Introduction
The novel coronavirus disease 2019 (COVID-19) is
elicited by the 2019 novel coronavirus (2019-nCoV), More than 388
million individuals have been infected worldwide, posing a serious
threat to human health (1). Pregnant
women infected with 2019-nCoV have been reported to be at an
increased risk of developing severe disease and having adverse
outcomes and fetuses can also be severely affected (2). As the gestational age increases, the
shape of a pregnant woman's uterus changes, lung capacity
decreases, hormone levels change and immune functions are
suppressed; thus, pregnant women who are infected with COVID-19 are
at a high risk of developing severe illness (3). During the period in which epidemic
prevention and lockdown measures were implemented, the author had
the opportunity to treat a pregnant woman with triplets who was
also infected with 2019-nCoV; her condition gradually developed
from asymptomatic infection to severe disease. Following treatment
with convalescent plasma from patients with COVID-19,
methylprednisolone, etc., all parameters measured were found to
return to normal values. By 32 weeks and 5 days of pregnancy, the
patient delivered by cesarean section due to intrahepatic
cholestasis of pregnancy (ICP), and the three premature infants
were not infected with 2019-nCoV. This case is reported herein and
in addition, a review of the relevant literature is also
presented.
Case report
General information History of present
illness
The patient was 29 years old and was admitted to the
Ruili Chinese Medicine and Dai Medical Hospital (Ruili, Yunnan,
China) due to her 2019-nCoV nucleic acid positive test 13 h prior
to admission. The patient was G1P0 and at 28 weeks of gestation and
pregnant with triplets, which were conceived naturally; regular
evaluations during the course of pregnancy yielded normal results.
Within 2 weeks prior to admission, the patient occasionally felt
fatigued and suffered from palpitations, no fever, cough, dyspnea,
chest tightness, abdominal pain or diarrhea, had a normal sense of
taste and smell, a normal spirit and normal sleep patterns. The
present study was approved by the Medical Ethics Committee of the
Dehong People's Hospital Affiliated to the Kunming Medical
University (Dehongzhou People's Hospital; approval no.
DHZYYLL2021-019), Kunming, China, and informed consent was obtained
from the parents of the infants and from the pregnant woman.
Past history. The patient was previously
healthy, had not been vaccinated against 2019-nCoV, and no
2019-nCoV infection had been reported in her family. Repeated
2019-nCoV nucleic acid tests were negative for 6 months prior to
admission.
Physical examination upon admission. The
patient's physical measurements were as follows: Height, 157 cm;
weight, 59 kg; body temperature, 36.8˚C; pulse rate, 102 beats/min;
respiration rate, 22 beats/min; blood pressure, 123/85 mmHg; and
oxygen saturation (without oxygen inhalation), 98%. The patient's
general conditions were normal, without any detected heart, lung or
abdomen abnormalities during the physical examination.
Additionally, occasional uterine contractions were observed upon an
obstetric examination. and triplet fetal heart auscultation was
normal. Furthermore, no vaginal bleeding or fluids were
detected.
Examination of the patient
Routine blood and biochemical testing, as well as
coagulation function testing were performed using a Roche Cobas
c701 fully automatic biochemical analyzer (Roche Diagnostics),
