Introduction
Acute ischemic stroke is a disease which is
associated with a high morbidity, high mortality and high
disability rate. Its occurrence also leads to a heavy economic
burden to society, families and individuals (1). Therefore, the treatment of acute
ischemic stroke is of particular importance. By improving cerebral
blood flow and saving brain tissue, recombinant tissue-type
plasminogen activator (rt-PA) is an effective clinical treatment
approved by the Food and Drug Administration (FDA). However, due to
the limited time window, only 2-5% of patients can receive rt-PA
thrombolysis treatment (2). It has
been demonstrated that thrombolysis therapy should be performed as
early as possible, and every 15 min of treatment acceleration can
improve the independent living ability of patients by 4% (3). Ischemic stroke can be divided into
anterior circulation stroke (ACS) and posterior circulation stroke
(PCS). Among all patients with ischemic stroke, patients with PCS
account for 5-10% (4). Of note,
5-19% of patients with PCS have received intravenous alteplase
thrombolytic therapy (5-8),
and 36% of these patients can achieve a National Institutes of
Health Stroke Scale (NIHSS) score >25(9). Since studies on intravenous rt-PA
thrombolysis in PCS are limited, the present retrospective study
aimed to investigate whether the term ‘time is brain’ is relevant
to PCS and whether patients with mild stroke receiving intravenous
rt-PA thrombolysis within 3 h may benefit more than others.
Patients and methods
Patient selection and treatment
The present study was a single-center retrospective
case study. Data were collected from patients who received rt-PA
thrombolytic therapy at the Shengli Oilfield Central Hospital from
January, 2016 to January, 2020, and the basic demographic
characteristics and ischemic stroke-related data were included in
this database. The present retrospective study was approved by the
Shengli Oilfield Central Hospital Ethics Committee. As this was a
retrospective study, the signing of relevant legal documents of
informed consent was not required.
Clinical evaluation
Basic demographic data, including a history of
hypertension, a history of type 2 diabetes mellitus, history of
hyperlipidemia, history of smoking, history of alcohol consumption
and a history of aspirin use (100 mg/day, the commonly used
antiplatelet drugs for secondary prevention of cerebrovascular
diseases are aspirin or clopidogrel, and the majority of patients
in China use aspirin alone), and atrial fibrillation (AF; the
patients enrolled in the study were patients treated with oral
warfarin. The use of any direct oral anticoagulant drugs within 24
h prior to thrombolytic therapy was considered a contraindication.
The enrolled patients with AF received an oral warfarin dose of
2.5-3 mg, and the International Normalized Ratio value was
monitored every 5 days with a control range of 2.0-3.0). NIHSS
scores were obtained upon admission, onset-to-needle time (ONT) and
at 24 h following thrombolysis. A brain computed tomography (CT)
scan prior to thrombolysis and at 24 h after thrombolysis is a
routine examination. Routine examinations of serum homocysteine
(Hcy) levels, which is related to H-type hypertension, were not
performed (results for Hcy levels were not available in the data
collected). The patients were treated with alteplase thrombolysis
at a dose of 0.9 mg/kg, and the maximum dose was not >90 mg. At
the beginning of thrombolysis, 10% of the total amount was
administered by intravenous injection, and the remaining amount was
pumped using a continuous micropump within 1 h. Generally, if the
patient has a cerebral hemorrhage following thrombolytic therapy,
in order to prepare for surgery, the patient's coagulation function
needs to be examined in order to guide the surgery, and often
report the urgent value of prothrombin time and the activated
partial thromboplastin time (APTT) index. A brain CT scan is
generally reviewed within 24 h following intravenous thrombolytic
therapy with alteplase. If there is no intracerebral hemorrhage,
patients should be administered aspirin (100 mg/day) + clopidogrel
(75 mg/day). This is consistent with the Chinese Guidelines for the
Diagnosis and Treatment of Acute Ischemic Stroke 2018(10).
