Introduction
Cystic neutrophilic granulomatous mastitis (CNGM) is
a rare subtype of mastitis with a distinct histological pattern
that is associated with the Corynebacterium species
(1-3).
The first well-documented compilation of disease associated with
this species was published in 1997 by Funke et al (4). The association of
Corynebacterium species with mastitis was first postulated
in a 2003 review of mastitis cases by Taylor et al (3). Corynebacterium is a lipophilic
Gram-positive bacillus with an affinity for adipose rich breast
tissue. The organism is fastidious to growth in culture media and
contemporary methods, including PCR for 16S ribosomal RNA and
matrix-assisted laser desorption/ionization time-of-flight mass
spectrometry (MALDI-TOF-MS) have facilitated more rapid and
reliable diagnoses (5). Gene
sequencing methods, such as PCR for 16S ribosomal RNA are
considered the reference for the validation of MALDI-TOF-MS
data.
To date, in the literature, a total of 141 cases of
CNGM presenting at a mean age of 35 years have been reported since
2002 and only one of these cases was of African descent (1). To the best of our knowledge, the
present study describes the first reported case of CNGM in a
patient of Afro-Caribbean descent. It is suspected these cases may
be underreported in the Caribbean and the scarcity of available
data render accurate diagnoses and appropriate management a
challenge in this population.
Cystic granulomatous mastitis predominates in the
minority ethnic groups of the geographical territories with the
largest published cohorts (1,6). The
first case control series demonstrating an association of CNGM with
the Corynebacterium species published in Auckland, New
Zealand included predominantly Māori and Pacific islanders
(3). A predilection of the disease
among the Hispanic population from Central America or Mexico has
been reported in North America (7).
These findings are consistent with the overall epidemiological
trend of granulomatous mastitis in minority ethnic groups,
including Hispanic, Black, Asian and African American women
(6,8). The case described herein had all the
characteristic clinicopathological features of CNGM and therefore
provides an ideal educational model to guide the diagnosis and
management of this unusual breast pathology in a resource-limited
Caribbean setting.
Case report
Case summary
A 32-year-old woman (gravida 2, para 2) with no
known chronic medical illnesses presented to the Barbados Cancer