Mindray BC-6800 auto hematology analyzer (Shenzhen Mindray
Bio-Medical Electronics Co., Ltd.) and a Sysmex CS5100 Coagulation
Analyzer (Sysmex Corporation), respectively. All corresponding test
results are presented in Table I,
Table II and Table III. An ABI 7500 real-time
fluorescent quantitative PCR instrument (Thermo Fisher Scientific,
Inc.) was used for 2019-nCoV RNA load determination. Zhijiang
Biotechnology Co., Ltd. was used for the nucleic acid test, and a
Ct value <43 was considered as a positive result; the 2019-nCoV
nucleic acid detection kit (fluorescent PCR method, National
Machinery Note: 20203400064) of Shengxiang Biotechnology Co., Ltd.
was used for nucleic acid testing, and a Ct value <40 was
considered as a positive result; each sample was analyzed with both
reagent tests, and the final report was the one with the highest
positive result. Subsequently, a GE 16-slice spiral CT OPTIMA
CT520Pro scanner (Cytiva) was used for the acquisition of patient
chest computed tomography (CT) scans. The patient was required to
lie in the supine position for the acquisition of the CT scans. The
corresponding scan parameters were as follows: Collimator, 0.625
mm; tube voltage, 120 kv; tube current, 139 mAs; Fov, 38 cm; W
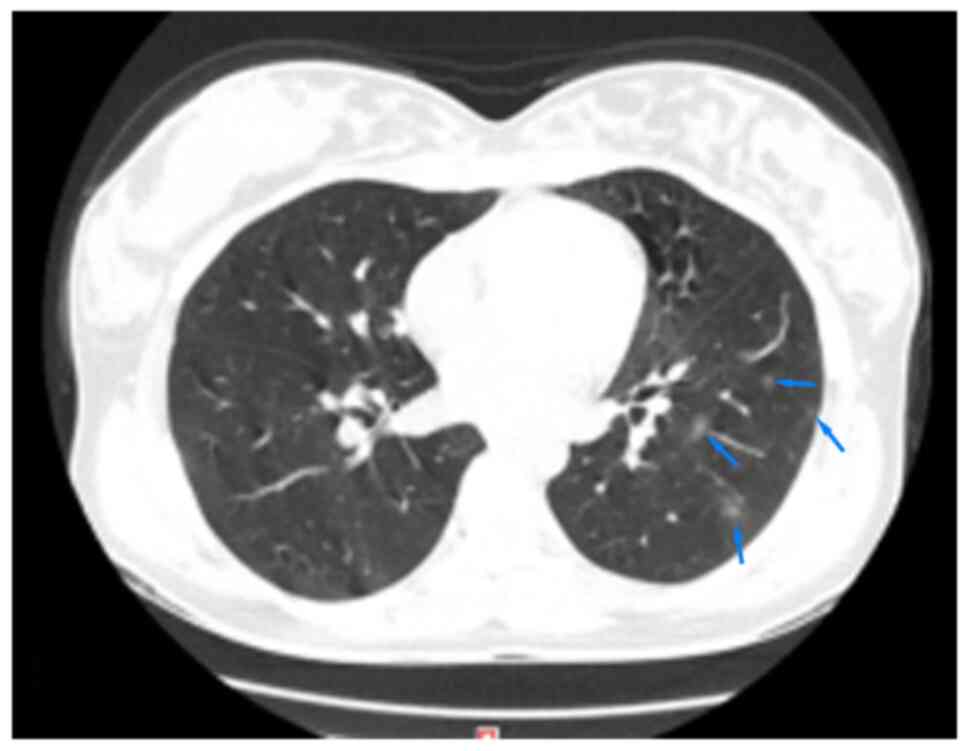
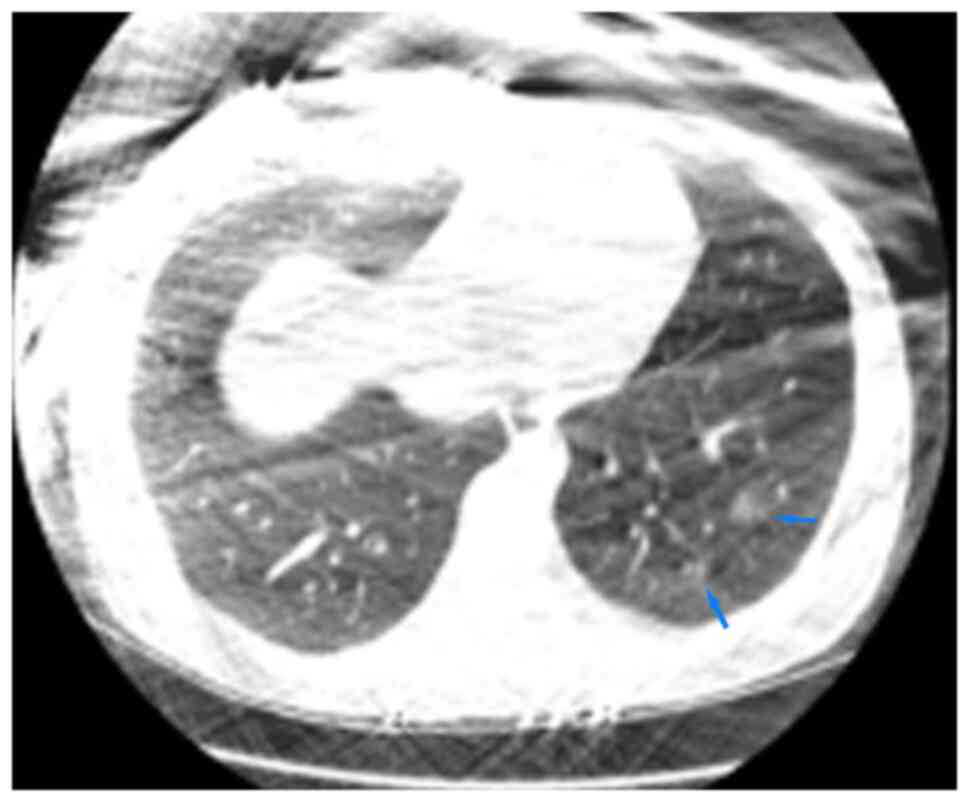
1,400 Hu; L-480 Hu. The CT images of the lungs are demonstrated in
Fig. 1. According to the CT report
on the 4th day of the disease course (Figs. 1 and 2), some lesions were covered by protective
lead clothing, affecting the observation. The thorax was
symmetrical. There were repeatedly small sections of ground glass
shadows in the upper, lower and right middle lobes of the lungs,
with some consolidation, thickened blood vessels and blurred edges,
being consistent with common COVID-19-related effects. CT imaging
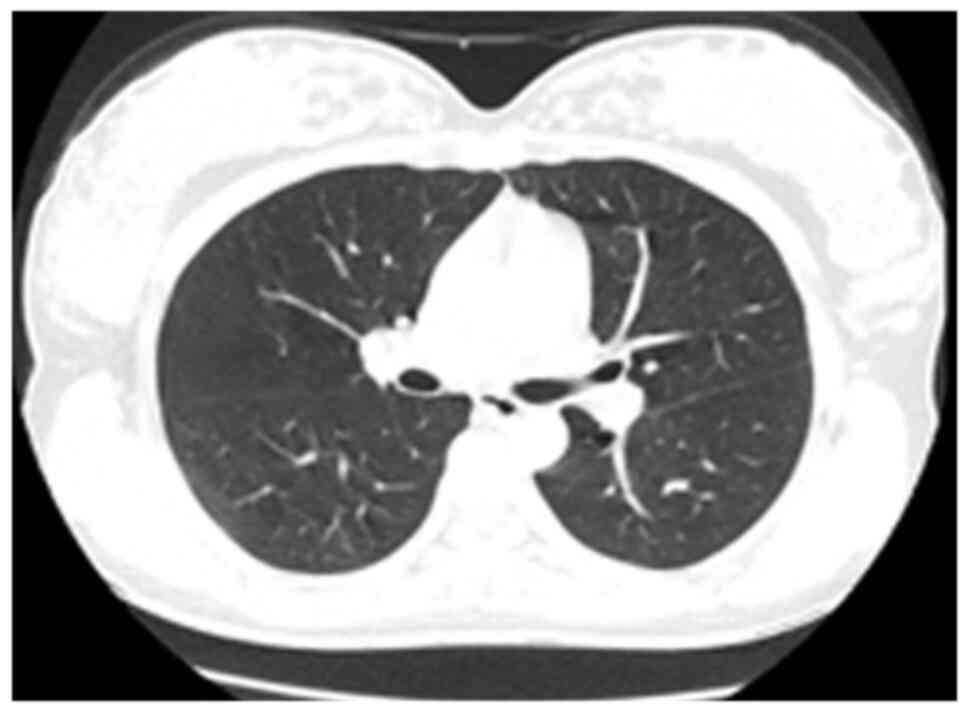
was also performed on the 52nd day of the disease course following
treatment (Fig. 3); at this time,
the lung lesions were absorbed and the conditions were
significantly improved in comparison with the initial clinical
observations. During hospitalization, the ultrasound examination of
the patient revealed pulmonary consolidation, abnormal pleural line
and pulmonary edema. The ultrasound reports of the premature
infants were normal. All three fetuses did not experience
intrauterine distress after repeated fetal heart monitoring.
 | Table IRoutine blood test results of the
woman pregnant with triplets infected with COVID-19. |
Table I
Routine blood test results of the
woman pregnant with triplets infected with COVID-19.
| |
Time
(day/month) |
|---|
| Item | Unit | 9/7 | 12/7 | 14/7 | 17/7 | 18/7 | 19/7 | 22/7 | 25/7 | 29/7 | 31/7 | 3/8 | 6/8 | 9/8 | 13/8 | 15/8 |
|---|
| Hb | g/l | 115 | 100 | 94 | 84 | 98 | 104 | 112 | 115 | 114 | 113 | 116 | 113 | 125 | 110 | 109 |
| WBC |
109/l | 10.4 | 6.69 | 6.67 | 7.48 | 6.3 | 6.83 | 3.69 | 6.34 | 6.36 | 5.41 | 4.48 | 4.62 | 4.52 | 6.31 | 6.46 |
| LYMPH% | % | 12.2 | 20 | 18.6 | 9.8 | 14.4 | 15.8 | 30 | 27.9 | 22.8 | 23.6 | 32.8 | 28.1 | 31.2 | 23.7 | 28.3 |
| LYMPH# |
109/l | 1.27 | 1.34 | 0.84 | 0.73 | 0.91 | 1.08 | 1.11 | 1.81 | 1.45 | 1.28 | 1.47 | 1.3 | 1.36 | 1.30 | 1.86 |
| PLT |
109/l | 211 | 151 | 126 | 163 | 188 | 210 | 298 | 349 | 310 | 285 | 228 | 167 | 182 | 221 | 207 |
 | Table IIBiochemical test of the woman
pregnant with triplets infected with COVID-19. |
Table II
Biochemical test of the woman
pregnant with triplets infected with COVID-19.