Clinical outcome
All patients that received rt-PA thrombolysis
therapy were divided into two groups, the 0-3 h (also termed the ≤3
h) group and the 3-4.5 h group, in order to observe whether there
were any differences in the NIHSS scores before thrombolysis and at
24 h after thrombolysis. In addition, the patients that received
rt-PA thrombolysis in the 0-3 h group were divided into the NIHSS
score ≤3 group and the NIHSS score >3 group, and the improvement
rate of the NIHSS score in the two groups was observed.
Statistical analysis
All statistical analyses were performed using SPSS
22.0 statistical software (SPSS, Inc.). Continuous data are
presented as the mean ± SD and statistically significant intergroup
differences were assessed using a t-test. Quantitative variables
are presented as a number and percentage [n (%)] and statistically
significant intergroup differences were assessed using the
χ2 test and Fisher's exact test. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
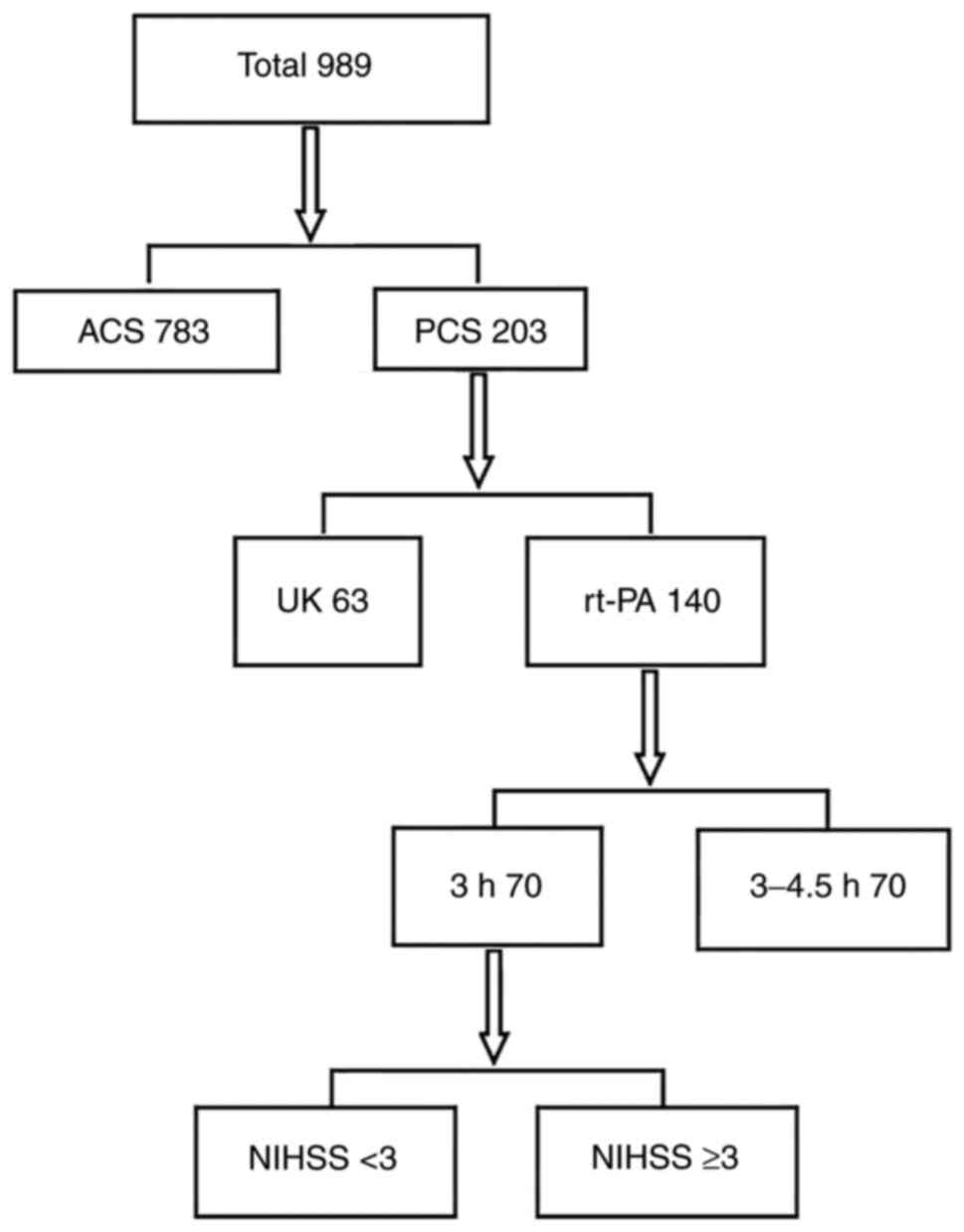
From January, 2016 to January, 2020, a total of 989
patients with acute ischemic stroke received intravenous
thrombolysis therapy at Shengli Oilfield Central Hospital. These
included 783 patients with ACS and 203 patients with PCS (of note,
2 patients had negative results from brain magnetic resonance
imaging). The present study aimed to observe patients with PCS. Of
the 203 patients with PCS, 63 received thrombolytic therapy with
urokinase (NS normal saline 100 ml + one million U urokinase
infusion pumped in 30 min) and 140 patients received intravenous
thrombolytic therapy with alteplase. Among these, 70 patients were
included in the 0-3 h group and 70 patients were included in the
3-4.5 h group (Fig. 1).
No statistically significant differences were
observed between the two groups as regards age, sex, history of
hypertension, history of diabetes mellitus, history of
hyperlipidemia, smoking history and history of aspirin use, and
there were no significant differences in the NIHSS scores between
the two groups before thrombolysis. Following thrombolysis therapy,
