao J, Tian Z and Yang X: Breakthrough:
Chloroquine phosphate has shown apparent efficacy in treatment of
COVID-19 associated pneumonia in clinical studies. Biosci Trends.
14:72–73. 2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu
P, Liu X, Zhao L, Dong E, Song C, et al: In vitro antiviral
activity and projection of optimized dosing design of
hydroxychloroquine for the treatment of severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 71:732–739.
2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Gautret P, Lagier JC, Parola P, Hoang VT,
Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE,
et al: Hydroxychloroquine and azithromycin as a treatment of
COVID-19: Results of an open-label non-randomized clinical trial.
Int J Antimicrob Agents. 56(105949)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Garcia-Cremades M, Solans BP, Hughes E,
Ernest JP, Wallender E, Aweeka F, Luetkemeyer AF and Savic RM:
Optimizing hydroxychloroquine dosing for patients With COVID-19: An
integrative modeling approach for effective drug repurposing. Clin
Pharmacol Ther. 108:253–263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Javelot H, El-Hage W, Meyer G, Becker G,
Michel B and Hingray C: COVID-19 and
(hydroxy)chloroquine-azithromycin combination: Should we take the
risk for our patients? Br J Clin Pharmacol. 86:1176–1177.
2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
McCreary EK and Pogue JM: Coronavirus
disease 2019 treatment: A review of early and emerging options.
Open Forum Infect Dis. 7(ofaa105)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Costanzo M, De Giglio MAR and Roviello GN:
SARS-CoV-2: Recent reports on antiviral therapies based on
lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine,
remdesivir, favipiravir and other drugs for the treatment of the
new Coronavirus. Curr Med Chem. 27:4536–4541. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A trial of
Lopinavir-ritonavir in adults hospitalized with severe Covid-19. N
Engl J Med. 382:1787–1799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
LaCourse SM, John-Stewart G and Adams
Waldorf KM: Importance of inclusion of pregnant and breastfeeding
women in COVID-19 therapeutic trials. Clin Infect Dis. 71:879–881.
2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS,
Myoung J, Kim BT and Kim SJ: Current status of epidemiology,
diagnosis, therapeutics, and vaccines for novel coronavirus disease
2019 (COVID-19). J Microbiol Biotechnol. 30:313–324.
2020.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Ahmed SF, Quadeer AA and McKay MR:
Preliminary identification of potential vaccine targets for the
COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological
studies. Viruses. 12(254)2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Liao J, He X, Gong Q, Yang L, Zhou C and
Li J: Analysis of vaginal delivery outcomes among pregnant women in
Wuhan, China during the COVID-19 pandemic. Int J Gynaecol Obstet.
150:53–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Röhr S, Müller F, Jung F, Apfelbacher C,
Seidler A and Riedel-Heller SG: Psychosocial impact of quarantine
measures during serious coronavirus outbreaks: A rapid review.
Psychiatr Prax. 47:179–189. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
99
|
Hiremath P, Suhas Kowshik CS, Manjunath M
and Shettar M: COVID 19: Impact of lock-down on mental health and
tips to overcome. Asian J Psychiatr. 51(102088)2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Fatke B, Hölzle P, Frank A and Förstl H:
COVID-19 crisis: Early observations on a pandemic's psychiatric
problems. Dtsch Med Wochenschr. 145:675–681. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
101
|
Rashidi Fakari F and Simbar M: Coronavirus
pandemic and worries during pregnancy; a letter to editor. Arch
Acad Emerg Med. 8(e21)2020.PubMed/NCBI
|
|
102
|
Bohlken J, Schömig F, Lemke MR, Pumberger
M and Riedel-Heller SG: COVID-19 pandemic: Stress experience of
healthcare workers-A short current review. Psychiatr Prax.
47:190–197. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
103
|
Chew NW, Lee GK, Tan BY, Jing M, Goh Y,
Ngiam NJ, Yeo LL, Ahmad A, Ahmed Khan F, Shanmugam GN, et al: A
multinational, multicentre study on the psychological outcomes and
associated physical symptoms amongst healthcare workers during
COVID-19 outbreak. Brain Behav Immun. 88:559–565. 2020.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Sun N, Wei L, Shi S, Jiao D, Song R, Ma L,
Wang H, Wang C, Wang Z, You Y, et al: A qualitative study on the
psychological experience of caregivers of COVID-19 patients. Am J
Infect Control. 48:592–598. 2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Vieira PR, Garcia LP and Maciel EL: The
increase in domestic violence during the social isolation: What
does it reveals? Rev Bras Epidemiol. 23(e200033)2020.PubMed/NCBI View Article : Google Scholar : (In
Portuguese).
|
|
106
|
Usher K, Bhullar N, Durkin J, Gyamfi N and
Jackson D: Family violence and COVID-19: Increased vulnerability
and reduced options for support. Int J Ment Health Nurs.