| |
Time
(day/month) |
|---|
| Item | Unit | 9/7 | 12/7 | 14/7 | 17/7 | 18/7 | 19/7 | 22/7 | 25/7 | 29/7 | 31/7 | 3/8 | 5/8 | 6/8 | 9/8 | 13/8 | 15/8 |
|---|
| ALT | U/l | 14.68 | 13.39 | 21.35 | 48.53 | 46.8 | | 23.31 | 27.3 | 87.86 | | 132.54 | 154.10 | 151.11 | 105.5 | 27.52 | |
| AST | U/l | 19.67 | 18.21 | 20.21 | 56.93 | 59.3 | | 19.11 | 24.72 | 71.54 | | 81.94 | 90.97 | 91.18 | 47.55 | 23.52 | |
| TBA | µmol/l | 1.85 | 6.23 | 6.67 | 3.67 | 3.12 | 1.93 | 4.64 | 12.38 | 8.19 | | 35.56 | 21.05 | 20.47 | 33.00 | 5.61 | |
| LDH | U/l | 144.27 | 128.56 | 125.75 | | | | | | | | 167.71 | 180.72 | | 146.47 | | |
| CK | U/l | 49.04 | 22.4 | 28.4 | | | | | | | | 22.19 | 16.78 | | 9.69 | | |
| GLU | mmol/l | 4.65 | 4.58 | 4.21 | 4.53 | 3.96 | 4.22 | 4.75 | 3.88 | 4.22 | 4.12 | 3.39 | 3.76 | 3.87 | | | |
| CRP | mg/l | <5.0 | 9.7 | 38.0 | 167.7 | 94.1 | 84.5 | 35.3 | <5.0 | 2.4 | 3.1 | 2.83 | | | 2.44 | 126.42 | 31.54 |
| PCT | ug/l | 0.22 | <0.10 | <0.10 | | | | 0.23 | | 0.2 | 0.25 | 0.31 | | | | | 0.07 |
| IL-6 | pg/ml | 32.8 | 16.2 | 80.9 | | 46.1 | 56.2 | 28.4 | | 23.15 | 3.85 | | | | 4.78 | 28.28 | 6.01 |
 | Table IIICoagulation function test results of
the woman pregnant with triplets infected with COVID-19. |
Table III
Coagulation function test results of
the woman pregnant with triplets infected with COVID-19.
| |
Time
(day/month) |
|---|
| Item | Unit | 9/7 | 12/7 | 14/7 | 17/7 | 18/7 | 22/7 | 29/7 | 31/7 | 3/8 | 6/8 | 13/8 |
|---|
| D-dimer | mg/l | 1.0 | 2.7 | 2.0 | 1.3 | 1.0 | 1.4 | 2.3 | 2.1 | 2.2 | 0.9 | 0.5 |
| PT | sec | 14.8 | 10.9 | 10.9 | 10.1 | 10.7 | 11.2 | 11.0 | 11.6 | 11.6 | 10.5 | 12.5 |
| APTT | sec | 35.7 | 35.1 | 38.6 | 42.2 | 35.9 | 32.8 | 33.1 | 33 | 29.5 | 27.0 | 32.0 |
| INR | / | 1.25 | 0.95 | 0.95 | 0.88 | 0.93 | 0.97 | 0.96 | 1.01 | 1.01 | 0.91 | 0.96 |
| FIB | g/l | 2.13 | 2.5 | 3.23 | 2.08 | 4.96 | 4.07 | 2.44 | 4.29 | 2.35 | 3.4 | 3.1 |
| TT | sec | 20.1 | 19.6 | 17.4 | 21.3 | 20 | 26.8 | 20 | 20.9 | 17.6 | 18.1 | 15.8 |
Diagnosis and treatment of the
patient
The joint diagnosis and treatment medical team
members were the following: The Chinese Epidemic Prevention and
Control Command Expert Group, Respiratory Science Group, Obstetrics
Group, Neonatology group and Critical Medicine Science Group. After
consultation with these expert groups, diagnosis and treatment were
conducted based on the patient's medical history, epidemiological
investigation, and changes in symptoms and signs. The diagnosis,
classification and main treatments administered are presented in
Table IV. Respiratory tract
isolation, contact isolation and daily fetal heart monitoring were
performed until the infant delivery was completed. The patient
began to develop skin itching, increased transaminase alanine
transaminase (ALT) and aspartate aminotransferase (AST) levels, and
increased bile acid levels at 31 weeks and 4 days of pregnancy.
Following the exclusion of other liver diseases, the patient was
diagnosed with severe ICP. Subsequently, analysis by the medical
staff of the joint diagnosis and treatment team revealed that
anti-COVID antibodies could be detected, and the nucleic acid Ct
values were close to negative, indicating that the patient
underwent the recovery period. The patient uterine height was
measured at 38 cm and the abdominal circumference was 104 cm.
Patient cardiopulmonary compression was also clearly detectable.
The gestational age was 32 weeks and 5 days. Premature infant
delivery was expected without any lethal incidents. Due to
pregnancy and COVID-19 infection, and as each of the three infants
was in a different position (cephalic, breech and transverse
position); it was decided for the pregnancy to be concluded by
performing a cesarean section. Cesarean section was performed in
the negative pressure ward at 32 weeks and 5 days of pregnancy,
under combined spinal-epidural anesthesia. The infants were born in
a single transfer single room with isolated treatment. Following a
24-h time period, with a negative nucleic acid test (three times),
the newborns were transferred to the same nursing unit for
treatment until discharge.
 | Table IVDiagnosis, classification and main
patient treatments. |
Table IV
Diagnosis, classification and main
patient treatments.
| Date
(day/month/year) | Conditions | Diagnosis | Treatment
measures |
|---|
| 09/07/2021 | Occasional fatigue
and palpitations. Vital signs were stable. | Asymptomatic
infection with COVID-19 | Dexamethasone
promotes fetal lung maturity (09/07/2021 to 10/07/2021). |
| 11/07/2021 | Mild sore throat,
cough and expectoration. Vital signs were stable. | COVID-19 (mild
type) | Magnesium sulfate
suppresses uterine contractions and protects cranial nerves of
fetuses (11/07/2021 to 13/07/2021). Reduced-molecular-weigh
heparint sodium prevents microvascular sodium thrombosis
(11/07/2021 to 16/07/2021). |
| 12/07/2021 | Sore throat, cough,
high fever, fatigue, palpitations, and occasional abdominal
distension | COVID-19 (ordinary
type) | Intermittent nasal
catheter low flow oxygen inhalation (12/07/2021 to 15/07/2021).
Methylprednisolone sodium succinate (15/07/2021 to 18/07/2021)
Infusion with COVID-19 convalescen plasma (1,800 ml of type A
virus) (13/07/2021 to 17/07/2021). |
| 16/07/2021 | High fever,
hemoptysis, difficulty breathing, and occasional contractions. | COVID-19 (severe
type) | High-flow oxygen
inhalation through the nose, restricting activities (16/07/2021 to
21/078/2021). Pumping morphine (0.5 mg/h) at night to improve
patient's sleep (16/07/2021 to 21/07/2021). Infusion A (positive)
RBC suspension 3U to improve anemia (16/07/2021 to 18/07/2021).
