Introduction
Ischemic stroke is one of the most common vascular
disorders worldwide and, despite notable advancements being made in
treatments and diagnostic imaging techniques, it is still
associated with high mortality and morbidity rates (1,2). Chronic
kidney disease (CKD) is also a serious public health concern with a
high incidence rate (3).
Atherosclerosis is the main cause of both ischemic stroke and CKD
(4,5). The immune response to atherosclerosis
causes the production of autoantibodies in the sera of patients
(6). These can potentially be used
as biomarkers for preventive diagnosis and for the identification
of the disease type of atherosclerosis-related diseases. Recent
studies have demonstrated that serum autoantibodies play a critical
role in various diseases (7,8). In previous studies, the authors
identified several autoantibodies associated with ischemic stroke,
including RPA2, PDCD11, ATP2B4, BMP-1, MMP1, CBX1, DNAJC2, AP3D1,
DIDO1 and SERPINE 1 (9-15).
These may all be applied to diagnosis and treatment. The present
study examines the use of the anti-thiosulfate
sulfurtransferase-like domain-containing 2 (TSTD2) autoantibody as
a novel biomarker for atherosclerosis-related cerebral infarction
and CKD in the sera of patients with atherosclerosis.
Materials and methods
Patient samples
Serum samples were obtained from patients who
suffered an ischemic stroke and from healthy donors (HDs). The
present study analyzed 684 serum samples, including 275 from
patients with acute ischemic stroke (AIS), 300 from patients with
CKD and 109 from HDs. The AIS group consisted of 196 patients with
acute cerebral infarction (aCI) and 79 patients with transient
ischemic attack (TIA). The serum samples of the patients with aCI
and TIA were collected at Chiba Prefectural Sawara Hospital, Chiba
Rosai Hospital, and Chiba Aoba Municipal Hospital. The aCI samples
were collected within 2 weeks of the diagnosis of atherothrombotic
brain infarction. The median (range) of age in years of the HD, aCI
and TIA subjects were 60 (45-90), 77 (58-85) and 73 (26-90),
respectively. The subject information of the Sawara stroke cohort
is summarized in Table SI.
The CKD serum samples were obtained from the
Kumamoto cohort (16,17), which included 145 samples from
patients with type-1 (diabetic kidney disease), 32 with type-2
(nephrosclerosis) and 123 with type-3 (glomerulonephritis) CKD. The
HD serum samples were collected from the Chiba Prefectural Sawara
Hospital, and Port Square Kashiwado Clinic (Chiba, Japan). The HDs
were individuals with no history of ischemic stroke or CKD, and no
abnormalities upon a physical examination or brain magnetic
resonance imaging. Those with autoimmune diseases were excluded
from the study. The median (range) of age in years of the HDs,
type-1 CKD, type-2 CKD and type-3 CKD subjects were 57 (44-76), 65
(38-93), 79 (54-90) and 63 (28-89), respectively. The subject
information of the CKD cohort is summarized in Table SII. Each sample was centrifuged at
3,000 x g for 10 min at 4˚C, and the supernatant was stored at
-80˚C until use. Repeated thawing and freezing were avoided.
The present study was conducted in accordance with
the principles of the 1913 revision of the Declaration of Helsinki
and with the approval of the Ethical Review Committee of Chiba
University, Graduate School of Medicine and cooperative hospitals
(approval no. 2018-320). The research on recombinant DNA was
conducted with the permission of the Graduate School of Medicine,
Chiba University, and following Japanese regulations. Each
participant provided written informed consent to participation and
publication.
ProtoArray® screening
The initial screening was performed using
ProtoArray® Human Protein Microarrays v.4.0 (Thermo
Fisher Scientific, Inc.), which are loaded with 9,480 proteins, as
previously described (18). A total
of 20 serum samples (10 each from HDs and patients with
atherosclerosis) were used to select antigenic proteins
specifically recognized by serum immunoglobin G (IgG) antibodies.
The results were analyzed using Prospector (Thermo Fisher
Scientific, Inc.) software based on M-statistics. When comparing
the two groups, a positive cutoff value corresponds to relative
fluorescence units above the M-statistic signal threshold
established for each antigenic protein. The percentage of the
samples in each group that exhibited an immune response above the
cutoff value was determined and the P-value for the significance of
the difference between the two groups was calculated, as previously
described (19).
Expression and purification of
glutathione-S-transferase-tagged antigenic proteins
The expression plasmid of the TSTD2 protein tagged
with glutathione-S-transferase (GST) was constructed by recombining
the cDNA sequence into a pGEX-4T-1 (Cytiva) plasmid vector. The
competent Escherichia coli (E. coli) BL-21 cells was
obtained from Nippon Gene, Co. Ltd. pGEX-4T-1-TSTD2 (0.2 µg in 1
µl) was mixed with the BL-21 cells (5x108 cells in 20
µl), incubated for 5 min at 0˚C, incubated for 45 sec at 42˚C, and
plated on agar-Luria broth plate containing 50 µg/ml ampicillin
according to the instructions of Nippon Gene, Co. Ltd. The
transformed cells were cultured in 200 ml Luria broth and treated
with 0.1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) reagent
for 3 h. The cells were then harvested, washed with
phosphate-buffered saline (PBS), and sonicated in BugBuster Master
Mix (Merck KGaA). The cell lysates were centrifuged at 13,000 x g
for 10 min at 4˚C, and the precipitates containing recombinant
proteins were dissolved in 8 M urea in TED buffer [50 mM Tris-HCl
(pH 8.0), 1 mM ethylenediaminetetraacetic acid (EDTA) and 1 mM
dithiothreitol]. The samples were then sequentially dialyzed in
steps of 2 h each against 4 and 2 M urea in TED buffer. The samples
were then dialyzed against TED buffer for 15 h to remove the urea
and centrifuged at 10,000 x g for 30 min at 4˚C. The GST-fused
recombinant protein recovered in the supernatant fraction was
directly purified by affinity chromatography using
glutathione-Sepharose (Cytiva) according to the manufacturer's
instructions. The purified protein was filtered using Amicon
Ultra-15 (Merck) centrifugal filter equipment to concentrate it
(9,20-22).
The concentration of the purified protein was quantified by
Bradford method using Bio-Rad Protein assay (#5000001JA, Bio-Rad
Laboratories, Inc.). The proteins were subjected to sodium dodecyl
sulfate-polyacrylamide (11%) gel electrophoresis (SDS-PAGE)
followed by staining with 0.05% Coomassie Brilliant Blue (Nakalai
Tesque, Inc.) in 10% methanol (FUJIFILM Wako Pure Chemical
Corporation) and 50% methanol (FUJIFILM Wako Pure Chemical
Corporation) for 1 h at 25˚C and destaining in 7.5% acetic acid and
5% methanol for longer than 16 h at 25˚C. The purity was calculated
using Personal Density Scanning Imager (Molecular Dynamics,
Inc.).