the NIHSS score of the two groups was statistically analyzed (the
NIHSS score in the 0-3 h group was 3.78±4.22 and that in the 3-4.5
h group was 6.70±9.17); there was a statistically significant
difference between the two groups (P<0.05; Table I).
 | Table IDemographic profiling and risk factors
in patients with PCS. |
Table I
Demographic profiling and risk factors
in patients with PCS.
| Baseline
variables | ONT (0-3 h),
n=70 | ONT (3-4.5 h),
n=70 | P-value |
|---|
| Demographics | | | |
|
Male | 50 (71.4%) | 50 (71.4%) | ns |
|
Age,
years | 62.90±11.30 | 65.57±11.66 | 0.171 |
| Risk factors | | | |
|
Hypertension | 46 (65.7%) | 45 (64.3%) | 0.859 |
|
Diabetes
mellitus | 18 (25.7%) | 26 (37.1%) | 0.145 |
|
Hyperlipidemia | 4 (5.7%) | 2 (2.9%) | 0.340 |
|
Coronary
artery disease | 12 (17.1%) | 15 (21.4%) | 0.520 |
|
Atrial
fibrillation | 4 (5.7%) | 3 (4.3%) | 0.500 |
|
Smoking | 25 (35.7%) | 25 (35.7%) | ns |
|
History of
alcohol consumptiona | 17 (24.3%) | 19 (27.1%) | 0.699 |
|
History of
oral aspirin use | 11 (15.7%) | 11 (15.7%) | ns |
| DNT (min) | 74.13±32.12 | 84.34±37.82 | 0.093 |
| ONT (min) | 131.72±34.88 | 229.58±24.07 | 0.01 |
| Baseline NIHSS
score | 6.63±6.94 | 8.0±8.62 | 0.302 |
| NIHSS score (24 h
after thrombolysis) | 3.78±4.22 | 6.70±9.17 | 0.016 |
The patients in the 0-3 h group who received rt-PA
intravenous thrombolysis were then divided into the NIHSS score
>3 group and NIHSS score ≤3 group. No statistically significant
differences were observed between the two groups as regards age,
sex, history of hypertension, history of diabetes mellitus, history
of hyperlipidemia, smoking history and history of aspirin use. The
improvement rate in the NIHSS score was examined prior to
thrombolysis therapy and at 24 h after intravenous thrombolysis
therapy; the comparison between the two groups revealed that the
improvement rate in the NIHSS score ≤3 group was high (the
improvement rate in the NIHSS score >3 group was 33.6%, and that
in the NIHSS score ≤3 group was 53.5%); the difference was
statistically significant (P<0.05; Table II).