Actively improve the preparations for cesarean section and
pre-start the follow-up treatment of premature (16/97/2021). |
| 22/07/2021 | Dry cough, no
fatigue, no palpitations, no dyspnea, no fever. | COVID-19 (ordinary
type) | Nasal cannula
oxygen inhalation (22/07/2021 to 27/07/2021) Low molecular weight
heparin sodium prevents microvascular thrombosis (27/07/2021 to
03/08/2021) |
| 03/08/2021 | Itchy skin, vital
signs were stable. | COVID-19 (ordinary
type) Severe intrahepatic cholestasis of pregnancy | Ursodeoxycholic
acid tablets (04/08/2021 to 11/08/2021) Cesarean section
(11/08/2021). Post-cesarean section nursing (11/08/2021 to
29/08/2021). |
| 29/08/2021 | No discomfort,
vital signs were stable. Discharge. | COVID-19 (mild
type) | Nucleic acid test
results were consecutively negative (22/08/2021, 23/08/2021,
28/08/2021, 29/08/2021). Examination of lung computed tomography
scan before discharge indicated that the lesions were absorbed
(29/08/2021). Completion of discharge procedures and leaving the
hospital (29/08/2021). |
Delivery and treatment of premature
infants
Three transfer teams were engaged in total, and each
transfer team was responsible for the transfer of one premature
infant. The preterm infants were transported using T-combination
resuscitator ventilation. The premature infants were born by
caesarean section. Each premature infant was separately transported
to a neonatal intensive care unit (NICU) for isolation treatment by
a neonatal escort delivery team wearing secondary protective
clothing. At the time of the laparotomy, the amniotic fluid of
three amniotic cavities was obtained for 2019-nCoV nucleic acid
test and the results were negative. The 2019-nCoV nucleic acid test
of the stool, urine, gastric juice, nasal swab and throat swab
samples were completed three times at 0.5, 12 and 24 h following
delivery. All three premature infants were negative for infection.
The three premature infants were transferred to the same nursing
unit for treatment until discharge. During hospitalization,
2019-nCoV nucleic acid testing was performed repeatedly and all
corresponding results were negative. The blood antibody test
results for all three premature infants 2019-nCoV IgM test results
were negative and IgG were positive many times after birth.
Treatment results and follow-up
Following delivery, bile acid and liver functions
gradually improved, the ICP was cured, and the nucleic acid tests
were negative (two times). The patient was then transferred to the
isolation point for 14 days for 2019-nCoV treatment, and was not
found positive at the end of this time period; the patient was thus
discharged. Monitoring continued until December, 2021, and after
that time point, the patient was continually found negative for
2019-nCoV infection. All three premature infants were discharged
after no signs of discomfort were detected and were negative
consecutively for two 2019-nCoV nucleic acid tests. Repeated
nucleic acid tests were negative, indicating that the three
premature infants were not infected with 2019-nCoV. The three
premature infants were monitored for growth and development
continuously until December, 2021. Eye, lung, heart, brain, liver,
kidney, intestine and other organ functions were also not found to
be abnormal.
Discussion
2019-nCoV testing and monitoring
In the present case report, combined with clinical
manifestations and imaging results, the diagnosis of COVID-19 was
confirmed. The three premature infants reported in the present case
report who were monitored until discharge, 2019-nCoV nucleic acid
test results were negative and 2019-nCoV IgM test results were
negative, but IgG results were positive. Nucleic acid and antibody
levels of the patient and of the three infants required continuous
monitoring.
It took only 28 days for the COVID-19 pathogen to be
identified in China (4).
Quantitative polymerase chain reaction (qPCR) is commonly used to
detect 2019-nCoV, and clinical gene sequencing is commonly used for
pathogen metagenomic sequencing (mNGS). Young patients, patients
with asymptomatic or mild clinical symptoms are more likely to be
subsequently re-infected with 2019-nCoV, whereas this is rare in
patients with severe and critical COVID-19(5). The majority of patients re-infected
with 2019-nCoV have no disease progression, and the majority of
patients do not present with obvious lung symptoms, thus requiring
no special treatment. Isolation and observation are recommended,
and the 2019-nCoV nucleic acid is required to be tested regularly
(6). By analyzing the causes of
2019-nCoV re-infection, a main cause has been reported to be
2019-nCoV residual re-infection (7),
or alternatively 2019-nCoV could be mainly concentrated in the
lower respiratory tract, and false negatives may occur when
nasopharyngeal swabs are collected (8). Multiple site sampling and repeated
screening for 2019-nCoV have been reported to increase the positive
diagnosis rate (9). Therefore,
strict self-isolation and long-term follow-up are crucial after
acquiring a negative result for 2019-nCoV (10). In the present case report, the
2019-nCoV nucleic acid analysis results were negative following
patient treatment, and no re-infection occurred. In addition, the
test results for 2019-nCoV infection of the premature infants were
negative.
2019-nCoV infection may cause adverse effects in
pregnant women, fetuses and newborns, thus continuous monitoring of
the fetus after birth has been reported to be crucial (11). In addition to 2019-nCoV nucleic acid
and antibody levels, the most commonly used method for disease
monitoring is low-dose chest CT scan. Previous research has
reported that that a low-dose chest CT scan repeated for up to 10
times during pregnancy will not cause fetal malformations (12), and a low-dose chest CT in patients
with COVID-19 plays a crucial role in screening, diagnosis, triage
and follow-up (13). The
characteristics of the pulmonary ultrasound imaging of COVID-19 are
lung consolidation, pulmonary edema, pleural effusion and abnormal
pleural line, amongst others. Lung ultrasound has been reported to
be of definite value in the diagnosis of pneumonia and to be
suitable for its application in lung disease screening in adults,
children and newborns (14,15). In the case presented herein, a
low-dose spiral CT scan was used on the patient and a lung
ultrasound examination was also performed. The results of the lung
ultrasound examination were compared and evaluated along with those
of previous lung ultrasound examinations, reducing the risk of
radiation injury.
No vertical intrauterine infection of
three premature infants born by cesarean section
The receptor of 2019-nCoV has been clearly
researched and it is the angiotensin converting enzyme 2 (ACE2). It
mainly exists in the respiratory and digestive tract. It is also
widely distributed in the lymph nodes, thymus, spleen, bone marrow,
liver, skin, brain and other organs. It cannot be fully excluded
that the infection of the organs with ACE2 receptors may cause
damage to corresponding organs (16). The respiratory tract and contact
transmission route have been confirmed to be the main routes of
transmission, while the fecal-oral transmission route and the
vertical transmission route from-mother-to-child remain
controversial. A previous study demonstrated that 2019-nCoV was
detected in the saliva of patients, and part of it was active,
suggesting that 2019-nCoV may still be transmitted directly or
indirectly through saliva (17).
Infection by touching the surface of contaminated objects is termed
contact infection (18). The
infection of cases in different regions of China suggests the
existence of fecal-oral transmission (19).
There are several previously published studies in
favor of the vertical transmission of 2019-nCoV from mother to
child not being possible. One study reported that 10 infants
delivered by pregnant women infected with 2019-nCoV were not
infected with 2019-nCoV (20).
Another study reported nine cases of 2019-nCoV-infected pregnant
women. However, 2019-nCoV nucleic acid testing for amniotic fluid,
fetal umbilical cord blood, neonatal nasopharyngeal swabs and
breast milk yielded negative results (21). Furthermore, in a study on 55 pregnant
women with COVID-19, no evidence of the vertical transmission of
2019-nCoV was found in 46 newborns (22). In another study, a total of 71
pregnant women infected with 2019-nCoV were examined, and none of
the newborns were found to be infected with 2019-nCoV (23). In addition, a study on 42 pregnant
women with COVID-19 in the third trimester revealed that newborns
were not infected with 2019-nCoV (24). In another study, 2019-nCoV RT-PCR
tests were performed on 30 samples of cord blood, 26 samples of
breast milk, 23 samples of amniotic fluid during childbirth and 12
samples of placenta submitted for examination, and the results were
all negative (25). The author
analyzed that the possible reasons for the absence of vertical
transmission may be the following: i) Prevention, isolation and
protection measures were taken for the pregnant women and children
before and during delivery; ii) treatment of pregnant women before
and during delivery minimized the pregnant women's viral load; iii)
timely treatment before delivery may cause the mother to produce
antibodies, and IgG antibody may exert a protective effect on the
fetus through the placenta.