Western blot analysis
The GST and GST fusion proteins (0.3 µg) were
separated by SDS-PAGE (11% polyacrylamide) and transferred to
nitrocellulose membranes (cat. no. 1620112, Advantec Toyo Kaisha,
Ltd.). The membranes were blocked with blocking solution [0.5% skim
milk powder in a buffer consisting of 20 mM Tris-HCl (pH 7.6), 137
mM NaCl, and 0.1% Tween-20], and subjected to specific primary
antibodies, i.e., 1:5,000-diluted antibodies against GST (cat. no.
600-101-200, Rockland Immunochemicals, Inc.) or 1:1,000-diluted
sera from subjects. Following incubation with 1:30,000-diluted
horseradish peroxidase (HRP)-conjugated secondary antibodies
[donkey anti-goat (cat. no. sc-2056), or anti-human IgG (cat. no.
sc-2453); both from Santa Cruz Biotechnology, Inc.],
immunoreactivity was measured using Immobilon Western HRP Substrate
(Merck KGaA) and LuminoGraph II (ATTO Co, Ltd.). The results were
detected as previously described (21,23).
Quantification of antibodies using
amplified luminescent proximity homogenous assay-linked
immunosorbent assay (AlphaLISA)
AlphaLISA was used for the quantitative measurement
of serum antibodies to purified proteins. Subsequently, 2.5 µl
serum diluted 1:100 in AlphaLISA buffer [25 mM hydroxyethyl
piperazine ethane sulfonic acid (Thermo Fisher Scientific, Inc.),
pH 7.4, 0.1% casein (Merck KGaA), 0.5% Triton X-100 (FUJIFILM Wako
Pure Chemical Corporation), 1 mg/ml dextran-500 (Merck KGaA) and
0.05% Proclin-300 (Merck KGaA)] and 2.5 µl GST or GST-fused TSTD2
proteins (10 µg/ml) was placed in 384-well microtiter plates (white
opaque OptiPlate, PerkinElmer, Inc.) and used for the experiments.
The reaction mixture was incubated at room temperature for 6-8 h,
after which anti-human IgG-conjugated acceptor beads (2.5 µl, 40
µg/ml; PerkinElmer, Inc.) and glutathione-conjugated donor beads
(2.5 µl, 40 µg/ml; PerkinElmer, Inc.) were added. The mixture was
incubated for 7-21 days at room temperature in the dark.
Chemiluminescence was read on an EnSpire Alpha (PerkinElmer, Inc.)
microplate reader (PerkinElmer, Inc.), as previously described
(13,22).
Statistical analyses
The Dunn's multiple comparison test following a
Kruskal-Wallis test was used to analyze continuous variables using
JMP Pro 14.2.0 software (SAS Institute Inc.). Correlations between
TSTD2 antibody levels and each clinical and demographic parameter
were evaluated using Spearman's correlation analysis. The cutoff
TSTD2 antibody level for predicting ischemic stroke was assessed to
maximize the sum of the sensitivity and specificity rates using a
receiver operating characteristic (ROC) curve analysis. Clinical
and demographic factors, including sex, age, smoking and alcohol
drinking habits, and the presence of medical conditions such as
hypertension, diabetes, cardiovascular disease, hyperlipidemia and
obesity [based on body mass index (BMI)] were examined in relation
to the serum TSTD2 antibody levels using a Mann-Whitney U test.
Spearman's correlation analyses, ROC analysis and analysis using
the Mann-Whitney U test were performed using GraphPad Prism 5
software (GraphPad, Inc.). All the tests were two-tailed, and a
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Identification of the TSTD2 antibody
using ProtoArray screening
The screening of ProtoArray® loaded with
9,480 proteins identified TSTD2 antibodies (accession no.
NM_139246.3) in eight of the 10 serum samples from patients with
atherosclerosis and in two of the 10 serum samples from the HDs.
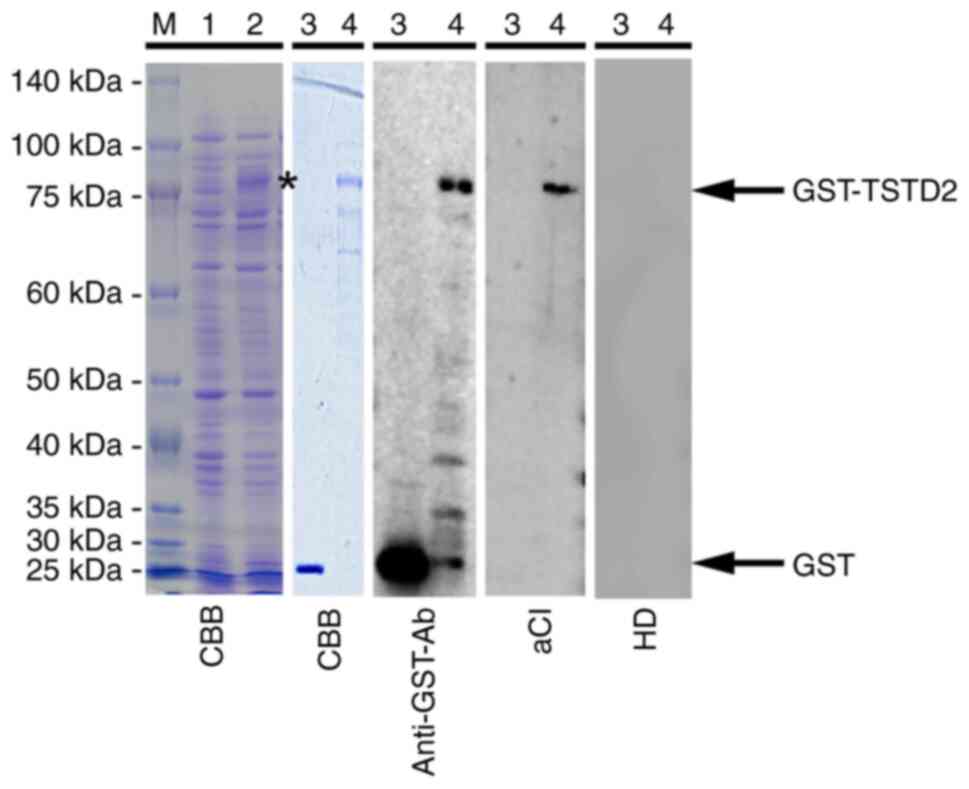
The expression of GST-fused TSTD2 protein was successively induced
in E. coli containing pGEX-4T-1-TSTD2 following treatment
with IPTG (Fig. 1, lanes 1 and 2 of
the CBB blot). Affinity-purified GST and GST-TSTD2 proteins were
also subjected to SDS-PAGE followed by staining with Coomassie
Brilliant Blue (Fig. 1, lanes 3 and
4 of the CBB blot). The purity of GST-TSTD2 protein was ~80%.
