Introduction
Meningitis/ventriculitis (MV) is a complication that
can occur following neurosurgical procedures (1-4).
Devices such as an external ventricular drain (EVD) and
intracranial pressure (ICP) monitors are critical for the
management of an elevated ICP, bleeding and monitoring in patients
undergoing neurosurgery (5).
However, the use of such devices is associated with considerable
complications, including misplacement, bleeding and infections
(2). The incidence of infection
among patients in which an EVD is used ranges between 0 and 22%,
while multidrug resistant (MDR) and extensively drug resistant
microorganisms have been reported (3). These infections are associated with
high morbidity and mortality rates, they significantly prolong the
duration of hospital stay, and thus also increase the costs and
often negatively affect the overall prognosis of patients (3,6,7).
The present study aimed to examine the risk factors
for central nervous system (CNS) infections associated with the use
of the EVD system and to assess the effects of an infection control
intervention targeting the improved management of cerebrospinal
fluid (CSF) drains.
Patients and methods
Study design
The present retrospective study performed in a
tertiary care academic neurosurgical center at the University
Hospital of Larissa (Larissa, Greece). The study included all
patients hospitalized between April, 2011 and August, 2018 at the
neurosurgical and intensive care unit (ICU) who had been receiving
therapy with EVD for developing hydrocephalus following different
neurosurgical interventions. In total, 20 patients (41.7%)
developed MV, and the cases that presented positive cultures or
Gram-positive stains in the CSF, but normal levels of glucose,
protein and cytology were considered to as contamination cases but
not infection cases, and were thus excluded from the study. In the
final pool, 48 patients out of the 65 patients were included and
these patients were divided into two groups. Data collection was
performed by two qualified research nurses (MC and CG), and the
data were reviewed and analyzed by two physicians (GF and VEG) on
the basis of a structured form that included questions related to
protocol. The protocol included points (listed and defined
statistically) related to a list of variables (items, ‘database
fields’) that were essential for the study to be completed, such as
the characteristics of the population (i.e., age, sex, medical
history), type of treatment, microbiology (including bacterial
susceptibility to antibiotics), hospital-related infections, and
the main end points (overall survival, adverse effects related to
treatment). The Institutional Review Board (IRB) of University of
Thessaly, Greece/The School of Medicine/School of Health Sciences
approved the study (IRB no. 28122/18-05-2018). The present study
was in line with the Declaration of Helsinki (1995; as revised in
Edinburgh 2000). Written informed was obtained from all the
included patients.
Following inclusion, the patients were classified
into two groups: The patients without MV (group A; n=28) and
patients who developed MV (group B; n=20).
Study endpoints
The primary outcome was in-hospital mortality.
Secondarily, the length of hospital stay, ICU stay and the Glasgow
Coma Scale were examined in patients who were discharged alive
(GCSexit).
Clinical data and definitions
The severity of the clinical condition according to
the Acute Physiological and Chronic Health Evaluation II (APACHE
II) scoring system was retrieved from records of the patients and
was then documented. The APACHE II score is a severity-of-disease
classification system that is applied within a 24-h timeframe
following the admission of a patient. An integer score from 0 to 71
is computed based on several measurements; higher scores correspond
to disease which is more severe disease and to a higher risk of
mortality (8). The findings from
computed tomography (CT) brain scans were used to describe the
cause of admission as follows: Traumatic brain injury, the presence
or absence of subarachnoid hemorrhage at the basal cisterns and
intraventicular involvement, intracerebral or/and intraventricular
hemorrhage. Nosocomial MV was defined according to definition
provide by the Centers for Disease Control and Prevention (CDC)
(9).
Infections of the CNS are diagnosed according to at
least one of the following two criteria: The presence of a
microorganism isolated from CSF and a fever >38˚C in the absence
of any other recognized cause, as well as any of the following: An
increased number of leukocytes (>10,000 per mm3 with
>50% polymorphonuclear leukocytes), increased protein (>45
mg/dl) and/or decreased glucose levels (40 mg/dl) in the CSF.
Herein, patients were considered to have a mixed bacterial
infection when two or more microorganisms were isolated from the
CSF cultures. A positive culture or Gram-positive stain in the CSF
with normal levels of glucose and protein and a normal number of
cells in the absence of symptoms was not considered an infection.
According to the institution's therapeutic protocol, patients were
considered to have been cured if they presented no fever over the
previous 10 days and no isolated microorganisms in the previous two
CNS cultures (10).
In the present study, CSF samples were obtained with
the use of an intraventricular catheter or lumbar puncture at rest.