Society-Breast Screening Programme with a 2-week history of a left
breast lump and breast pain. The pain had resolved by the time of
presentation, but the lump persisted. There was no associated
nipple discharge or trauma to the breast. She had no previous
breast surgeries. A physical examination revealed a healthy-looking
young woman. There was a 6x6 cm mobile, non-tender left breast mass
between the 2 to 4 o'clock positions 4 cm from the nipple. The
right breast and axilla were normal.
In view of the persistence of the mass, a breast
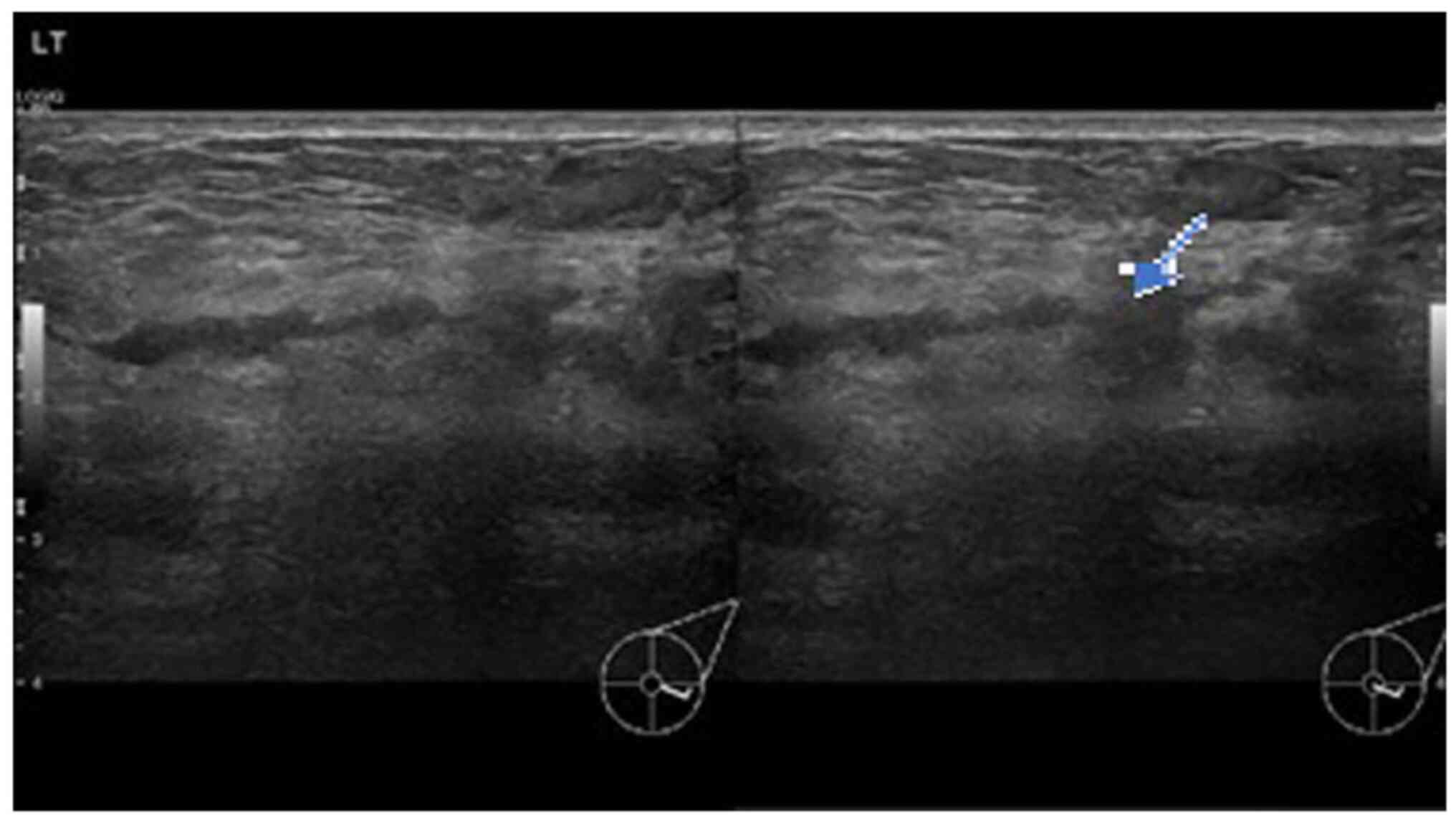
ultrasound was performed. The initial examination yielded normal
results. A second ultrasound repeated after 10 days (Fig. 1) revealed an area of architectural
distortion with dilated ducts measuring up to 0.3 cm in diameter
located at the 3 to 4 o'clock position of the left breast. There
was no discrete mass or significant axillary lymphadenopathy. The
findings were consistent with mastitis. The other differential
diagnosis was inflammatory breast carcinoma. The patient was
treated with a course of oral Augmentin (amoxicillin and clavulanic
acid) and although the swelling decreased in size, it did not
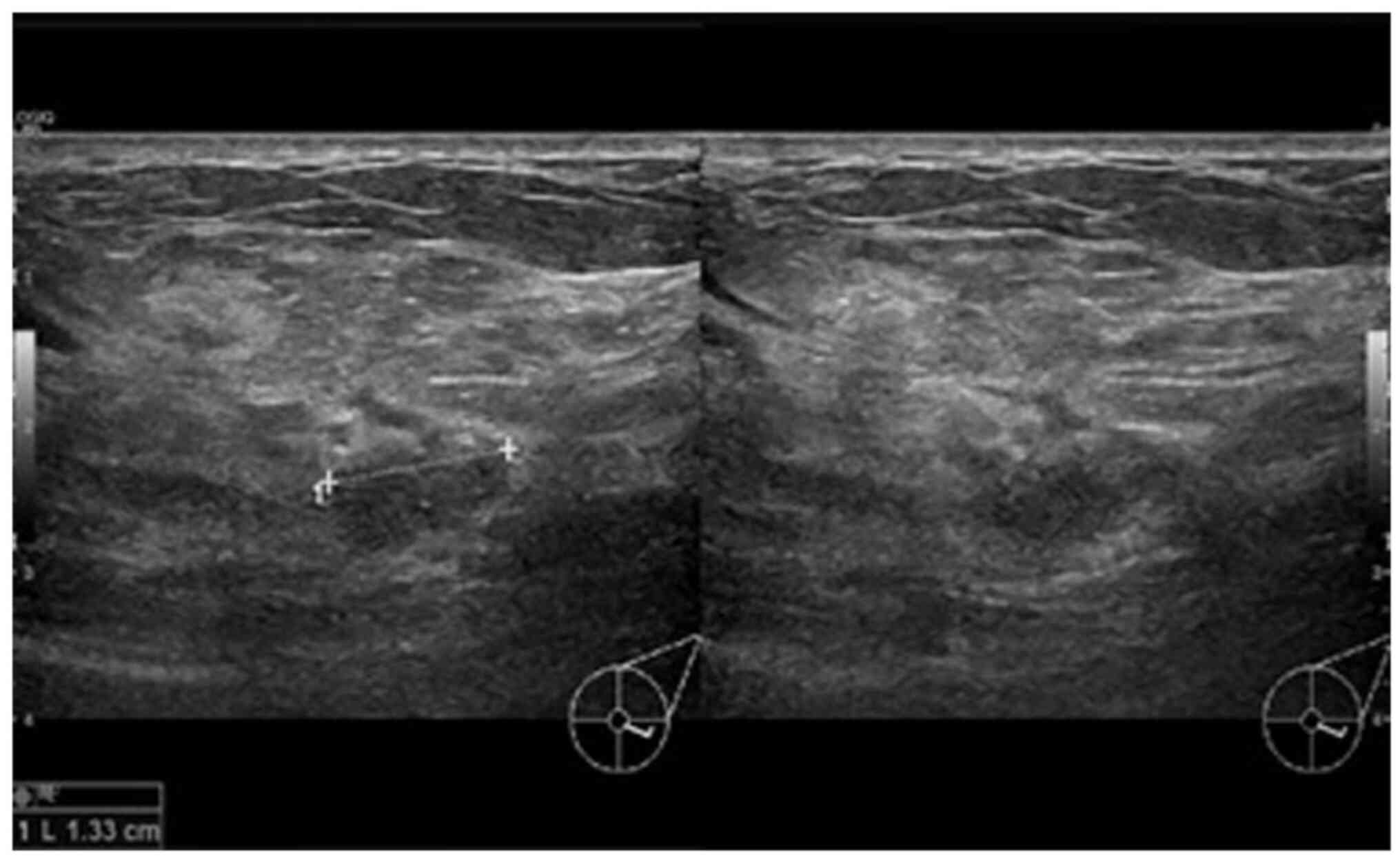
completely resolve. A third ultrasound (Fig. 2) after 2 weeks of treatment was