Elevated TSTD2 antibody levels in
patients with AIS
To investigate the association between TSTD2
autoantibodies and AIS, serum TSTD2 antibody levels were examined
in the HD, aCI and TIA groups using AlphaLISA. Compared with the HD
group, the aCI group exhibited significantly higher TSTD2 antibody
levels (P=0.0191); however, no significant difference was observed
between the TIA and HD groups (P=0.2030), or between the TIA and
aCI groups (P=0.2589) (Fig. 2A).
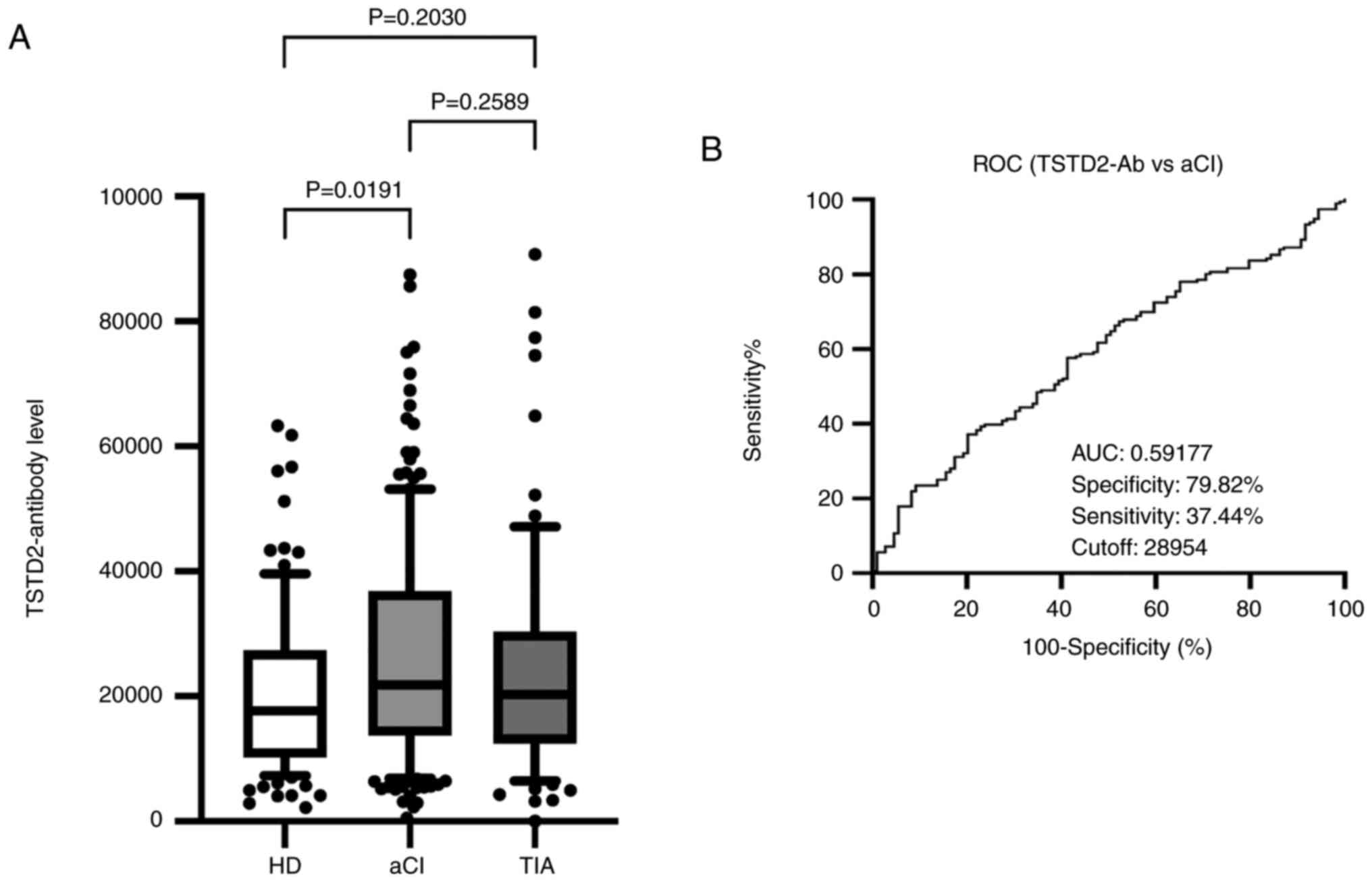 | Figure 2Comparison of the serum levels of
TSTD2 antibodies in HDs, and in patients with aCI and TIA. (A) The
figure illustrates the levels of serum TSTD2-Abs examined using
amplified luminescence proximity homogeneous assay (Alpha)-linked
immunosorbent assay. Antibody levels are represented by Alpha
photon counts and shown in a box-whisker plot. The horizontal lines
represent medians, and the boxes represent the 25 and 75th
percentiles. The whiskers represent the 10 and 90th percentiles,
and the dots represent outliers. P-values were calculated using the
Kruskal-Wallis test. A ROC curve analysis was performed to assess
the ability of serum TSTD2-Abs to detect aCI. (B) The numbers in
the graph are the AUC, specificity, sensitivity and cutoff values
for the marker levels. TSTD2, thiosulfate sulfurtransferase-like
domain-containing 2; TSTD2-Ab, TSTD2 antibody; HD, healthy donor;
aCI, acute cerebral infarction; TIA, transient ischemic attack;
ROC, receiver operating characteristic; AUC, area under the
curve. |
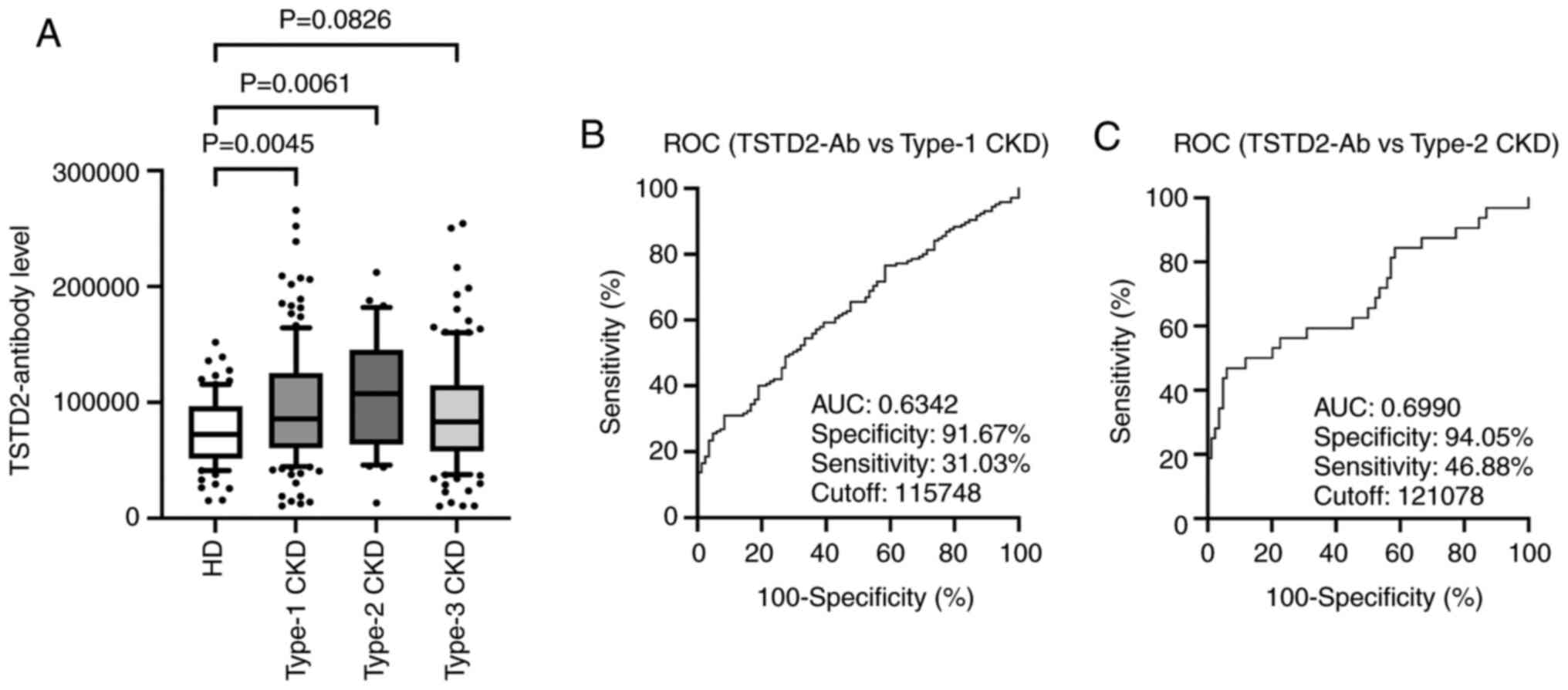
Elevation of TSTD2 antibody levels in
patients with CKD
The present study then examined antibody levels in
the sera of patients with CKD. This group was divided into three
subgroups according to CKD type as follows: Type-1 (diabetic kidney
disease), type-2 (nephrosclerosis) and type-3 (glomerulonephritis).
The type-1 and type-2 CKD subgroups had significantly higher serum
TSTD2-Ab levels than the HDs. The type-3 CKD subgroup exhibited
higher levels than the HD group, although the difference was not
significant (P=0.0826) (Fig.
3A).
ROC analysis of TSTD2 antibody levels
in patients with aCI and type-1 and type-2 CKDs
A ROC analysis of the TSTD2 antibody levels in aCI
and type-1 and type-2 CKDs was also performed. The cutoff values
for these levels in aCI and type-1 and type-2 CKDs were 28,954
(sensitivity, 37.4%; specificity, 79.8%), 11,5748 (sensitivity,
31.03%; specificity, 91.67%) and 12,1078 (sensitivity, 46.88%;
specificity, 94.05%), respectively (Figs. 2B and 3B and C).
The area under the curve (AUC) values were 0.592, 0.6342 and 0.6990
for aCI and type-1 and type-2 CKDs, respectively (Figs. 2B, 3B
and C). The AUC value for type-2 CKD
was higher than that for type-1 CKD, although the sample numbers
examined diffed.