Gram-positive MDR bacteria were methicillin-resistant
Staphylococcus aureus and Enterococcus faecium.
Colistin-resistant bacteria were considered isolates with minimal
inhibitory concentrations >2 µg/ml by both broth microdilution
and E-test methods.
The morphology, Gram stain and reactions with the
Vitek 2 GNI card (bioMe'rieuxVitek, Australia, Pty Ltd., Generic
Network Interface) were all used to identify the microbial clinical
isolates.
Statistical analysis
Data are expressed as the mean ± SD. Data were
assessed for normality using the Shapiro-Wilkes test. Nominal data
were analyzed using the Fisher's exact test. Continuous data were
analyzed using an unpaired Student's t-test or the Mann-Whitney
U-test, as appropriate. The discriminative ability of significant
variables was evaluated by using the area under the receiver
operating characteristic curve (ROC) (AUC). A P-value <0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed with the use of Statistical
Product and Service Solutions (SPSS) software, version 15 (SPSS
Inc.).
Results
A total of 48 patients were hospitalized during the
study period and were treated using an EVD. A total of 20 patients
(41.7%) developed bacterial MV, and 12 of them (60%) had MDR, which
could be treated with an intraventricular administration of
colistin, tygecycline or amikacin. Of the 48 patients, 44 (91.6%)
received intravenous (IV) antimicrobial treatment, and 12 (25%)
received IV and intraventricular antimicrobial treatment via EVD
catheters. The baseline characteristics of the patients are
presented in Tables I, II and III. In the baseline demographic and
pathophysiological characteristics of the patients, the parameters,
incidence of diabetes mellitus and the sum of EVDs changed
(Tables I and II), exhibited statistically significant
differences between the groups (P<0.05). However, following
univariate analysis for the survivors and non-survivors, the same
parameters did not exhibit any statistically significant difference
(Table IV).
 | Table IBaseline demographic characteristics
of the patients. |
Table I
Baseline demographic characteristics
of the patients.
| Parameter | All patients, n=48
(100%) | Group A, n=28
(58.3%) | Group B, n=20
(41.7%) | P-value |
|---|
| Age, mean ± SD
(years) | 55.2±17 | 47.3±16 | 60.8±17 | <0.05 |
| Sex (male), n
(%) | 32 (66.6) | 17 (35.4) | 15 (31.2) | 0.301 |
| Cause of
admission | | | | |
|
ICH + or
IVT, n (%) aSAH, n (%) | 26 (54.1) | 14 (29.1) | 12(25) | 0.363 |
|
Anterior
circulation aneurysm, n (%) | 14 (29.1) | 8 (16.6) | 6 (12.5) | |
|
Posterior
circulation aneurysm, n (%) | 4 (8.3) | 2 (4.1) | 2 (4.1) | |
|
TBI,
n (%) | 4 (8.3) | 4 (8.3) | 0 (0) | |
| Diabetes mellitus, n
(%) | 9 (18.7) | 8 (16.6) | 1 (2.0) | <0.05 |
| Hypertension, n
(%) | 31 (64.5) | 20 (41.6) | 11 (22.9) | 0.241 |
| Hyperlipidemia, n
(%) | 15 (31.2) | 11 (22.9) | 4 (8.3) | 0.155 |
| GCS of admission,
mean ± SD | 7.4±3 | 7.6±3 | 7.2±2 | 0.735 |
| APACHE II score, mean
± SD | 19.3±7 | 19.9±7 | 18.4±6 | 0.745 |
| Hemodialysis, n
(%) | 10 (20.8) | 5 (10.4) | 5 (10.4) | 0.548 |
| EVD, n (%) | 35 (72.9) | 20 (41.6) | 15 (31.2) | 0.784 |
| Distance from the
wound exit side to the burr hole, mean ± SD (mm) | 24.1±25 | 27.2±32 | 19.7±10 | 0.494 |
| Duration, mean ± SD
(days) | 8.6±6 | 7.5±6 | 10.2±7 | 0.193 |
| DC, n (%) | 18 (37.5) | 10 (20.8) | 8 (16.6) | 0.762 |
 | Table IIPathophysiological characteristics of
the patients. |
Table II
Pathophysiological characteristics of
the patients.