On the contrary, other previously published studies
have suggested that 2019-nCoV transmission may occur vertically
from mother to child. In a previous study on which 33 cases of
neonates delivered by pregnant women with COVID-19 were examined,
three cases of neonates positively tested for SARSCoV-2 in the
upper respiratory tract and anus were confirmed to be infected with
SARS-CoV-2; however, due to strict compliance with infection
control and preventive measures during childbirth, vertical
mother-to-child transmission was not fully excluded (26). Another study reported a case of a
pregnant woman with COVID-19. In that study, the 2019-nCoV antibody
IgG levels stated were: 140.32 AU/ml, IgM: 45.83 AU/ml, 2 h after
the birth of the newborn delivered by cesarean section, due to
strict compliance with infection control and preventive measures
during childbirth, being in support of the possibility of
intrauterine vertical infection (27). The possible causes of vertical
transmission in those cases of viral transmission are analyzed as
follows: i) The treatment was not provided timely before delivery,
and the pregnant women's viral load was too high; ii) regardless of
the treatment being timely before delivery or not, the treatment
was not effective, and the pregnant women's viral load was too
high; iii) the immune function of the pregnant women was reduced
since the SARS-CoV-2 does not cause immunity or induces weak
immunity; and iv) prevention, isolation and protection measures
were not taken during delivery. Based on the aformentioned studies,
it can be suggested that the mother-to-child vertical transmission
of 2019-nCoV may occur; however, the probability of transmission is
minimal.
Body stimulation by pathogens has been reported to
induce the production of large amounts of IgG. It has been
demonstrated that IgG is able to traverse through the placenta;
thus, IgG can be detected after birth, and IgG continues to exist
for a long period of time. However, IgM, having a greater molecular
weight, is not able to traverse through the placenta, and the IgM
half-life is reduced. Therefore, newborns positive for IgM may
indicate the possibility of vertical transmission in the uterus
(28). Some researchers have
suggested that even if a mother suffers from COVID-19, it is not
impossible to rule out the possibility of other viral infections;
thus, it is also considered that only IgM positivity is not
sufficient evidence of 2019-nCoV congenital infection (27-29).
The three premature infants described in the present case report
were negative for 2019-nCoV nucleic acid monitoring, and 2019-nCoV
IgM test results were negative, but the IgG results were positive.
It was thus considered that vertical intrauterine infection did not
occur. Additionally, it has not yet been determined throughout the
literature, to the best of our knowledge, whether vaginal delivery
or cesarean delivery is the safest method. A previous study on 42
pregnant women suffering from COVID-19 in the third trimester of
pregnancy reported that 52.4% of pregnant women gave birth
vaginally, and the newborns were not infected with 2019-nCoV
(24), suggesting that vaginal
delivery may not increase the risk of vertical transmission. It is
uncertain whether breastfeeding leads to the transmission of
2019-nCoV. A study on 48 breast milk samples revealed that one
sample was positive 2019-nCoV, and none of the other samples were
positive for 2019-nCoV (30). The
three premature infants reported in the present study were fed with
formula milk after birth, and no 2019-nCoV infection was found.
Following discharge, the mother's nucleic acid test results were
negative, and breast milk test results were negative repeatedly.
Breastfeeding was introduced, and formula milk was added when the
breast milk was insufficient.
Convalescent plasma from patients with
COVID-19 combined with methylprednisolone are effective for
COVID-19 treatment and persistent high fever, and timely treatment
for complications are helpful for the treatment of COVID-19
Several therapeutics have been suggested for the
treatment of 2019-nCoV infection and COVID-19; however, their
effectiveness and safety, particularly the safety of medication
during pregnancy, remain to be further investigated (19,31,32).
According to the literature, the mortality rate of COVID-19 may be
range between 3.1-7.2%; however, the range in mortality rate in
cases of severe COVID-19 infection is between 15.7-25.5%, and in
critical cases, it has been reported to be as high as 39.0-61.5%
(33). In addition to timely
symptomatic treatment, respiratory and circulatory support
treatment, severe and critical COVID-19 patients can also use the
COVID-19 patient's convalescent plasma treatment. Previous research
has reported that treatment with convalescent plasma from patients
with COVID-19 may reduce mortality (34). It is suggested that patients with
severe and critical COVID-19 infection, particularly patients with
rapid progression, be treated with convalescent plasma from
patients with COVID-19 as soon as possible. A study suggested that
the majority of patients with COVID-19 were treated most
effectively with convalescent plasma from patients with COVID-19,
following the onset of symptoms for 6-14 days, particularly after
the onset of 3-5 days (35).
Previous studies have revealed that the use of convalescent plasma
from patients with COVID-19 may increase survival rates for
critically ill patients with rapid disease progression and a
continuously high viral load following antiviral and hormonal
therapy, including pregnant women. Convalescent plasma may inhibit
viral replication, early inflammatory response and organ tissue
damage. The use of convalescent plasma from patients with COVID-19
has been recommended at an earlier stage instead of using it solely
as remedial treatment (36,37).
The effects of convalescent plasma from patients
with COVID-19 in the treatment of passive immunity include viral
neutralization and immune regulation. The suggested mechanisms of
action are, according to Hung et al (38), as follows: i) The most important
component of convalescent plasma is the γ-globulin molecule, which
has been reported to clear the viral load and relieve viremia; ii)
the neutralizing antibody and non-neutralizing antibody of
convalescent plasma may enhance the adaptive immune response
mediated by T-lymphocytes; iii) the important complement regulatory
proteins, including CD55 contained in the COVID-19 patient's
convalescent plasma may inhibit the activity of complement C3 and
C5, accelerate their decomposition, and reduce inflammatory damage
to host cells. The general principle of the COVID-19 patient's
convalescent plasma treatment infusion is a high antibody titer and
repeated administration, being favorable for viral neutralization,
without any accompanying side-effects according to a previously
published study (39). In China, it
is recommended that the infusion dose of convalescent plasma from
patients with COVID-19 is usually 200-500 ml (4-5 ml/kg); if the
COVID-19 patient's convalescent plasma antibody titer is relatively
low, an increased dose will be necessary (40). The adverse reactions from treatment
with convalescent plasma of COVID-19 patients are mild, with an
incidence of 12.9%, and an incidence of 3.1% has been attributed to
transfusion (41). Although
treatment with convalescent plasma from patients with COVID-19
theoretically poses a risk of infection with 2019-nCoV, there are
currently no reported cases of infection due to the use of such
plasma, at least to the best of our knowledge.
Methylprednisolone inhibits the release of cytokines
and inflammatory mediators, and exerts anti-inflammatory effects,
and methylprednisolone acts on the hypothalamic body temperature
regulation center and the heat-producing organs, resulting in the
restoration of body temperature to normal levels (42,43).