Analysis of correlations between TSTD2
antibody levels and clinical and demographic parameters in the
stroke cohort
Spearman's correlation analyses to identify the
correlations between the TSTD2 antibody levels, and the clinical
and demographic variables in the Sawara stroke cohort. The patient
information is presented in Table
SI, which included hematological examination results, smoking
status and alcohol consumption status, age, sex, height, weight,
BMI and intima-media thickness (IMT) of the carotid artery.
Positive correlations were found between the TSTD2 antibody levels
and maximum IMT (Rho=0.1703, P=0.0028), C-reactive protein levels
(Rho=0.1395, P=0.0221), blood sugar (Rho=0.1542, P=0.039), smoking
duration (Rho=0.1938, P=0.0002) and alcohol consumption frequency
(Rho=0.1174, P=0.0241). Negative correlations were found between
the TSTD2 antibody levels and cholinesterase levels (Rho=-0.1960,
P=0.0009), albumin levels (Rho=-0.1727, P=0.0007) and total
cholesterol (Rho=-0.1567, P=0.0046) (Table I).
 | Table ISpearman's correlation analysis for
the correlation between the serum TSTD2 antibody levels and
clinical features of patients with aCI. |
Table I
Spearman's correlation analysis for
the correlation between the serum TSTD2 antibody levels and
clinical features of patients with aCI.
| Variables | Spearman's rank
correlation coefficient (Rho) | P-value |
|---|
| Age, years | 0.0954 | 0.0624 |
| BMI | 0.0193 | 0.7075 |
| Maximum IMT | 0.1703 | 0.0028 |
| AST | 0.0054 | 0.9161 |
| ALT | -0.0428 | 0.4051 |
| ALP | 0.0647 | 0.228 |
| LDH | 0.086 | 0.0986 |
| tBil | -0.0226 | 0.6642 |
| CHE | -0.1960 | 0.0009 |
| γ-GTP | 0.0586 | 0.2677 |
| TP | -0.0910 | 0.0797 |
| ALB | -0.1727 | 0.0007 |
| BUN | 0.034 | 0.5082 |
| Creatinine | -0.0079 | 0.8783 |
| eGFR | -0.0124 | 0.8154 |
| UA | 0.069 | 0.2558 |
| AMY | -0.0764 | 0.2537 |
| T-CHO | -0.1567 | 0.0046 |
| HDL-C | -0.0673 | 0.3106 |
| TG | -0.1109 | 0.0814 |
| Na | -0.0139 | 0.7877 |
| K | -0.0994 | 0.054 |
| Cl | -0.0989 | 0.055 |
| CRP | 0.1395 | 0.0221 |
| WBC | 0.0905 | 0.0783 |
| RBC | -0.0673 | 0.1912 |
| HGB | -0.0564 | 0.2737 |
| HCT | -0.0626 | 0.2243 |
| PLT | -0.0481 | 0.3503 |
| BS | 0.1542 | 0.0039 |
| HbA1c | -0.0250 | 0.6695 |
| Smoking duration
(years) | 0.1938 | 0.0002 |
| Frequency of
alcohol consumption (times/week) | 0.1174 | 0.0241 |
Antibody levels were compared between two groups
using the Mann-Whitney U test using the cutoff values determined by
ROC analysis, and were found to be significantly higher in males
than in females (P=0.036), those who were ≥65 years of age compared
with those <65 of age (P=0.047), in those with hypertension
compared with those without this condition (P=0.036), in smokers
compared with non-smokers (P=0.003), and in those who consumed
alcohol compared with those who did not (P=0.042) (Fig. 4).