| Parameter | All patients, n=48
(100%) | Group A, n=28
(58.3%) | Group B, n=20
(41.7%) | P-value |
|---|
| Fever of 10 days
duration before MV, n (%) | 12(25) | 1 (2.0) | 11 (22.9) | <0.05 |
| CSF leak, n (%) | 5 (10.4) | 2 (4.1) | 3 (6.2) | 0.380 |
| Sepsis, n (%) | 27 (56.2) | 8 (16.6) | 19 (39.5) | <0.05 |
| VAP, n (%) | 27 (56.2) | 13 (27.0) | 14 | 0.105 |
| UTI, n (%) | 13 (27.0) | 5 (10.4) | 8 (16.6) | 0.089 |
| EVD | | | | |
|
Sum of EVDs
changed, mean ± SD | 1.6±1 | 1.2±0.6 | 2.3±1 | <0.05 |
|
Duration,
mean ± SD (days) | 17.6±12 | 10.8±5 | 27.1±14 | <0.05 |
|
IVentrT, n
(%) | 12(25) | 0(0) | 12(25) | <0.05 |
|
IVentrT
duration of colistin, mean ± SD (days) | 4.0±8 | 0.0±0 | 9.7±10 | <0.05 |
|
IVentrT
duration of tygecycline, mean ± SD (days) | 2.2±5 | 0.0±0 | 5.4±8 | <0.05 |
|
IventrT
duration of amikacin, mean ± SD (days) | 0.4±3 | 0.0±0 | 1.0±4 | 0.237 |
| Intravenous
antimicrobial treatment | | | | |
|
Colistin, n
(%) | 18 (37.5) | 1 (2.0) | 17 (35.4) | <0.05 |
|
Meropenem, n
(%) | 4 (8.3) | 0 (0) | 4 (8.3) | <0.05 |
|
Amikacin, n
(%) | 1 (2.0) | 0 (0) | 1 (2.0) | 0.232 |
|
Linezold, n
(%) | 1 (2.0) | 0 (0) | 1 (2.0) | 0.232 |
|
Tygecycline,
n (%) | 11 (22.9) | 1 (2.0) | 10 (20.8) | <0.05 |
|
Ceftazidime/avibactam,
n (%) | 2 (4.1) | 0 (0) | 2 (4.1) | 0.087 |
|
Trimethoprim/sulfamethoxazole,
n (%) | 1 (2.0) | 0 (0) | 1 (2.0) | 0.232 |
|
Ampicillin/sulbactam
n (%) | 5 (10.4) | 0 (0) | 5 (10.4) | <0.05 |
|
Gentamicin,
n (%) | 1 (2.0) | 0 (0) | 1 (2.0) | 0.232 |
 | Table IIIIsolated microorganisms from CSF of
patients with EVD-related infections. |
Table III
Isolated microorganisms from CSF of
patients with EVD-related infections.
| Microorganism | All 20 (100%) |
|
Acinetobacter
baumanii, n (%) | 12(60) |
|
Klebsiella
pneumoniae, n (%) | 6(30) |
|
Staphylococcus
haemolyticus, n (%) | 1(5) |
|
Polymicrobial
(Acinetobacter baumanii and Enterobacter Enterobacter
cloacae), n (%) | 1(5) |
 | Table IVUnivariate analysis for survivors and
non-survivors. |
Table IV
Univariate analysis for survivors and
non-survivors.
| Parameter | Survivors, n=31
(64.6%) | Non-survivors, n=17
(35.4%) | P-value |
|---|
| Group A, n (%) | 18 (64.3) | 10 (35.7) | 0.951 |
| Group B, n (%) | 13(65) | 7(35) | |
| Age, mean ±SD
(years) | 54.2±19 | 55.7±31 | 0.829 |
| Sex (male), n
(%) | 21 (43.7) | 11 (22.9) | 0.301 |
| Cause of
admission | | | |
|
ICH + or
IVT, n (%) | 17(35.4) | 9(18.7) | 0.792 |
|
aSAH, n
(%) | | | |
|
Anterior
circulation aneurysm, n (%) | 10 (20.8) | 4 (8.3) | |
|
Posterior
circulation aneurysm, n (%) | 2 (4.1) | 2 (4.1) | |
|
TBI,
n (%) | 2 (4.1) | 2 (4.1) | |
| Diabetes mellitus,
n (%) | 5 (10.4) | 4 (8.3) | 0.530 |
| Hypertension, n
(%) | 18 (37.5) | 13 (27.0) | 0.202 |
| Hyperlipidemia, n
(%) | 7 (14.5) | 8 (16.6) | 0.080 |