From the above, it is known that methylprednisolone exerts
anti-inflammatory and fever-limiting effects. However, it may
inhibit the immune function of patients, delay virus clearance and
also cause sodium retention, body fluid retention, osteoporosis and
others (42,43). Therefore, the use of corticosteroids
is not recommended for the routine and long-term treatment of
COVID-19. Methylprednisolone has been commonly used in China for a
short-term period (3-5 days) and reduced-dose (1-2 mg/kg/day)
treatment, particularly for patients with dyspnea and severe
hypoxemia. The possible causes of anemia in patients with COVID-19
may involve bone marrow hematopoietic function possibly being
inhibited to reduce red blood cell (RBC) production, or the
activation of mononuclear macrophages to produce extravascular
hemolysis leads to increased RBC destruction, and anemia may thus
occur. Breathing difficulties become more intense, and severe cases
may lead to breathing failure, lung damage, all of which can
aggravate COVID-19. Additionally, in patients with a
well-functioning immune system, the 2019-nCoV viral load has been
reported to be more easily cleared, with the quality of sleep
directly affecting immune efficiency further (44-46).
Sudden COVID-19 disease has been reported to affect the
psychological, physical and social functions of patients, and may
lead to a gradual increase in the levels of cortisol and
norepinephrine. The increase in the levels of these hormones may
affect the patient's disposition, resulting in increased anxiety,
affecting sleep and disease prognosis. It has also been reported
that a functioning immune system is important for viral load
reduction and for the promotion of a timely patient recovery, with
the quality of sleep being a crucial factor affecting immunity. The
immunity of patients with sleep disorders has been demonstrated to
be severely damaged, leading to the weakening of the body's
resistance and viral load elimination efficacy (47,48).
In the present case report, the patient developed
persistent high fever, fatigue, and heart palpitations. Combined
with the findings of CT imaging, it was suggested that the
patient's clinical condition was aggravated. The patient was then
treated with convalescent plasma from patients with COVID-19;
however, the patient's clinical condition did not improve.
Methylprednisolone was also added to the treatment combination, and
subsequently, her body temperature returned to normal levels,
following 6 days of treatment. It was thus suggested that
convalescent plasma from patients with COVID-19 combined with
methylprednisolone was effective in treating COVID-19 and the
persistent high fever. During the course of the illness, the
patient was anxious and suffered from insomnia; the effect of
psychotherapy was not obvious. Following morphine treatment and a
moderate exercise program, the ability to sleep was improved.
During the course of the disease, blood transfusion was used to
correct anemia treatment, and the patient changed from severe to
normal COVID-19. It is suggested that timely treatment with
complications, including anxiety, insomnia, and anemia are
favorable for COVID-19 treatment.
Patient with COVID-19, pregnant with
triplets exhibits increased C-reactive protein (CRP), increased
interleukin (IL)-6, and decreased hemoglobin (Hb) levels with the
worsening of the clinical conditions
The majority of patients with COVID-19 have normal
white blood cell counts, increased CRP levels, and a decreased
number of lymphocytes. Lung CT scans demonstrate typical punctate
and ground glass changes near the pleura, and some patients have
liver damage. CRP is often used for the evaluation of infectious
diseases. It is a sensitive indicator of inflammation, and the
majority of patients with COVID-19 present with increased CRP
levels. In patients with severe COVID-19 infection, levels of ≥10
mg/l account for 81.5% (49,50). Patients with severe COVID-19
infection often also present with increased levels of inflammatory
factors (31). The early increase in
the levels of D-dimer in patients with COVID-19 may be related to
the inflammatory response. A sharp and sudden increase in D-dimer
levels may indicate an acute inflammatory response storm,
indicating that the disease may be exacerbated. The average
increasement rate during COVID-19 infection has been reported to be
46.4% (51). In severe COVID-19
cases of pregnant woman, the hematopoietic function of bone marrow
may be inhibited in order to reduce RBC production, or the
activation of mononuclear macrophages. As a result, extravascular
hemolysis may occur, possibly leading to increased RBC destruction
and anemia.
In addition to its coagulation biological effects,
platelets are also a main innate immunity component. In some
patients with COVID-19, increased levels of liver enzymes and
myocardial enzymes have been reported, and in several patients with
critical COVID-19 infection, troponin levels may also increase
(33,52). In another study, it was revealed that
the blood urea and blood creatinine levels of non-surviving
patients before death increased rapidly compared with those of
survivors (51). It has also been
reported that the average glucose/blood sugar (Glu) levels of
patients with COVID-19 are 7.4 mmol/l, and 52% of patients present
with increased Glu levels (52).
Additionally, it has been reported that the majority of patients
with COVID-19 exhibit normal serum procalcitonin (PCT) levels when
admitted to hospital, and an increase in secondary bacterial
infections (33,52). Therefore, it is recommended that
patients with COVID-19 be monitored for Glu, CRP and PCT levels,
biochemical indicators, coagulation function, arterial blood gas
analysis, chest imaging, cytokines.
In the present case report, the condition of the
woman pregnant with triplets and infected with COVID-19 worsened
gradually; the CRP and IL-6 levels increased, and the Hb levels
notably decreased. Increased CRP, increased IL-6 and decreased Hb
levels are more sensitive markers than the white blood cell count;
the absolute value of lymphocytes, Hb, PLT and blood coagulation
may indicate the aggravation of the disease. The white blood cell
count, absolute value of lymphocytes, Hb, platelet count and
coagulation function were not markedly altered during the course of
the disease and treatment. This may be attributed to the timely
treatment when the conditions worsened.
Pregnant women with COVID-19 are more likely to
suffer from ICP, leading to liver damage (53), which may be directly caused by the
virus or by the systemic inflammatory response induced by the virus
(33). The occurrence and
exacerbation of the ICP may be related to estrogen, thyroid
hormones, environment, genetics, immune factors. ICP may have
adverse effects on pregnant women and fetuses.
In the present case report, the patient presented
with increased total biliary acid (TBA), abnormal liver function
and myocardial enzymes, and skin itching at 31 weeks and 4 days of
gestation, and was diagnosed with severe ICP. Subsequent treatment
with ursodeoxycholic acid tablets was not effective. The cesarean
section was performed at 32 weeks and 5 days of gestation following
the completion of glucocorticoid-induced lung maturation. The
purpose was to decrease or avoid the toxic effects of high bile
acids. Following delivery, TBA decreased, liver function improved,
and ICP was cured. It was suggested that the ICP of the woman with
COVID-19 who was pregnant with triplets may progress rapidly; the
therapeutic effect of ursodeoxycholic acid tablets was ineffective,
and childbirth was an effective treatment method.
In brief, it has been previously stated COVID-19
should not be diagnosed from clinical manifestations alone. For
individuals with an epidemiological history and obvious clinical
manifestations, if a nucleic acid test is negative, multiple tests
need to be repeated to eliminate the possibility of false
negatives. Effective sampling can ensure that the samples have a
sufficient viral load. After sampling, the 2019-nCoV nucleic acid
test is performed in due time, combined with the main clinical
symptoms such as fever or cough for diagnosis, so false negatives
are reduced (54). Additionally,
COVID-19 may cause a normal flora imbalance in the patient's
respiratory tract, which can be combined with multiple viral
infections. Among these combined viral infections, there are more
blood-borne viruses, and blood indicators monitoring needs to be
intensified (55,56).