suggestive of a 1.3-cm hypoechoic mass with indistinct borders at
the 4 0'clock position. A decision was made to proceed with a
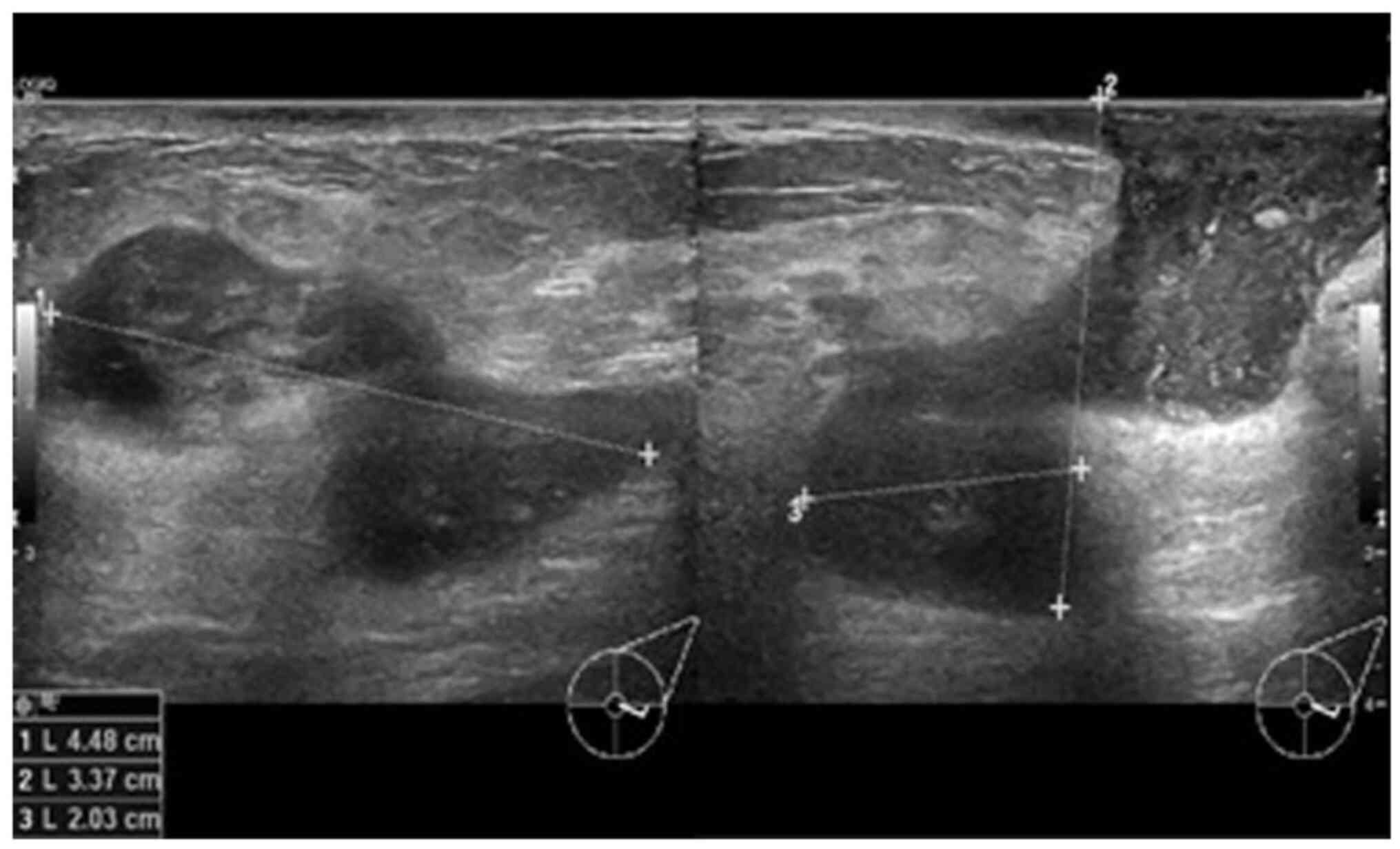
biopsy. A repeat ultrasound (Fig. 3)
was conducted after 6 months, which revealed hypoechoic tubular
collections and fistulous tracts towards the skin.
Histological findings
Sections (4-µm-thick) were cut from
paraffin-embedded tissue that was fixed in 10% neutral buffered
formalin for 12 h. The Gram-Twort modified method for staining
bacteria was followed and all solutions were freshly prepared by a
histotechnologist according to the laboratory's standard protocol
at room temperature. In summary, the tissue sections were stained
with Lillie's crystal violet for 4 min and treated with Lugol's
iodine solution for 4 min. The second stain was Twort's working
solution for 5 min; 2% acetic acid in absolute alcohol was used for
decolorization. All reagents were sourced from Stat Lab Medical
Products. The stained slides were then mounted and viewed using an
Olympus pathology light microscope (Olympus Corporation). The
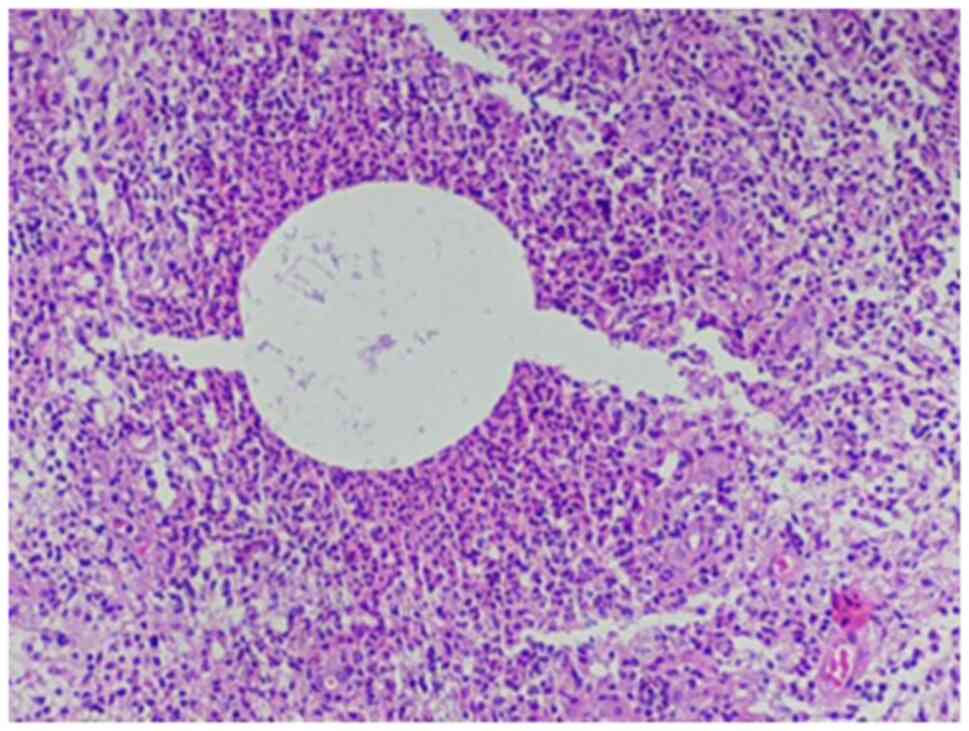
histopathological analysis of the biopsy sample using hematoxylin
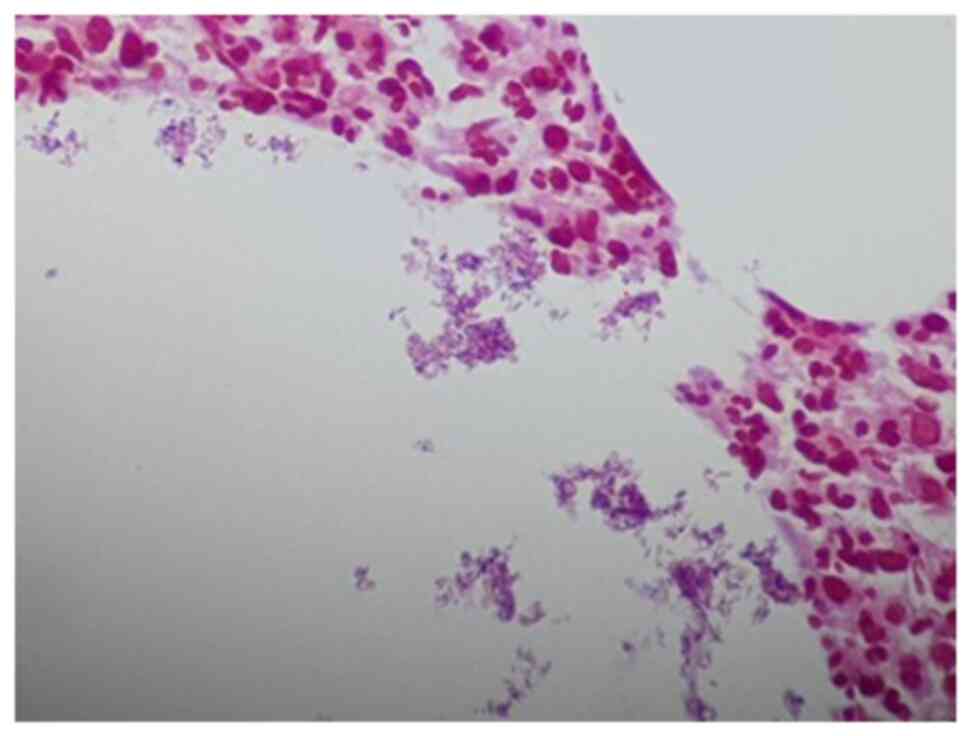
and eosin staining (Figs. 4 and
5) revealed cystic spaces surrounded
by neutrophils. The Gram stain (Fig.
6) revealed Gram-positive blue rods within these spaces
morphologically consistent with Corynebacterium species. The
features were consistent with CNGM.
Discussion
Granulomatous mastitis is a heterogenous group of
diseases with a diverse clinical picture and association (9). It was first clearly described in 1972
by Kessler and Wolloch (10) as a
lobulocentric pattern of granulomatous inflammation not associated
with trauma, infection, or exogenous material. Granulomatous
mastitis, as described by Kessler and Wolloch (10), is termed idiopathic granulomatous
mastitis. However, there is significant overlap in the literature
and a number of cases reviewed in publications on idiopathic
granulomatous mastitis would meet the diagnostic criteria for CNGM
(11,12).
The link between CNGM and Corynebacterium
infection has been reported in multiple studies (1-3,7).
The identification of this association places it outside the
category of idiopathic granulomatous mastitis (11,12). It
is fitting that this should be recognized as a distinct diagnostic
entity in order to avoid overlap with other subtypes of
granulomatous mastitis.