| GCS of admission,
mean ± SD | 7.9±3 | 6.5±2 | 0.174 |
| APACHE II score,
mean ± SD | 17.5±6 | 22.5±6 | <0.05 |
| Hemodialysis, n
(%) | 4 (8.3) | 6 (12.5) | 0.068 |
| EVD, n (%) | 21 (43.7) | 14 (29.1) | 0.276 |
|
Distance
from the wound exit side to the burr hole, mean ± SD (mm) | 15.4±4 | 39.8±38 | <0.05 |
|
Duration,
mean ± SD (days) | 8.6±7 | 8.7±5 | 0.974 |
| DC, n (%) | 12(25) | 6 (12.5) | 0.815 |
| Fever of 10 days
duration before MV, n (%) | 8 (16.6) | 4 (8.3) | 0.862 |
| CSF leak, n
(%) | 3 (6.2) | 2 (4.1) | 0.821 |
| Sepsis, n (%) | 19 (39.5) | 8 (16.6) | 0.342 |
| VAP, n (%) | 17 (35.4) | 10 (20.8) | 0.790 |
| UTI, n (%) | 9 (18.7) | 4 (8.3) | 0.682 |
| EVD | | | |
|
Sum of EVDs
changed, mean ± SD | 1.6±0.9 | 1.8±1 | 0.742 |
|
Duration,
mean ± SD (days) | 17.5±11 | 17.7±15 | 0.503 |
|
IVentrT, n
(%) | 7 (14.5) | 5 (10.4) | 0.601 |
|
IVentrT
duration of colistin, mean ± SD (days) | 4.3±8 | 3.5±8 | 0.832 |
|
IVentrT
duration of tygecycline, mean ± SD (days) | 1.4±4 | 3.6±7 | 0.200 |
|
IventrT
duration of amikacin, mean ± SD (days) | 0.0±0 | 1.2±5 | 0.177 |
| Intravenous
antimicrobial treatment | | | |
|
Colistin, n
(%) | 12(25) | 6 (12.5) | 0.815 |
|
Meropenem, n
(%) | 2 (4.1) | 2 (4.1) | 0.524 |
|
Amikacin, n
(%) | 0 (0) | 1 (2.0) | 0.172 |
|
Linezold, n
(%) | 1 (2.0) | 0 (0) | 0.454 |
|
Tygecycline,
n (%) | 7 (14.5) | 4 (8.3) | 0.940 |
|
Ceftazidime/avibactam,
n (%) | 1 (2.0) | 1 (2.0) | 0.660 |
|
Trimethoprim/sulfamethoxazole,
n (%) | 0 (0) | 1 (2.0) | 0.172 |
|
Ampicillin/sulbactam
n (%) | 3 (6.2) | 2 (4.1) | 0.821 |
|
Gentamicin,
n (%) | 1 (2.0) | 0 (0) | 0.454 |
| Hospital stay, mean
± SD (days) | 49.7±32 | 27.2±27 | <0.05 |
| ICU stay, mean ± SD
(days) | 34.4±21 | 21.4±16 | <0.05 |
In 12 out of the 20 (60%) patients in group B, the
isolated microorganism in the CSF was Acinetobacter
baumannii; in 6 patients (30%), Klebsiella pneumoniae
was detected; and in 1 patient (5%), Staphylococcus
haemolyticus was detected (Table
III).
Study outcomes
The clinical outcomes of the patients are presented
in Table V. The durations of
hospital stay and ICU stay were significantly lower in group A
(32.4±24 and 21.1±11 days, respectively) compared to group B
(54.7±37 and 42±24 days, respectively) (P=0.027 and P=0.001,
respectively). The characteristics of the univariate analysis for
survivors and non-survivors are presented in Table IV. The APACHE II score was
significantly lower, and the EVD distance from the wound exit side
to the burr hole was significantly shorter in the survivors
compared to the non-survivors (17.5±6 and 15.4±4 mm vs. 22.5±6 and
39.8±38 mm, respectively) (Table
IV).
 | Table VOutcomes of the patients with
external ventricular drainage. |
Table V
Outcomes of the patients with
external ventricular drainage.