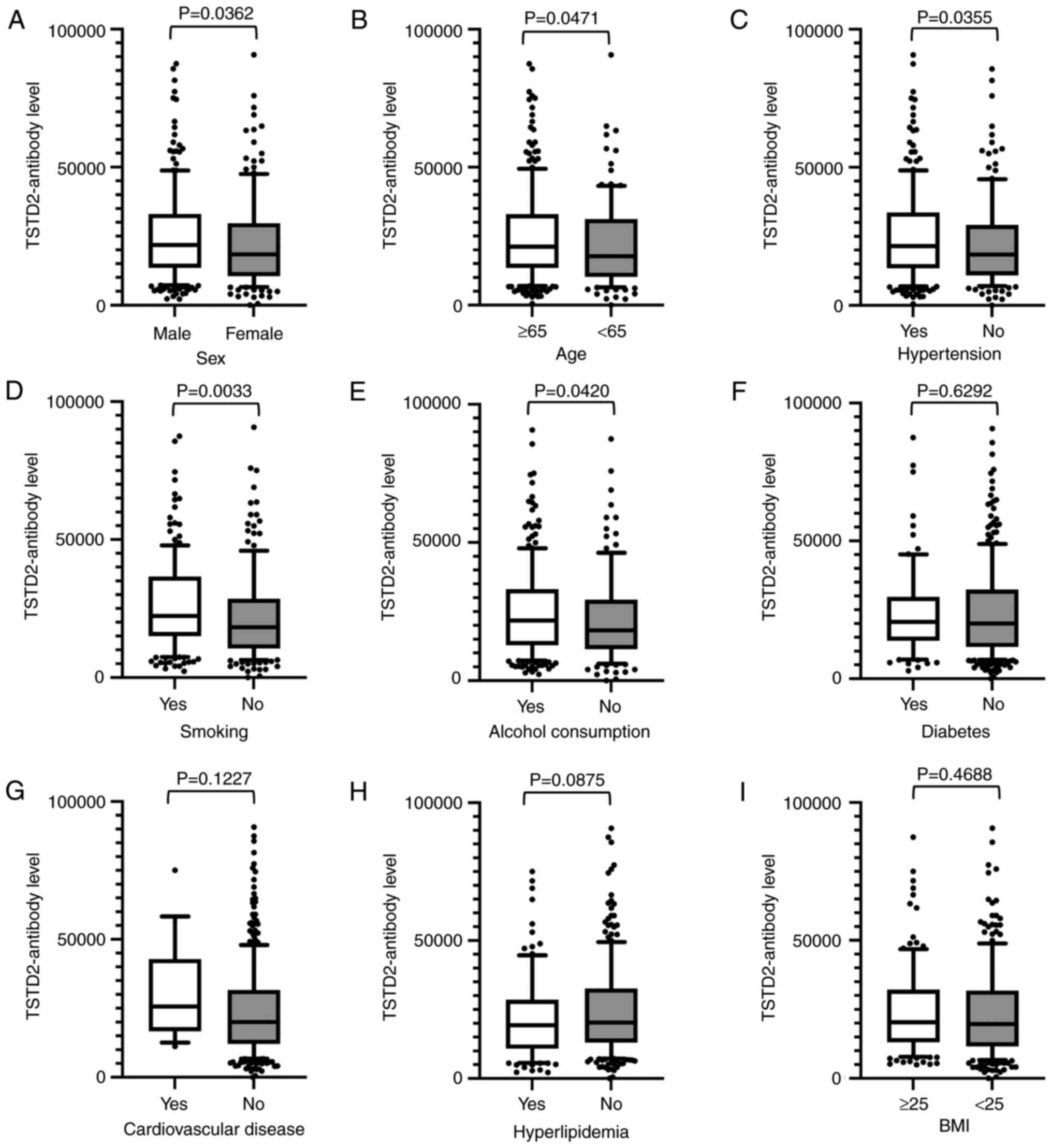 | Figure 4Associations between TSTD2 antibody
levels and clinical and demographic variables in the serum of acute
cerebral infarction patients. The associations between TSTD2
antibody levels and (A) sex, (B) age, (C) hypertension, (D) smoking
status, (E) alcohol consumption, (F) diabetes, (G) cardiovascular
disease, (H) hyperlipidemia, and (I) BMI were examined in the acute
cerebral infarction cohort. The TSTD2 antibody levels obtained
using amplified luminescence proximity homogeneous assay-linked
immunosorbent assay are shown in box-whisker plots. The P-values
were calculated using Mann-Whitney U tests. TSTD2, thiosulfate
sulfurtransferase-like domain-containing 2; BMI, body mass
index. |
Analysis of correlations between TSTD2
antibody levels and clinical and demographic parameters in the CKD
cohort
Subsequently, Spearman's correlation analysis was
performed to identify the correlations between the serum TSTD2
antibody levels and the clinical features of patients in the
Kumamoto CKD cohort. The patient information is presented in
Table SII. Positive correlations
were found between TSTD2 antibody levels and plaque scores
(Rho=0.1608, P=0.0056), maximum IMT (Rho=0.1266, P=0.0295),
cardio-ankle vascular index (CAVI) of the right side (Rho=0.1430,
P=0.0165) and the left side (Rho=0.1472, P=0.0132), parathyroid
hormone (Rho=0.1539, P=0.0076), aspartate aminotransferase
(Rho=0.1654, P=0.0041), lactate dehydrogenase (Rho=0.1749,
P=0.0024), and C-reactive protein (Rho=0.1638, P=0.0044) levels. A
negative correlation was found between TSTD2 antibody levels and
BMI (Rho=-0.1509, P=0.0089) (Table
II).
 | Table IISpearman's correlation analysis of
the correlation between the serum TSTD2 antibody levels and
clinical features of patients with CKD. |
Table II
Spearman's correlation analysis of
the correlation between the serum TSTD2 antibody levels and
clinical features of patients with CKD.