In conclusion, in the present study, although the
pregnant woman with triplets was infected with COVID-19, the three
premature infants delivered by cesarean section did not present
with intrauterine vertical infection, and abnormal growth and
development were not observed. Treatment with convalescent plasma
from patients with COVID-19 combined with methylprednisolone was
effective for the treatment of COVID-19 and the persistent high
fever, and the timely treatment of complications including anxiety
and insomnia were helpful for the treatment of COVID-19. Increased
CRP, increased IL-6, and decreased Hb levels possibly indicated
that the patient's condition worsened. In addition, increased CRP,
increased IL-6, and decreased Hb levels are more sensitive
indicators of disease severity than routine blood white blood cell
counts, lymphocyte ratio and absolute lymphocyte value, the Hb and
PLT levels, and coagulation function. The ICP of the woman pregnant
with triplets infected with COVID-19 progressed rapidly, and the
use of ursodeoxycholic acid tablets was not an effective treatment
for ICP; however, delivery proved to be safe and effective.
The present case report described the disease
changes, treatment methods and treatment effects of a female
patient with COVID-19 bearing triplets. In all three premature
infants delivered by cesarean section, vertical intrauterine
transmission did not occur. In comparison with other similar case
reports, the present study has certain limitations: The
characteristics of an individual case only were presented.
Therefore, previously the published literature was reviewed, aiming
to strengthen the understanding of the majority of medical workers
on the treatment of pregnant women with triplets, infected with
COVID-19 and also in relation to the outcomes of preterm
infants.
The diagnosis and treatment experience and related
literature review hereby reported may provide future reference for
medical practitioners. The present study suggested that pregnant
women with multiple babies should be treated promptly if they are
suffering from COVID-19, and treatments including convalescent
plasma from patients with COVID-19 and methylprednisolone, are
effective for those who are suffering from COVID-19. It is also
strongly suggested that the 2019-nCoV nucleic acid level, CRP, IL-6
and Hb levels should be monitored in patients with COVID-19.
Additionally, the delivery of triplets from a pregnant woman
infected with COVID-19 does not necessarily lead to the
transmission of 2019-nCoV infection to the infants or induce infant
growth and development issues. Thus, COVID-19 infection is not
necessarily associated with pregnancy loss.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Education
Department of Yunnan Province, China (grant no. 2019J1316).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
ZF independently completed the following work:
Conception and design of the study; Acquisition, analysis, and
interpretation of data; drafting the manuscript and revising it
critically for content; final approval of the manuscript to be
published; agreement to be accountable for all aspects of this
study in ensuring that questions related to the accuracy or
integrity of any part of this manuscript are appropriately
investigated and resolved. The author has read and approved the
final manuscript. ZF confirms the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Dehong People's Hospital Affiliated to the Kunming
Medical University (Dehongzhou People's Hospital; approval no.
DHZYYLL2021-019), Kunming, China, and informed consent was obtained
from the parents of the children and pregnant women.
Patient consent for publication
The present case report obtained the informed
consent of the patient, and the report/publishing was carried out
with her authorization.
Competing interests
The author declares that there are no competing
interests.
References
|
1
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Perlman S: Another decade, another
coronavirus. N Engl J Med. 382:760–762. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Martinez-Portilla R, Smith ER, He S,
Torres J, Espino S, Solis-Paredes JM and Poon LC: Young pregnant
women are also at an increased risk of mortality and severe illness
due to COVID-19: Analysis of the Mexican national surveillance
program. Am J Obstet Gynecol. 224:404–407. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cheng VCC, Wong SC, To KKW, Ho PL and Yuen
KY: Preparedness and proactive infection control measures against
the emerging novel coronavirus in China. J Hosp Infect.
104:254–255. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
An J, Liao X, Xiao T, Qian S, Yuan J, Ye
H, Qi F, Shen C, Wang L, Liu Y, et al: Clinical characteristics of
recovered COVID-19 patients with re-detectable positive RNA test.
Ann Transl Med. 8(1084)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Atzrodt CL, Maknojia I, McCarthy RDP,
Oldfield TM, Po J, Ta KTL, Stepp HE and Clements TP: A guide to
COVID-19: A global pandemic caused by the novel coronavirus
SARS-CoV-2. FEBS J. 287:3633–3650. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang B, Liu S, Dong Y, Zhang L, Zhong Q,
Zou Y and Zhang S: Positive rectal swabs in young patients
recovered from coronavirus disease 2019 (COVID-19). J Infect.
81:e49–e52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yao XH, He ZC, Li TY, Zhang HR, Wang Y,
Mou H, Guo Q, Yu SC, Ding Y, Liu X, et al: Pathological evidence
for residual SARS-CoV-2 in pulmonary tissues of a
ready-for-discharge patient. Cell Res. 30:541–543. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Deng W, Guang TW, Yang M, Li JR, Jiang DP,
Li CY and Wang DX: Positive results for patients with COVID-19
discharged form hospital in Chongqing, China. BMC Infect Dis.
20(429)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fu W, Chen Q and Wang T: Letter to the
editor: Three cases of redetectable positive SARS-CoV-2 RNA in
recovered COVID-19 patients with antibodies. J Med Virol.
92:2298–2301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dang D, Wang L, Zhang C, Li Z and Wu H:
Potential effects of SARS-CoV-2 infection during pregnancy on
fetuses and newborns are worthy of attention. J Obstet Gynaecol
Res. 46:1951–1957. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Williams PM and Fletcher S: Health effects
of prenatal radiation exposure. Am Fam Physician. 82:488–493.
2010.PubMed/NCBI
|
|
13
|
Chung M, Bernheim A, Mei X, Zhang N, Huang
M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, et al: CT imaging
features of 2019 novel coronavirus (2019-nCoV). Radiology.
295:202–207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu J: Lung ultrasonography for the
diagnosis of neonatal lung disease. J Matern Fetal Neonatal Med.
27:856–861. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kurepa D, Zaghloul N, Watkins L and Liu J:
Neonatal lung ultrasound exam guidelines. J Perinatol. 38:11–22.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou P, Yang XL, Wang XG, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature.
579:270–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
To KK, Tsang OT, Yip CC, Chan KH, Wu TC,
Chan JM, Leung WS, Chik TS, Choi CY, Kandamby DH, et al: Consistent
detection of 2019 novel coronavirus in Saliva. Clin Infect Dis.
71:841–843. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
de Graaf M, Beck R, Caccio SM, Duim B,
Fraaij P, Le Guyader FS, Lecuit M, Le Pendu J, de Wit E and
Schultsz C: Sustained fecal-oral human-to-human transmission
following a zoonotic event. Curr Opin Virol. 22:1–6.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Holshue ML, DeBolt C, Lindquist S, Lofy
KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural
A, et al: First case of 2019 novel coronavirus in the United
States. N Engl J Med. 382:929–936. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhu H, Wang L, Fang C, Peng S, Zhang L,
Chang G, Xia S and Zhou W: Clinical analysis of 10 neonates born to
mothers with 2019-nCoV pneumonia. Transl Pediatr. 9:51–60.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen H, Guo J, Wang C, Luo F, Yu X, Zhang
W, Li J, Zhao D, Xu D, Gong Q, et al: Clinical characteristics and
intrauterine vertical transmission potential of COVID-19 infection
in nine pregnant women: A retrospective review of medical records.
Lancet. 395:809–815. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li
S, Biswas A, Choolani M, Mattar C and Su LL: Coronavirus disease
2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol.