| Parameter | All patients, n=48
(100%) | Group A n=28 | Group B n=20 | P-value |
|---|
| Mortality, n
(%) | 17 (35.4) | 10 (35.7) | 7(35) | 0.959 |
| Hospital stay, mean
± SD (days) | 41.7±32 | 32.4±24 | 54.7±37 | <0.027 |
| ICU stay, mean ± SD
(days) | 29.8±20 | 21.1±11 | 42±24 | <0.001 |
| GCSexit | 9.3±5 | 9.2±5 | 9.4±5 | 0.885 |
| Cured, n (%) | 31 (64.5) | 18 (64.3) | 13(65) | 0.959 |
ROC analysis demonstrated that the durations of
hospital stay [AUC, 0.822; standard error (SE), 0.760; 95%
confidence interval (CI), 0.673-0.970; P<0.05], ICU stay (AUC,
0.737; SE, 0.079; 95% CI, 0.583-0.892; P<0.05) and GCSexit (AUC,
0.968; SE, 0.026; 95% CI, 0.916-1.000; P<0.05) could predict
poor outcomes (Table VI). Notably,
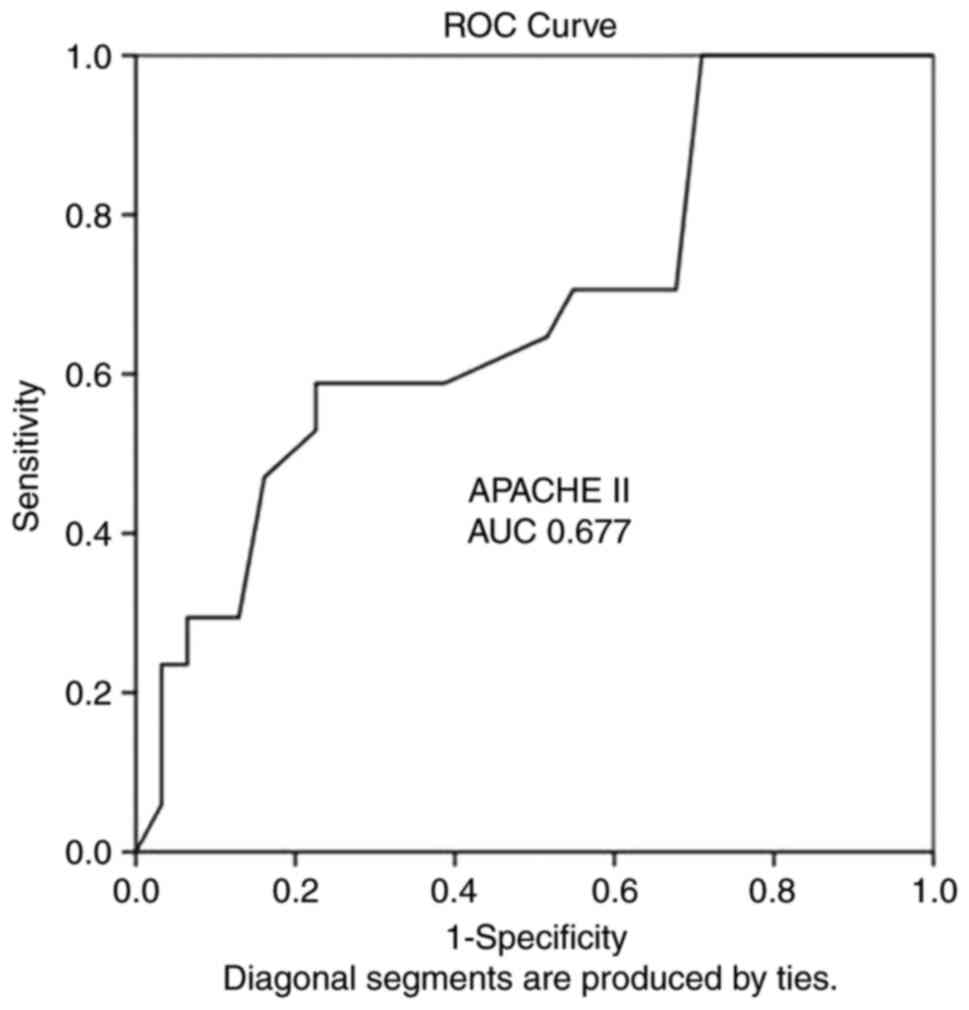
ROC analysis also revealed that the APACHE II score (AUC, 0.677;
SE, 0.082; 95% CI, 0.516-0.839; P=0.044) and with a cut-off value
of 14 could predict poor outcomes (mortality) with a sensitivity of
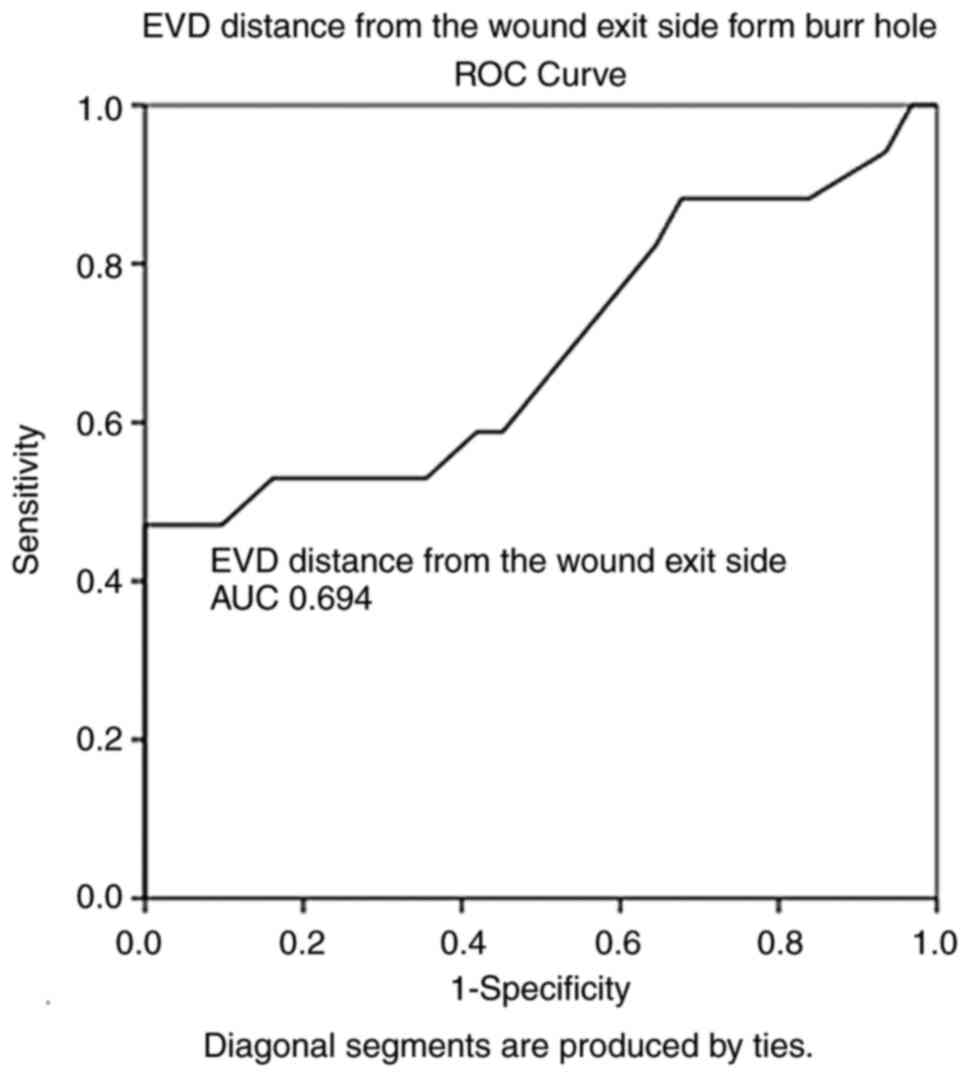
100% and a specificity of 71%. In addition, the EVD distance from
the wound exit side from the burr hole (AUC, 0.694; SE, 0.088; 95%
CI, 0.521-0.866; P=0.028) and with a cut-off of 11.5 mm could
predict poor outcomes (mortality) with a sensitivity of 88% and a
specificity of 83% (Figs. 1 and
2, and Table VI).
 | Table VIROC analysis (outcome:
Mortality). |
Table VI
ROC analysis (outcome:
Mortality).
| Parameter | AUC | SE | 95% CI,
lower-upper | P-value |
|---|
| APACHE II
score | 0.677 | 0.082 | 0.516-0.839 | <0.0044 |
| EVD; distance from
the wound exit side to the burr hole | 0.694 | 0.088 | 0.521-0.866 | <0.028 |
| Duration of