| Variables | Spearman's rank
correlation coefficient (Rho) | P-value |
|---|
| Age, years | 0.0545 | 0.3468 |
| Height | -0.0044 | 0.9401 |
| Weight | -0.1152 | 0.0461 |
| BMI | -0.1509 | 0.0089 |
| Plaque score | 0.1608 | 0.0056 |
| Maximum IMT | 0.1266 | 0.0295 |
| ABI (right) | -0.0122 | 0.8353 |
| ABI (left) | -0.0124 | 0.8319 |
| CAVI (right) | 0.1430 | 0.0165 |
| CAVI (left) | 0.1472 | 0.0132 |
| HbA1c | -0.0077 | 0.9260 |
| PTH | 0.1539 | 0.0076 |
| Fe | -0.0984 | 0.0889 |
| Ferritin | 0.1110 | 0.0547 |
| TSAT ratio | -0.0615 | 0.2882 |
| Kt/V | -0.0874 | 0.1308 |
| RBC | -0.0680 | 0.2406 |
| PLT | -0.0743 | 0.1994 |
| TP | -0.0568 | 0.3272 |
| ALB | -0.0758 | 0.1907 |
| UA | -0.0153 | 0.7912 |
| Na | 0.1010 | 0.0807 |
| K | -0.0112 | 0.8462 |
| Cl | 0.0522 | 0.3676 |
| Ca | 0.0130 | 0.8223 |
| IP | -0.0017 | 0.9770 |
| Mg | 0.0544 | 0.3476 |
| AST | 0.1654 | 0.0041 |
| ALT | 0.0975 | 0.0920 |
| LDH | 0.1749 | 0.0024 |
| γ-GTP | 0.0895 | 0.1219 |
| AP | 0.0557 | 0.3366 |
| tBil | -0.0105 | 0.8559 |
| AMY | -0.0448 | 0.4394 |
| Creatinin | -0.0354 | 0.5415 |
| T-CHO | -0.0300 | 0.6047 |
| HDL-C | -0.0741 | 0.2004 |
| LDL-C | -0.0058 | 0.9197 |
| TG | 0.0383 | 0.5086 |
| CRP | 0.1638 | 0.0044 |
The presence of autoantibodies against
purified protein in the sera of aCI patients
Western blot analysis was performed to determine the
presence of anti-TSTD2 antibodies in the serum samples. Both GST
and GST-TSTD2 protein reacted with commercial anti-GST antibodies
whereas GST-TSTD2, but not GST was recognized by antibodies in the
sera of patients with aCI (Fig. 1,
lanes 3 and 4 of the anti-GST-Ab, aCI, and HD blots). HD serum
contained no TSTD2 antibodies.
Discussion
Atherosclerosis is one of the major causes of
ischemic stroke (24) and the
primary cause of CKD (5).
Autoantibodies have been found to develop alongside atherosclerosis
(13,14). Some of these autoantibodies may have
causal or suppressive effects on disease development. For example,
anti-GRP78 autoantibodies accelerate the development of
atherosclerotic lesions (25).
Therefore, the present study performed ProtoArray®
screening and identified a novel autoantibody marker and selected
the TSTD2 autoantibody as a candidate marker for atherosclerosis.
AlphaLISA was then used to quantify anti-TSTD2 antibodies in
patient sera, and a significant increase in the antibody levels was
found in samples from patients with aCI and type-1 and type-2 CKD,
as compared with those from the HDs (Figs. 2A and 3A). Western blot analysis was performed and
this confirmed the presence of TSTD2 antibodies in aCI sera and
their absence in HD sera (Fig.
1).
Subsequently, the correlation between clinical
factors and anti-TSTD2 antibody levels was examined in the aCI
cohort. A significant elevation in antibody levels was observed in
males, and in those with hypertension, who were older, and with a
smoking history and a history of alcohol consumption (Fig. 4), all of which are risk factors for
atherosclerosis (26-28).
These findings were consistent with the results of the Spearman's
correlation analyses, which revealed a positive correlation between
the serum TSTD2 antibody levels and maximum IMT and smoking
(Table I). Maximum IMT, plaque score
and CAVI (left and right) were also associated with serum TSTD2
antibody levels in the CKD cohort (Table II). IMT and plaque scores are widely
accepted as indicators of atherosclerosis (29,30).
Thus, the TSTD2 antibody may predominantly reflect the development
of atherosclerosis, and may thus be associated with the presence of
aCI and CKD.
Spearman's correlation analysis revealed that blood
sugar, a typical diabetes mellitus (DM) marker, was related to the
serum TSTD2 antibody levels (Table
I). This correlation did not remain significant when the
presence of DM was accounted for (Fig.
4F). The AUC of type-2 CKD (nephrosclerosis) was higher than
that for type-1 CKD (diabetic kidney disease) (Fig. 3B and C). Consequently, it can be concluded that
the serum TSTD2-Abs levels do not directly reflect DM, but may be
related to DM-induced atherosclerotic disorders, including CKD.
This is in contrast to anti-SH3BP5 antibody marker, which is mainly
related to DM and, along with that, with type-1 CKD (31). Anti-DIDO1 antibody marker has been
found to be equally associated with all three types of CKD, but has
shown no association with DM (14).
TSTD2 is a thiosulfate sulfurtransferase (31). Although the function of TSTD2 has not
yet been fully elucidated, its expression in the soleus feed artery
can be upregulated by endurance exercise training (32). TSTD2 can function in the downstream
region of the (pro)renin receptor signaling pathway (33). The renin-angiotensin system plays a
key regulatory role in hypertension (34). This is consistent with the finding of
the present study that the TSTD2 antibody levels were strongly
associated with hypertension (Fig.
4C). If TSTD2 plays a causal role in the regulation of
hypertension, it may be used as a therapeutic target to prevent the
development of atherosclerosis. Among the other correlated factors
in Fig. 4, the habit of smoking is
one of the major risk factors for the development of
atherosclerosis (35). Age may be
indirectly associated with atherosclerosis as a confounding factor.
The male sex and alcohol consumption may also be associated with
the smoking habit and indirectly with atherosclerosis.
Reactive oxygen species (ROS) are considered to
contribute to vascular inflammation in atherosclerosis as a result
of mediating various signaling pathways (36,37). As
a result, there are increasing reports that the excessive
production of ROS is implicated in the pathogenesis of acute and
chronic cardiovascular diseases and renal dysfunction (38-40).
TSTD2 is one of the TST-like domains and is known as rhodanese
(41). It is a mitochondrial enzyme
that catalyzes sulfur transfer through multiple molecular pathways
and is known for its ability to reduce the antioxidants glutathione
and thioredoxin, detoxify ROS and regulate cellular homeostasis
(42,43). Although, to the best of our
knowledge, there have been no reports to date demonstrating a
direct association between TSTD2 function and ROS, TST has
antioxidant effects, and smoking, alcohol consumption, and
hyperglycemia have the potential to cause vascular endothelial
damage due to oxidative stress and the production of free radicals.
It was hypothesized speculated that the levels of TSTD2, which is
considered to contribute to the detoxification of ROS, are
increased in blood, resulting in an elevation in the levels of
anti-TSTD2 antibodies.