222:521–531. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Prabhu M, Cagino K, Matthews KC,
Friedlander RL, Glynn SM, Kubiak JM, Yang YJ, Zhao Z, Baergen RN,
DiPace JI, et al: Pregnancy and postpartum outcomes in a
universally tested population for SARS-CoV-2 in New York City: A
prospective cohort study. BJOG. 127:1548–1556. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Marín Gabriel MA, Cuadrado I, Álvarez
Fernández B, González Carrasco E, Alonso Díaz C, Llana Martín I,
Sánchez L, Olivas C, de Las Heras S and Criado E: Neo-COVID-19
Research Group. Multicentre Spanish study found no incidences of
viral transmission in infants born to mothers with COVID-19. Acta
Paediatr. 109:2302–2308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Elshafeey F, Magdi R, Hindi N, Elshebiny
M, Farrag N, Mahdy S, Sabbour M, Gebril S, Nasser M, Kamel M, et
al: A systematic scoping review of COVID-19 during pregnancy and
childbirth. Int J Gynaecol Obstet. 150:47–52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao
J and Zhou W: Neonatal early-onset infection with SARS-CoV-2 in 33
neonates born to mothers with COVID-19 in Wuhan, China. JAMA
Pediatr. 174:722–725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dong L, Tian J, He S, Zhu C, Wang J, Liu C
and Yang J: Possible vertical transmission of SARS-CoV-2 from an
infected mother to her newborn. JAMA. 323:1846–1848.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang
W and Long X: Antibodies in infants born to mothers with COVID-19
pneumonia. JAMA. 323:1848–1849. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kimberlin DW and Stagno S: Can SARS-CoV-2
infection be acquired in utero? More definitive evidence is needed.
JAMA. 323:1788–1789. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lackey KA, Pace RM, Williams JE, Bode L,
Donovan SM, Järvinen KM, Seppo AE, Raiten DJ, Meehan CL, McGuire MA
and McGuire MK: SARS-CoV-2 and human milk: What is the evidence?
Matern Child Nutr. 16(e13032)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chan JF, Yuan S, Kok KH, To KK, Chu H,
Yang J, Xing F, Liu J, Yip CC, Poon RW, et al: A familial cluster
of pneumonia associated with the 2019 novel coronavirus indicating
person-to-person transmission: A study of a family cluster. Lancet.
395:514–523. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H,
Wu Y, Zhang L, Yu Z, Fang M, et al: Clinical course and outcomes of
critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:
A single-centered, retrospective, observational study. Lancet
Respir Med. 8:475–481. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hung IF, To KK, Lee CK, Lee KL, Chan K,
Yan WW, Liu R, Watt CL, Chan WM, Lai KY, et al: Convalescent plasma
treatment reduced mortality in patients with severe pandemic
influenza A (H1N1) 2009 virus infection. Clin Infect Dis.
52:447–456. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
ToK K, Tsang OT, Leung WS, Tam AR, Wu TC,
Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, et al: Temporal profiles
of viral load in posterior oropharyngeal saliva samples and serum
antibody responses during infection by SARS-CoV-2: An observational
cohort study. Lancet Infect Dis. 20:565–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan
J, Wang F, Li D, Yang M, Xing L, et al: Treatment of 5 critically
ill patients with COVID-19 with convalescent plasma. JAMA.
323:1582–1589. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pan Y, Zhang D, Yang P, Poon LLM and Wang
Q: Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis.
20:411–412. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hung IFN, To KKW, Lee CK, Lee KL, Yan WW,
Chan K, Chan WM, Ngai CW, Law KI, Chow FL, et al: Hyperimmune IV
immunoglobulin treatment: A multicenter double-blind randomized
controlled trial for patients with severe 2009 influenza A(H1N1)
infection. Chest. 144:464–473. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gutfraind A and Meyers LA: Evaluating
large-scale blood transfusion therapy for the current Ebola
epidemic in Liberia. J Infect Dis. 211:1262–1267. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Joyner MJ, Wright RS, Fairweather D,
Senefeld JW, Bruno KA, Klassen SA, Carter RE, Klompas AM, Wiggins
CC, Shepherd JR, et al: Early safety indicators of COVID-19
convalescent plasma in 5000 patients. J Clin Invest. 130:4791–4797.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nguyen FT, van den Akker T, Lally K, Lam
H, Lenskaya V, Liu STH, Bouvier NM, Aberg JA, Rodriguez D, Krammer
F, et al: Transfusion reactions associated with COVID-19
convalescent plasma therapy for SARS-CoV-2. Transfusion. 61:78–93.
2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Corral-Gudino L, Bahamonde A,
Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, García-Ibarbia
C, Mora V, Cerezo-Hernández A, Hernández JL, López-Muñíz G, et al:
Methylprednisolone in adults hospitalized with COVID-19 pneumonia:
An open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr.
133:303–311. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu J, Zheng X, Huang Y, Shan H and Huang
J: Successful use of methylprednisolone for treating severe
COVID-19. J Allergy Clin Immunol. 146:325–327. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Besedovsky L, Lange T and Born J: Sleep
and immune function. Pflug Arch Eur J Physiol. 463:121–137.
2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Almeida C and Malheiro A: Sleep, immunity
and shift workers: A review. Sleep Sci. 9:164–168. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Irwin MR: Why sleep is important for
health: A psychoneuroimmunology perspective. Annu Rev Psychol.
66:143–172. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Huang X, Li H, Meyers K, Xia W, Meng Z, Li
C, Bai J, He S, Cai W, Huang C, et al: Burden of sleep disturbances
and associated risk factors: A cross-sectional survey among
HIV-infected persons on antiretroviral therapy across China. Sci
Rep. 7(3657)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Taylor DJ, Kelly K, Kohut ML and Song KS:
Is insomnia a risk factor for decreased influenza vaccine response?
Behav Sleep Med. 15:270–287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Groeneveld GH, van 't Wout JW, Aarts NJ,
van Rooden CJ, Verheij TJM, Cobbaert CM, Kuijper EJ, de Vries JJC
and van Dissel JT: Prediction model for pneumonia in primary care
patients with an acute respiratory tract infection: Role of
symptoms, signs, and biomarkers. BMC Infect Dis.
19(976)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Guan W, Ni ZY, Hu Y, Ling WH, Qu CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of 2019 novel coronavirus infection in China. MedRxiv.
2(6)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Anness A and Siddiqui F: COVID-19
complicated by hepatic dysfunction in a 28-week pregnant woman. BMJ
Case Rep. 13(e237007)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zandi M, Farahani A, Zakeri A, Akhavan
Rezayat S, Mohammadi R, Das U, Dimmock JR, Afzali S, Nakhaei MA,
Doroudi A, et al: Clinical symptoms and types of samples are
critical factors for the molecular diagnosis of symptomatic
COVID-19 patients: A systematic literature review. Int J Microbiol.
2021(5528786)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Malekifar P, Pakzad R, Shahbahrami R,
Zandi M, Jafarpour A, Rezayat SA, Akbarpour S, Shabestari AN,
Pakzad I, Hesari E, et al: Viral coinfection among COVID-19 patient
groups: An update systematic review and meta-analysis. Biomed Res
Int. 2021(5313832)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Soltani S, Zakeri A, Zandi M, Kesheh MM,
Tabibzadeh A, Dastranj M, Faramarzi S, Didehdar M, Hafezi H,
Hosseini P and Farahani A: The role of bacterial and fungal human
respiratory microbiota in COVID-19 patients. Biomed Res Int.
2021(6670798)2021.PubMed/NCBI View Article : Google Scholar
|