hospital stay | 0.822 | 0.760 | 0.673-0.970 | <0.05 |
| Duration of ICU
stay | 0.737 | 0.079 | 0.583-0.892 | <0.05 |
| GCSexit | 0.968 | 0.026 | 0.916-1.000 | <0.05 |
The cure rates did differ not significantly between
the groups: 18 out of 28 (64.3%) vs. 13 out of 20 (65%) (P=0.951).
The GCS exit was 9.2±5 in group A and 9.4±5 in group B (P=0.885)
(Table V).
Discussion
The findings of the present study suggest that in
patients receiving therapy with EVD for developed hydrocephalus,
the severity of the clinical condition according to the APACHE II
score was associated with survival. In addition, it was found that
the EVD-related distance from the wound exit side to the burr hole
could predict poor outcomes (mortality) in these patients. Notably,
EVD duration over a mean of ≥17 days does not affect the outcomes
of patients. Furthermore, changing EVDs between 9 and 12 days is a
safe procedure without any risk for the development of MV. As
regards the pathophysiological characteristics of the patients
(Table II), the mean duration of
EVD change was 17.6 days for all patients, 10.8 days for group A
and 27.1 days for group B; the difference between the groups was
statistically significant. Thus, the EVD duration, with a mean time
of ≥17 days, did not affect the outcomes of patients. However, the
mean duration of EVD change for survivors and non-survivors was
8.6±7 and 8.7±5 days and the different was not statistically
significant (Table IV). Thus, a
mean change of EVD between 9 and 12 days is a safe procedure
without any risk for the development of MV and an EVD change after
>12 or (8.7±5) days is critical for avoiding drain-related
infections in the CNS.