 | Table IIDemographic profiling and risk factors
in patients with PCS (ONT ≤3 h). |
Table II
Demographic profiling and risk factors
in patients with PCS (ONT ≤3 h).
| Baseline
variables | NIHSS score ≤3,
n=33 | NIHSS score >3,
n=37 | P-value |
|---|
| Demographics | | | |
|
Male | 23 (69.7%) | 27 (73.0%) | 0.762 |
|
Age,
years | 63.00±10.15 | 67.86±12.56 | 0.081 |
| Risk factors | | | |
|
Hypertension | 21 (63.6%) | 25 (67.6%) | 0.729 |
|
Diabetes
mellitus | 12 (36.4%) | 6 (16.2%) | 0.054 |
|
Hyperlipidemia | 1 (3.0%) | 3 (8.1%) | 0.352 |
|
Coronary
artery disease | 6 (18.2%) | 6 (16.2%) | 0.828 |
|
Atrial
fibrillation | 2 (6.0%) | 3 (8.1%) | 0.555 |
|
Smoking | 8 (24.2%) | 17 (45.9%) | 0.059 |
|
History of
alcohol consumptiona | 6 (18.2%) | 11 (29.7%) | 0.261 |
|
History of
oral aspirin use | 6 (18.2%) | 5 (13.5%) | 0.417 |
| DNT (min) | 66.37±34.74 | 75.00±28.81 | 0.267 |
| ONT (min) | 130.34±42.14 | 132.94±27.43 | 0.761 |
| NIHSS score
improvement rate (24 h after thrombolysis) | 0.5354 | 0.3359 | 0.038 |
Discussion
The present retrospective study demonstrated that
patients with PCS could benefit more from intravenous thrombolysis
performed at an earlier stage. In addition, in patients with mild
stroke who received intravenous thrombolysis therapy at an earlier
stage, the improvement rate was more pronounced. Some researchers
have found that the risk of bleeding following intravenous rt-PA
thrombolysis in PCS (RR 0.49) is significantly lower than that in
ACS (11). A previous study in China
also found that the safety and efficacy of intravenous thrombolysis
in patients with PCS was also higher than that in patients with ACS
(12). It is important to determine
the risk of bleeding following intravenous thrombolysis in PCS. The
Get With the Guidelines Stroke (GWTG-S) Registry found that the
probability of bleeding after intravenous rt-PA thrombolysis in PCS
was 4.4%, and the probability in China was 4.87-7.3% (13-15).
All patients in the present study did not suffer from intracerebral
hemorrhage following intravenous thrombolysis therapy. Of note, the
probability of intracerebral hemorrhage after thrombolysis in
patients with PCS is lower than that in those with ACS. In
addition, the NIHSS score can indicate more severe conditions, and
patients who require thrombolysis + intravascular interventional
therapy are admitted to neurology intensive care units. However,
from the perspective of pathogenesis, the question remains as to
why the probability of the occurrence of intracerebral hemorrhage
after thrombolysis therapy in PCS is lower than that in ACS. The
small volume of the infarction and the posterior circulation
supplied by bilateral vertebral arteries are considered to be
related (16,17).
In the present study, in the patients who received
thrombolysis, a NIHSS score of 3 was considered as the threshold
for patients with mild stroke. However, it remains to be confirmed
whether it is reasonable to use a NIHSS score of 3 as the
threshold. Currently, there is no clear definition of mild stroke.