29:549–552. 2020.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Dmitrieva K: Job Losses Deepen in Pandemic
with U.S. Tally topping 30 million, 2020. https://www.bloomberg.com/news/articles/2020-04-30/another3-8-million-in-u-s-filed-for-jobless-benefits-last-week.
|
|
108
|
HSE: Hospital service disruptions and
visiting restrictions (COVID-19), 2020. https://www2.hse.ie/services/hospital-service-disruptions/hospital-servicedisruptions-covid19.html.
|
|
109
|
Dore B: Covid-19: Collateral damage of
lockdown in India. BMJ. 369(m1711)2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
FIGO: Fertility Treatment and COVID-19,
2020. https://www.figo.org/fertility-treatment-andcovid-19.
|
|
111
|
Cannistra SA, Haffty BG and Ballman K:
Challenges faced by medical journals during the COVID-19 pandemic.
J Clin Oncol. 38:2206–2207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Bothou M, Tsikouras P, Iatrakis G,
Tsatsaris G, Platanisiotis N, Chatzigianni E, Anthoulaki X and
Bothou A: Social and psychological effects of coronavirus disease
(COVID-19). Rev Clin Pharmacol Pharmacokinet Int Ed. 34:117–120.
2020.
|
|
113
|
Iatrakis G, Tsikouras P and Nikolettos N:
Viral Infections/SARS-COV-2. Obstetrics/ISBN978-618-84118-3-8
Athens, Greece Desmos DigitaLLKE,P.370. https://service.eudoxus.gr/search/#a/id:94645778/0.
|
|
114
|
Girolamo RD, Khalil A, Alameddine S,
D'Angelo E, Galliani C, Matarrelli B, Buca D, Liberati M, Rizzo G
and D'Antonio F: Placental histopathology after SARS-CoV-2
infection in pregnancy: A systematic review and meta-analysis. Am J
Obstet Gynecol MFM. 3(100468)2021.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Di Toro F, Gjoka M, Di Lorenzo G, De Santo
D, De Seta F, Maso G, Risso FM, Romano F, Wiesenfeld U,
Levi-D'Ancona R, et al: Impact of COVID-19 on maternal and neonatal
outcomes: A systematic review and meta-analysis. Clin Microbiol
Infect. 27:36–46. 2021.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Galang RR, Newton SM, Woodworth KR,
Griffin I, Oduyebo T, Sancken CL, Olsen EO, Aveni K, Wingate H,
Shephard H, et al: Risk factors for illness severity among pregnant
women with confirmed severe acute respiratory syndrome coronavirus
2 infection-surveillance for emerging threats to mothers and babies
network, 22 state, local, and territorial health departments, 29
March 2020-5 March 2021. Clin Infect Dis. 73 (Suppl 1):S17–S23.
2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
NIH: Coronavirus Disease 2019 (COVID-19).
Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/.
Accessed October 1, 2021.
|
|
118
|
Shimabukuro TT, Kim SY, Myers TR, Moro PL,
Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang
KT, et al: Preliminary findings of mRNA Covid-19 vaccine safety in
pregnant persons. N Engl J Med. 384:2273–2282. 2021.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Royal College of Obstetricians and
Gynaecologists: Coronavirus (COVID-19), pregnancy and women's
health. https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-covid-19-pregnancy-and-womens-health/.
Accessed October 1, 2021.
|
|
120
|
Wong YP, Khong TY and Tan GC: The effects
of COVID-19 on placenta and pregnancy: What do we know so far?
Diagnostics (Basel). 11(94)2021.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Blakeway H, Prasad S, Kalafat E, Heath PT,
Ladhani SN, Le Doare K, Magee LA, O'brien P, Rezvani A, Dadelszen
PV and Khalil A: COVID-19 vaccination during pregnancy: Coverage
and safety. Am J Obstet Gynecol: Aug 10, 2021 (Epub ahead of
print).
|
|
122
|
Subbaraman N: Pregnancy and COVID: What
the data say. Nature. 591:193–195. 2021.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Shanes ED, Otero S, Mithal LB, Mupanomunda
CA, Miller ES and Goldstein JA: Severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of
immunity and placental histopathology. Obstet Gynecol. 138:281–283.
2021.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Stafford IA, Parchem JG and Sibai BM: The
coronavirus disease 2019 vaccine in pregnancy: Risks, benefits, and
recommendations. Am J Obstet Gynecol. 224:484–495. 2021.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Dinnes J, Deeks JJ, Berhane S, Taylor M,
Adriano A, Davenport C, Dittrich S, Emperador D, Takwoingi Y,
Cunningham J, et al: Rapid, point-of-care antigen and
molecular-based tests for diagnosis of SARS-CoV-2 infection. Rapid,
point-of-care antigen and molecular-based tests for diagnosis of
SARS-CoV-2 infection. Cochrane Database Syst Rev: Aug 26, 2020
(Epub ahead of print).
|
|
126
|
Deeks JJ, Dinnes J, Takwoingi Y, Davenport
C, Spijker R, Taylor-Phillips S, Adriano A, Beese S, Dretzke J,
Ferrante di Ruffano L, et al: Antibody tests for identification of
current and past infection with SARS-CoV-2. Cochrane Database Syst
Rev: Jun 25, 2020 (Epub ahead of print).
|