EVD-related infections may arise from catheter
colonization by the insertion of skin flora during placement
(11). In the present study, the EVD
distance from the wound exit side to the burr hole was related to
poor outcomes. Conversely, a distance >11.5 mm could predict a
better outcome.
Previous studies have reported various lengths of
hospital stays in patients with MV following EVD placement, with a
mean duration of 42-128 days (12-14).
Post-surgical intracranial infection is a severe complication
secondary to neurosurgical procedures, usually defined as bacterial
(MV) following neurosurgery. It is main cause of prolonged hospital
stay, increased medical costs, severe neurological dysfunction and
even mortality (1). Although the
incidence of MV is not high, the case fatality rate is usually
>20% once MV occurs due to the special anatomical and
physiological structure of the CNS. Effectively reducing the risk
factors of MV and early identification and treatment is of utmost
clinical significance to reduce the incidence and mortality rates
associated with post-operative MV and to improve the prognosis of
patients (1). In the present study,
the duration of hospital stay was 32.4±24 days, which was shorter
than that in the existing literature (1,12-14).
Furthermore, previous studies have reported that a
longer drainage duration does not represent an increased risk of
developing MV infection (15-17).
On the other hand, other studies have reported that the risk of
becoming infected with MV is significantly lower after day 9 of EVD
(1,18).
Of note, other studies have concluded that a longer
drainage duration affects the chances of infection (17,19).
However, in the present study, the mean EVD duration was 17.6±12
days (10.8±5 days for those who had not developed MV and 27.1±14
days for patients with MV), and there was no association with poor
outcomes. This may indicate that EVD duration, with a mean time of
≥17 days, does not affect the outcomes of patients. In addition,
for the periods mentioned above, the sum of EVDs change was 1.6±1
in the total sample (1.2±0.6 and 2.3±1 for the patients without MV
and those with MV, respectively). Thus, an EVD changed between 9
and 12 days is safe and does not pose any risk for the development
of MV.
Studies have reported that the APACHE-II scoring
system is useful for classifying patients according to their
disease severity. Furthermore, a previous study demonstrated a
negative relationship between a high score and the length of the
hospital stay (20). In the present
study, the value of the APACHE II score was determined as a mean
score of 17.5±6 and was associated with an increased survival.
The present study had some limitations which should
be mentioned. The main limitation was that it was conducted in a
single center, and its retrospective nature was associated with
possible errors in collecting and interpreting the data from the
clinical history.
In conclusion, the present study determined the
value of the APACHE II score and its association with an increased
survival in patients treated with EVD. In addition, it was found
that the EVD-related distance from the wound exit side of the burr
hole could predict poor outcomes in these patients. Notably, EVD
duration, with a mean time of ≥17 days, did not affect the outcomes
of patients. As a result, avoiding an EVD change after >12 days
is critical for avoiding drain-related infections in the CNS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CG and GF conceptualized the study. CG, VEG, MC,
DAS, GF, DM and KNF made a substantial contribution to data
interpretation and analysis, and wrote and prepared the draft of
the manuscript. CG and GF analyzed the data and provided critical
revisions. CG and GF confirm the authenticity of all the raw data.
All authors contributed to manuscript revision, and have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board (IRB) of University
of Thessaly, Greece/The School of Medicine/School of Health
Sciences approved the study (IRB no. 28122/18-05-2018). The present
study was in line with the Declaration of Helsinki (1995; as
revised in Edinburgh 2000). Written informed was obtained from all
the included patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li C, Zhou P, Liu Y and Zhang L: Treatment
of ventriculitis and meningitis after neurosurgery caused by
Carbapenem-Resistant enterobacteriaceae (CRE): A challenging topic.
Infect Drug Resist. 16:3807–3818. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hussein K, Rabino G, Feder O, Eghbaryeh H,
Zayyad H, Sviri G, Benenson R and Paul M: Risk factors for
meningitis in neurosurgical patients with cerebrospinal fluid
drains: Prospective observational cohort study. Acta Neurochir
(Wien). 161:517–524. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fotakopoulos G, Makris D, Chatzi M,
Tsimitrea E, Zakynthinos E and Fountas K: Outcomes in
meningitis/ventriculitis treated with intravenous or
intraventricular plus intravenous colistin. Acta Neurochir (Wien).