Some researchers have indicated that mild stroke refers to patients
with a NIHSS score of 0-5(18). Of
note however, other researchers have defined mild stroke as a NIHSS
score of 0-3(19). Thus, there
remains some discrepancy as to the definition of mild stroke. A
number of symptoms of PCS cannot be scored and measured using the
NIHSS score (20), such as dizziness
and walking instability. In addition, the NIHSS score may be low in
PCS, and it may not fully reflect the actual condition of the
disease. The present study mainly introduced acute PCS. Therefore,
mild stroke was defined as a patient with a NIHSS score of
<3.
The present retrospective study analyzed the NIHSS
scores before thrombolysis and at 24 h after thrombolysis therapy,
which provides evidence for emergency rt-PA thrombolysis following
admission for acute PCS. Currently, early neurological
deterioration (END) has been put forward as a concept (21). The NIHSS score 24 h after
thrombolysis and a NIHSS score at the time of intravenous
thrombolysis of >4 met the definition of END (22). In the present study, among the 140
patients with PCS who received rt-PA treatment, 5 patients met the
definition of END 24 h after receiving thrombolysis therapy.
However, it appears that a NIHSS score of <4 is also meaningful
for patients with mild stroke (23).
It was previously reported that the incidence of END was 16.3%
(24) and 17.6% (25), respectively. A recent large cohort
study calculated the incidence of END as 6% (26), and the probability of END in the
present study was 3.6%. The reason may be that the present
retrospective study only examined patients with PCS, and only
examined the patients within 4.5 h of onset. In addition, some
patients with higher NIHSS scores were directly admitted to the
Neurology Intensive Care Unit of our hospital. Therefore, the
probability of the occurrence of END in the present study was lower
than that of other studies. For patients who were receive rt-PA
thrombolysis therapy, the incidence of END caused by symptomatic
cerebral hemorrhage accounts for ~20% (27). In addition, it has also been noted
that the edema following thrombolysis can also lead to the
occurrence of END. Currently, 70% of cases of END are of unknown
cause (28).
The present study had a number of limitations.
Firstly, there were a number of patients with mild or rapidly
improved stroke who did not receive intravenous thrombolysis
treatment, although they were within the intravenous thrombolysis
window time frame. In previous studies on such patients who were
treated with intravenous thrombolysis, the outcome was not shown to
markedly improve (29); however,
other studies have demonstrated a significant improvement in such
patients who were received this treatment (30). In the present study, a higher
proportion of these patients should have received rt-PA intravenous
thrombolysis. Secondly, the patients were not followed-up for the
first three months; thus, to date, it cannot be determined whether
there were any adverse conditions or a repeat of symptoms. Thirdly,
there was some bias in the inclusion of patients in the present
study. The present study did not include patients with cardiogenic
cerebral infarction, particularly those with posterior circulation
cerebral infarction. In such patients, the disease is severe and
the NIHSS score is high. Such patients were directly admitted to
the neurological intensive care unit of our hospital. Furthermore,
some patients received mechanical recanalization therapy and were
thus not included, and finally, the number of patients with atrial
fibrillation was minimal in the present study. Patients who
received mechanical thrombectomy therapy were also excluded.
Finally, the present retrospective study was a single-center study;
thus, some of the data may not be accurate for all patients.
In conclusion, the present retrospective study found
that patients with acute PCS could benefit more from early-stage
intravenous thrombolysis therapy. In addition, in patients who
received intravenous rt-PA thrombolysis within 3 h of the onset of
ischemic stroke, it was found that the milder ischemic stroke, the
more prominent he improvement.
Acknowledgements
Not applicable.
Funding
Funding: The present study WAS supported by the China Key
RESEARCH and Development Program (grant no. 2016YFC1301502).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (YH, HZ, XC and ZG) contributed to the
study conception and design. Material preparation, data collection,
experiment design, experiment implementation and analysis were
performed by ZG. YH wrote the first draft of the manuscript. XC and
HZ confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
Shengli Oilfield Central Hospital Ethics Committee. As this was a
retrospective study, the signing of relevant legal documents of
informed consent was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Writing Group Members. Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Executive summary: Heart disease and
stroke statistics-2016 update: A report from the American heart
association. Circulation. 133:447–454. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roth JM: Recombinant tissue plasminogen
activator for the treatment of acute ischemic stroke. Proc (Bayl
Univ Med Cent). 24:257–259. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saver JL, Fonarow GC, Smith EE, Reeves MJ,
Grau-Sepulveda MV, Pan W, Olson DM, Hernandez AF, Peterson ED and
Schwamm LH: Time to treatment with intravenous tissue plasminogen
activator and outcome from acute ischemic stroke. JAMA.