The early stages of atherosclerosis are usually
accompanied by elevated autoantibody levels (44). Autoantibodies are stable and easy to
detect in patients' sera (45).
Hence, atherosclerosis-induced autoantibodies can be used for the
early diagnosis of atherosclerosis-related diseases. The present
study identified the TSTD2 antibody as a novel biomarker that may
be used for the detection of atherosclerosis-related aCI and CKD.
However, further research is required to evaluate the sensitivity
and specificity using validation cohorts, including
atherosclerosis-related diseases, as well as disease controls.
Supplementary Material
Subject information of the Sawara
stroke cohort.
Subject information of the Kumamoto
CKD cohort.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported, in part, by research
grants from the Japan Science and Technology Agency (Exploratory
Research No. 14657335) and JSPS KAKENHI (grant nos. 20K17953,
22K09227, 17K16626, 22K07273 and 19K09451).
Availability of data and materials
All results of the ProtoArray® Human
Protein Microarrays are available in the Figshre database
(https://doi.org/10.6084/m9.figshare.21510009.v1).
Authors' contributions
MK, TMac, HK, YI and TH conceived and designed the
study. MK, BSZ, SYL, HW and AAd performed the experiments and
acquired the data. YY, TMac, SM, IK, TW, AAo, KK and HT contributed
the reagents, materials, analysis tools or patient data. YY, AAd
and TMat analyzed and interpreted the data. BSZ, TMat and HK
performed the statistical analyses. MK, BSZ, SYL, TMac, YI and TH
drafted the manuscript. SM, TW and HT confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the principles of the 1913 revision of the Declaration of Helsinki
and with the approval of the Ethical Review Committee of Chiba
University, Graduate School of Medicine and cooperative hospitals
(approval no. 2018-320). The research on recombinant DNA was
conducted with the permission of the Graduate School of Medicine,
Chiba University, and following Japanese regulations. Each
participant provided written informed consent to participation and
publication.
Patient consent for publication
Not applicable.
Competing interests
The present study was performed in collaboration
with Fujikura Kasei Co., Ltd., and HK is an employee of Fujikura
Kasei Co., Ltd.
References
|
1
|
Virani SS, Alonso A, Benjamin EJ,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Delling FN, et al: Heart disease and stroke
statistics-2020 update: A report from the american heart
association. Circulation. 141:e139–e596. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mathers CD and Loncar D: Projections of
global mortality and burden of disease from 2002 to 2030. PLoS Med.
3(e442)2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McClellan WM and Plantinga LC: A public
health perspective on CKD and obesity. Nephrol Dial Transplant. 28
(Suppl 4):iv37–iv42. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Baldassarre D, Castelnuovo S, Frigerio B,
Amato M, Werba JP, De Jong A, Ravani AL, Tremoli E and Sirtori CR:
Effects of timing and extent of smoking, type of cigarettes, and
concomitant risk factors on the association between smoking and
subclinical atherosclerosis. Stroke. 40:1991–1998. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Drüeke TB and Massy ZA: Atherosclerosis in
CKD: differences from the general population. Nat Rev Nephrol.
6:723–735. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nilsson J, Hansson GK and Shah PK:
Immunomodulation of atherosclerosis: implications for vaccine
development. Arterioscler Thromb Vasc Biol. 25:18–28.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Miyashita K, Lutz J, Hudgins LC, Toib D,
Ashraf AP, Song W, Murakami M, Nakajima K, Ploug M, Fong LG, et al:
Chylomicronemia from GPIHBP1 autoantibodies. J Lipid Res.
61:1365–1376. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Obaid AH, Zografou C, Vadysirisack DD,
Munro-Sheldon B, Fichtner ML, Roy B, Philbrick WM, Bennett JL,
Nowak RJ and O'Connor KC: Heterogeneity of acetylcholine receptor
autoantibody-mediated complement activity in patients with
myasthenia gravis. Neurol Neuroimmunol Neuroinflamm.
9(e1169)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Machida T, Kubota M, Kobayashi E, Iwadate
Y, Saeki N, Yamaura A, Nomura F, Takiguchi M and Hiwasa T:
Identification of stroke-associated-antigens via screening of
recombinant proteins from the human expression cDNA library
(SEREX). J Transl Med. 13(71)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yoshida Y, Wang H, Hiwasa T, Machida T,
Kobayashi E, Mine S, Tomiyoshi G, Nakamura R, Shinmen N, Kuroda H,
et al: Elevation of autoantibody level against PDCD11 in patients
with transient ischemic attack. Oncotarget. 9:8836–8848.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang H, Zhang XM, Tomiyoshi G, Nakamura R,
Shinmen N, Kuroda H, Kimura R, Mine S, Kamitsukasa I, Wada T, et
al: Association of serum levels of antibodies against MMP1, CBX1,
and CBX5 with transient ischemic attack and cerebral infarction.
Oncotarget. 9:5600–5613. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yoshida Y, Zhang XM, Wang H, Machida T,
Mine S, Kobayashi E, Adachi A, Matsutani T, Kamitsukasa I, Wada T,
et al: Elevated levels of autoantibodies against DNAJC2 in sera of
patients with atherosclerotic diseases. Heliyon.
6(e04661)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li SY, Yoshida Y, Kobayashi E, Kubota M,
Matsutani T, Mine S, Machida T, Maezawa Y, Takemoto M, Yokote K, et
al: Serum anti-AP3D1 antibodies are risk factors for acute ischemic
stroke related with atherosclerosis. Sci Rep.
11(13450)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hiwasa T, Wang H, Goto KI, Mine S, Machida
T, Kobayashi E, Yoshida Y, Adachi A, Matsutani T, Sata M, et al:
Serum anti-DIDO1, anti-CPSF2, and anti-FOXJ2 antibodies as
predictive risk markers for acute ischemic stroke. BMC Med.
19(131)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kubota M, Yoshida Y, Kobayashi E,
Matsutani T, Li SY, Zhang BS, Mine S, Machida T, Takizawa H, Hiwasa
T and Iwadate Y: Serum anti-SERPINE1 antibody as a potential
biomarker of acute cerebral infarction. Sci Rep.
11(21772)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nishiura R, Fujimoto S, Sato Y, Yamada K,
Hisanaga S, Hara S, Nakao H and Kitamura K: Elevated
osteoprotegerin levels predict cardiovascular events in new
hemodialysis patients. Am J Nephrol. 29:257–263. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Komatsu H, Fujimoto S, Hara S, Fukuda A,
Fukudome K, Yamada K, Sato Y and Kitamura K: Recent therapeutic
strategies improve renal outcome in patients with IgA nephropathy.