158:603–610. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tsolaki V, Karvouniaris M, Manoulakas E,
Kotlia P, Karadontas V, Fotakopoulos G, Zakynthinos E and Makris D:
Intraventricular CNS treatment with Colistin-Tigecycline
combination: A case series. J Crit Care. 47:338–341.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lane PL, Skoretz TG, Doig G and Girotti
MJ: Intracranial pressure monitoring and outcomes after traumatic
brain injury. Can J Surg. 43:442–448. 2000.PubMed/NCBI
|
|
6
|
Neuberger A, Shofty B, Bishop B, Naffaa
ME, Binawi T, Babich T, Rappaport ZH, Zaaroor M, Sviri G, Yahav D
and Paul M: Risk factors associated with death or neurological
deterioration among patients with Gram-negative postneurosurgical
meningitis. Clin Microbiol Infect. 22:573.e1–e4. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Georgakopoulou VE, Gkoufa A,
Aravantinou-Fatorou A, Trakasi I, Trakas N, faropoulos K, Paterakis
K and Fotakopoulos G: Lower respiratory tract infections due to
multi-drug resistant pathogens in central nervous system injuries
(Review). Biomed Rep. 18(30)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
İşeri Nepesov M, Kılıç Ö and Dinleyici EÇ:
Successful intraventricular colistin treatment in resistant
Klebsiella pneumoniae meningitis. J Infect Dev Ctries.
16:1226–1229. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Horan TC, Andrus M and Dudeck MA: CDC/NHSN
surveillance definition of health care-associated infection and
criteria for specific types of infections in the acute care
setting. Am J Infect Control. 36:309–332. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nseir S, Favory R, Jozefowicz E, Decamps
F, Dewavrin F, Brunin G, Di Pompeo C, Mathieu D and Durocher A: VAT
Study Group. Antimicrobial treatment for ventilator-associated
tracheobronchitis: A randomized, controlled, multicenter study.
Crit Care. 12(R62)2008.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Tsang ACO and Leung GKK: External
ventricular drain infections. 2012 doi: 10.5772/32669.
|
|
12
|
De Bonis P, Lofrese G, Scoppettuolo G,
Spanu T, Cultrera R, Labonia M, Cavallo MA, Mangiola A, Anile C and
Pompucci A: Intraventricular versus intravenous colistin for the
treatment of extensively drug resistant Acinetobacter baumannii
meningitis. Eur J Neurol. 23:68–75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Remeš F, Tomáš R, Jindrák V, Vaniš V and
Setlík M: Intraventricular and lumbar intrathecal administration of
antibiotics in postneurosurgical patients with meningitis and/or
ventriculitis in a serious clinical state. J Neurosurg.
119:1596–1602. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang JH, Lin PC, Chou CH, Ho CM, Lin KH,
Tsai CT, Wang JH, Chi CY and Ho MW: Intraventricular antimicrobial
therapy in postneurosurgical Gram-negative bacillary meningitis or
ventriculitis: A Hospital-based retrospective study. J Microbiol
Immunol Infect. 47:204–210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pfisterer W, Mühlbauer M, Czech T and
Reinprecht A: Early diagnosis of external ventricular drainage
infection: results of a prospective study. J Neurol Neurosurg
Psychiatry. 74:929–932. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lyke KE, Obasanjo OO, Williams MA, O'Brien
M, Chotani R and Perl TM: Ventriculitis complicating use of
intraventricular catheters in adult neurosurgical patients. Clin
Infect Dis. 33:2028–2033. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Hoefnagel D, Dammers R, Ter Laak-Poort MP
and Avezaat CJ: Risk factors for infections related to external
ventricular drainage. Acta Neurochir (Wien). 150:209–214.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Edwards JR, Peterson KD, Andrus ML, Tolson
JS, Goulding JS, Dudeck MA, Mincey RB, Pollock DA and Horan TC:
NHSN Facilities. National Healthcare Safety Network (NHSN) Report,
data summary for. 2006, issued June 2007. Am J Infect Control.
35:290–301. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schade RP, Schinkel J, Visser LG, Van Dijk
JM, Voormolen JH and Kuijper EJ: Bacterial meningitis caused by the
use of ventricular or lumbar cerebrospinal fluid catheters. J
Neurosurg. 102:229–234. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Naved SA, Siddiqui S and Khan FH:
APACHE-II score correlation with mortality and length of stay in an
intensive care unit. J Coll Physicians Surg Pak. 21:4–8.
2011.PubMed/NCBI
|