309:2480–2488. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mazya MV, Lees KR, Collas D, Rand VM,
Mikulik R, Toni D, Wahlgren N and Ahmed N: IV thrombolysis in very
severe and severe ischemic stroke: Results from the SITS-ISTR
Registry. Neurology. 85:2098–2106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sung SF, Chen CH, Chen YW, Tseng MC, Shen
HC and Lin HJ: Predicting symptomatic intracerebral hemorrhage
after intravenous thrombolysis: Stroke territory as a potential
pitfall. J Neurol Sci. 335:96–100. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Forster A, Gass A, Kern R, Griebe M,
Hennerici MG and Szabo K: Thrombolysis in posterior circulation
stroke: Stroke subtypes and patterns, complications and outcome.
Cerebrovasc Dis. 32:349–353. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dornak T, Král M, Hazlinger M, Herzig R,
Veverka T, Buřval S, Šaňák D, Zapletalová J, Antalíková K and
Kaňovský P: Posterior vs. anterior circulation infarction:
Demography, outcomes, and frequency of hemorrhage after
thrombolysis. Int J Stroke. 10:1224–1228. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Breuer L, Huttner HB, Jentsch K, Blinzler
C, Winder K, Engelhorn T and Köhrmann M: Intravenous thrombolysis
in posterior cerebral artery infarctions. Cerebrovasc Dis.
31:448–454. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sarikaya H, Arnold M, Engelter ST, Lyrer
PA, Mattle HP, Georgiadis D, Bonati LH, Fluri F, Fischer U,
Findling O, et al: Outcomes of intravenous thrombolysis in
posterior versus anterior circulation stroke. Stroke. 42:2498–2502.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chinese Society of Neurology, Chinese
Stroke Society. Chinese Guidelines for the Diagnosis and Treatment
of Acute Ischemic Stroke 2018. Chin J Neurol. 51:666–682. 2018.
|
|
11
|
Keselman B, Gdovinová Z, Jatuzis D, Melo
TPE, Vilionskis A, Cavallo R, Frol S, Jurak L, Koyuncu B, Nunes AP,
et al: Safety and outcomes of intravenous thrombolysis in posterior
versus anterior circulation stroke: Results from the safe
implementation of treatments in stroke registry and meta-analysis.
Stroke. 51:876–882. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tong X, Liao X, Pan Y, Cao Y, Wang C, Liu
L, Zheng H, Zhao X, Wang C, Wang Y, et al: Intravenous thrombolysis
is more safe and effective for posterior circulation stroke: Data
from the thrombolysis implementation and monitor of acute ischemic
stroke in China (TIMS-China). Medicine (Baltimore).
95(e3848)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim JT, Fonarow GC, Smith EE, Reeves MJ,
Navalkele DD, Grotta JC, Grau-Sepulveda MV, Hernandez AF, Peterson
ED, Schwamm LH and Saver JL: Treatment with tissue plasminogen
activator in the golden hour and the shape of the 4.5-hour
time-benefit curve in the National United States get with the
guidelines-stroke population. Circulation. 135:128–139.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu M, Pan Y, Zhou L and Wang Y:
Predictors of post-thrombolysis symptomatic intracranial hemorrhage
in Chinese patients with acute ischemic stroke. PLoS One.
12(e0184646)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guo Y, Yang Y, Zhou M and He L: Risk
factors of haemorrhagic transformation for acute ischaemic stroke
in Chinese patients receiving intravenous recombinant tissue
plasminogen activator: A systematic review and meta-analysis.