Am J Nephrol. 30:19–25. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hamanaka S, Nakagawa T, Hiwasa T, Ohta Y,
Kasamatsu S, Ishigami H, Taida T, Okimoto K, Saito K, Maruoka D, et
al: Investigation of novel biomarkers for predicting the clinical
course in patients with ulcerative colitis. J Gastroenterol
Hepatol. 33:1975–1983. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vermeulen N, de Béeck KO, Vermeire S, Van
Steen K, Michiels G, Ballet V, Rutgeerts P and Bossuyt X:
Identification of a novel autoantigen in inflammatory bowel disease
by protein microarray. Inflamm Bowel Dis. 17:1291–1300.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nakashima K, Shimada H, Ochiai T,
Kuboshima M, Kuroiwa N, Okazumi S, Matsubara H, Nomura F, Takiguchi
M and Hiwasa T: Serological identification of TROP2 by recombinant
cDNA expression cloning using sera of patients with esophageal
squamous cell carcinoma. Int J Cancer. 112:1029–1035.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Matsutani T, Hiwasa T, Takiguchi M, Oide
T, Kunimatsu M, Saeki N and Iwadate Y: Autologous antibody to
src-homology 3-domain GRB2-like 1 specifically increases in the
sera of patients with low-grade gliomas. J Exp Clin Cancer Res.
31(85)2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li SY, Yoshida Y, Kobayashi E, Adachi A,
Hirono S, Matsutani T, Mine S, Machida T, Ohno M, Nishi E, et al:
Association between serum anti-ASXL2 antibody levels and acute
ischemic stroke, acute myocardial infarction, diabetes mellitus,
chronic kidney disease and digestive organ cancer, and their
possible association with atherosclerosis and hypertension. Int J
Mol Med. 46:1274–1288. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Muto M, Mori M, Hiwasa T, Takiguchi M,
Iwadate Y, Uzawa A, Uchida T, Masuda H, Sugimoto K and Kuwabara S:
Novel serum autoantibodies against talin1 in multiple sclerosis:
Possible pathogenetic roles of the antibodies. J Neuroimmunol.
284:30–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gutierrez J, Turan TN, Hoh BL and
Chimowitz MI: Intracranial atherosclerotic stenosis: Risk factors,
diagnosis, and treatment. Lancet Neurol. 21:355–368.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Crane ED, Al-Hashimi AA, Chen J, Lynn EG,
Won KD, Lhoták Š, Naeim M, Platko K, Lebeau P, Byun JH, et al:
Anti-GRP78 autoantibodies induce endothelial cell activation and
accelerate the development of atherosclerotic lesions. JCI Insight.
3(e99363)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Colafella KMM and Denton KM: Sex-specific
differences in hypertension and associated cardiovascular disease.
Nat Rev Nephrol. 14:185–201. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lechner K, von Schacky C, McKenzie AL,
Worm N, Nixdorff U, Lechner B, Kränkel N, Halle M, Krauss RM and
Scherr J: Lifestyle factors and high-risk atherosclerosis: pathways
and mechanisms beyond traditional risk factors. Eur J Prev Cardiol.
27:394–406. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Matsuda H: Health risk assessment of
long-term weight history. Nihon Koshu Eisei Zasshi. 37:817–824.
1990.PubMed/NCBI
|
|
29
|
Nezu T, Hosomi N, Aoki S and Matsumoto M:
Carotid intima-media thickness for atherosclerosis. J Atheroscler
Thromb. 23:18–31. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ojima S, Kubozono T, Kawasoe S, Kawabata
T, Miyata M, Miyahara H, Maenohara S and Ohishi M: Association of
risk factors for atherosclerosis, including high-sensitivity
C-reactive protein, with carotid intima-media thickness, plaque
score, and pulse wave velocity in a male population. Hypertens Res.
43:422–430. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cipollone R, Ascenzi P and Visca P: Common
themes and variations in the rhodanese superfamily. IUBMB Life.
59:51–59. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Padilla J, Jenkins NT, Thorne PK, Martin
JS, Rector RS, Davis JW and Laughlin MH: Transcriptome-wide RNA
sequencing analysis of rat skeletal muscle feed arteries. II.
Impact of exercise training in obesity. J Appl Physiol (1985).
116:1033–1047. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zaade D, Schmitz J, Benke E, Klare S,
Seidel K, Kirsch S, Goldin-Lang P, Zollmann FS, Unger T and
Funke-Kaiser H: Distinct signal transduction pathways downstream of
the (P)RR revealed by microarray and ChIP-chip analyses. PLoS One.
8(e57674)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang Z, Chen L, Zhong J, Gao P and Oudit
GY: ACE2/Ang-(1-7) signaling and vascular remodeling. Sci China
Life Sci. 57:802–808. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Siasos G, Tsigkou V, Kokkou E, Oikonomou
E, Vavuranakis M, Vlachopoulos C, Verveniotis A, Limperi M,
Genimata V, Papavassiliou AG, et al: Smoking and atherosclerosis:
Mechanisms of disease and new therapeutic approaches. Curr Med
Chem. 21:3936–3948. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Witztum JL: The oxidation hypothesis of
atherosclerosis. Lancet. 344:793–795. 1994.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Madamanchi NR, Vendrov A and Runge MS:
Oxidative stress and vascular disease. Arterioscler Thromb Vasc
Biol. 25:29–38. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ochoa CD, Wu RF and Terada LS: ROS
signaling and ER stress in cardiovascular disease. Mol Aspects Med.
63:18–29. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jha JC, Banal C, Chow BS, Cooper ME and
Jandeleit-Dahm K: Diabetes and kidney disease: Role of oxidative
stress. Antioxid Redox Signal. 25:657–684. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Aranda-Rivera AK, Cruz-Gregorio A,
Aparicio-Trejo OE and Pedraza-Chaverri J: Mitochondrial redox
signaling and oxidative stress in kidney diseases. Biomolecules.
11(1144)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nakajima T: Roles of sulfur metabolism and
rhodanese in detoxification and anti-oxidative stress functions in
the liver: Responses to radiation exposure. Med Sci Monit.
21:1721–1725. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Libiad M, Motl N, Akey DL, Sakamoto N,
Fearon ER, Smith JL and Banerjee R: Thiosulfate
sulfurtransferase-like domain-containing 1 protein interacts with
thioredoxin. J Biol Chem. 293:2675–2686. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nandi DL, Horowitz PM and Westley J:
Rhodanese as a thioredoxin oxidase. Int J Biochem Cell Biol.
32:465–473. 2000.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Iseme RA, McEvoy M, Kelly B, Agnew L,
Walker FR, Handley T, Oldmeadow C, Attia J and Boyle M: A role for
autoantibodies in atherogenesis. Cardiovasc Res. 113:1102–1112.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Anderson KS and LaBaer J: The sentinel
within: Exploiting the immune system for cancer biomarkers. J
Proteome Res. 4:1123–1133. 2005.PubMed/NCBI View Article : Google Scholar
|