Stroke Vasc Neurol. 3:203–208. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lindgren A, Norrving B, Rudling O and
Johansson BB: Comparison of clinical and neuroradiological findings
in first-ever stroke. A population-based study. Stroke.
25:1371–1377. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Menon BK, O'Brien B, Bivard A, Spratt NJ,
Demchuk AM, Miteff F, Lu X, Levi C and Parsons MW: Assessment of
leptomeningeal collaterals using dynamic CT angiography in patients
with acute ischemic stroke. J Cereb Blood Flow Metab. 33:365–371.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Logallo N, Kvistad CE, Naess H,
Waje-Andreassen U and Thomassen L: Mild stroke: Safety and outcome
in patients receiving thrombolysis. Acta Neurol Scand. Suppl
37-40:2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Willey JZ, Khatri P, Khoury JC, Merino JG,
Ford AL, Rost NS, Gonzales NR, Ali LK, Meyer BC and Broderick JP:
Variability in the use of intravenous thrombolysis for mild stroke:
Experience across the SPOTRIAS network. J Stroke Cerebrovasc Dis.
22:318–322. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sato S, Toyoda K, Uehara T, Toratani N,
Yokota C, Moriwaki H, Naritomi H and Minematsu K: Baseline NIH
Stroke scale score predicting outcome in anterior and posterior
circulation strokes. Neurology. 70 (24 Pt 2):2371–2377.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Siegler JE and Martin-Schild S: Early
neurological deterioration (END) after stroke: The END depends on
the definition. Int J Stroke. 6:211–212. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Seners P, Turc G, Oppenheim C and Baron
JC: Incidence, causes and predictors of neurological deterioration
occurring within 24 h following acute ischaemic stroke: A
systematic review with pathophysiological implications. J Neurol
Neurosurg Psychiatry. 86:87–94. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Saver JL, Gornbein J and Starkman S:
Graphic reanalysis of the two NINDS-tPA trials confirms substantial
treatment benefit. Stroke. 41:2381–2390. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alexandrov AV, Felberg RA, Demchuk AM,
Christou I, Burgin WS, Malkoff M, Wojner AW and Grotta JC:
Deterioration following spontaneous improvement: Sonographic
findings in patients with acutely resolving symptoms of cerebral
ischemia. Stroke. 31:915–919. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Grotta JC, Welch KM, Fagan SC, Lu M,
Frankel MR, Brott T, Levine SR and Lyden PD: Clinical deterioration
following improvement in the NINDS rt-PA stroke trial. Stroke.
32:661–668. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Simonsen CZ, Schmitz ML, Madsen MH,
Mikkelsen IK, Chandra RV, Leslie-Mazwi T and Andersen G: Early
neurological deterioration after thrombolysis: Clinical and imaging
predictors. Int J Stroke. 11:776–782. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim JM, Moon J, Ahn SW, Shin HW, Jung KH
and Park KY: The etiologies of early neurological deterioration
after thrombolysis and risk factors of ischemia progression. J
Stroke Cerebrovasc Dis. 25:383–388. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Seners P, Turc G, Tisserand M, Legrand L,
Labeyrie MA, Calvet D, Meder JF, Mas JL, Oppenheim C and Baron JC:
Unexplained early neurological deterioration after intravenous
thrombolysis: Incidence, predictors, and associated factors.
Stroke. 45:2004–2009. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Frank B, Grotta JC, Alexandrov AV, Bluhmki
E, Lyden P, Meretoja A, Mishra NK, Shuaib A, Wahlgren NG, Weimar C,
et al: Thrombolysis in stroke despite contraindications or
warnings? Stroke. 44:727–733. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yeo LLL, Ho R, Paliwal P, Rathakrishnan R
and Sharma VK: Intravenously administered tissue plasminogen
activator useful in milder strokes? A meta-analysis. J Stroke
Cerebrovasc Dis. 23:2156–2162. 2014.PubMed/NCBI View Article : Google Scholar
|