The case described herein was a nonlactating
multiparous woman of 32 years of age, which fits the demographic
profile for idiopathic granulomatous mastitis (12). The radiological findings in this
patient included architectural distortion, with dilated ducts in
the pre-treatment breast ultrasound and a hypoechoic mass with
indistinct borders post-treatment with antibiotics. Radiological
findings in CNGM have been sporadically reported in case reports
and case series, and include a spectrum of findings (1,13). A
mass is the most common radiographical feature followed by dilated
ducts (1). Edema with no mass,
abscesses and sinuses are also observed. The majority of cases are
reported as Breast Imaging-Reporting and Data System (BIRADS) score
4 (suspicious). Malignancy is often suspected based on the finding
of an irregular or ill-defined hypoechoic mass with shadowing
observed on a breast ultrasound (13). The finding of a hypoechoic mass with
indistinct borders in the case described in the present study is
congruent with the reported radiological findings for granulomatous
lobular mastitis (GLM) and CNGM (1,13,14). The
literature reviewed from case reports and series has documented one
radiological finding per patient. To the best of our knowledge, the
present study describes the first case documenting radiological
findings before and after treatment (1,13). The
spectrum of findings reported for the case described herein,
including dilated ducts followed by a mass and then fistulous
tracts suggests a progression of the disease, despite therapy. The
initial finding of dilated ducts was consistent with mastitis;
however, the presence of a mass despite antibiotic therapy
necessitated biopsy in this patient in order to exclude neoplasia.
Inflammatory breast carcinoma was considered as a differential
diagnosis for this case. Both inflammatory breast carcinoma and GLM
can reveal hypoechoic masses and the ultrasound findings of the two
can overlap such that biopsy of the lesion is required for a
definitive diagnosis (14).
In the present study, a histopathological evaluation
revealed the characteristic lobulocentric lipogranulomatous
inflammation with neutrophils and multiple bacterial rods confirmed
on a Gram stain. These rods exhibited a palisading arrangement with
the formation of cuneiform shapes and grouping into V shapes
considered to be morphologically consistent with coryneform species
(13) (Fig. 6). The case described herein
illustrates how well-defined light microscopic pathological
features can distinguish CNGM from cases that may overlap
clinically with idiopathic granulomatous mastitis.
Pathological diagnosis can be challenging, and some
cases have a palisading pattern of granulomatous inflammation
without the characteristic microcysts or lipid vacuoles. These
cases may represent an early phase in the evolution of the disease
(15). Gram stains are used to
identify bacterial organisms, although these can yield
false-negative results. The rate of false-negative diagnoses with a
Gram stain can be reduced by focusing more closely on the lipid
vacuoles that form microcysts to detect sparsely occurring
Gram-positive rods. In addition, the use of multiple stains or
thicker sections that increases the number of bacteria on the slide
can also reduce false-negative diagnoses (15). A careful search for organisms is
necessary in all cases to avoid the misclassification of CNGM as
non-infectious or idiopathic and facilitate appropriate
antimicrobial management for patients.
Microbial cultures would be ideal in all cases
demonstrating clinical and radiological features suggestive of
granulomatous mastitis. False-negative Gram stain results can occur
due to the low sensitivity of histochemical stains (15). In addition, the organism is difficult
to culture and additional molecular diagnostic tests, including
PCR, next-generation sequencing and MALDI-TOF can improve
diagnostic accuracy, if available (5,7,10,15,16).
The differential diagnoses for CNGM includes
infectious and non-infectious diseases. Non-infectious causes of
lipogranulomatous inflammation, including fat necrosis and silicone
implants can be distinguished from CNGM by the absence of abundant
neutrophils and the presence of polarizable material in giant cells
(1). Autoimmune causes of
necrotizing granulomatous inflammation, including granulomatosis
with polyangiitis and rheumatoid arthritis have been reported in
the breast. Granulomatosis with polyangiitis can be distinguished
microscopically from CNGM by the presence of necrotizing vasculitis
(1,17). Rheumatoid nodules presenting as
granulomatous mastitis have a central area of fibrinoid necrosis
palisaded by histiocytes and plasma cells. Abundant neutrophils
have been described in rheumatoid nodules, but lipogranulomas are
not present (18). Serology for ANCA
antibodies and rheumatoid factor supports the diagnosis of these
specific autoimmune causes of granulomatous mastitis (1,17,18).
Infectious causes of granulomatous mastitis,
including tuberculosis can usually be excluded by the
characteristic light microscopic features of the granulomatous
inflammation. The granulomas of primary tuberculosis of the breast
are well-formed, necrotizing and lack the neutrophils that
characterize CNGM (13,19,20).
Tuberculous granulomas of the breast also involve both ducts and
lobules, while CNGM is confined to the lobule. Ziehl-Neelsen stain,
PCR and culture would support the diagnosis of tuberculosis and
other mycobacterial infections of the breast (19,20).
Sarcoidosis is an idiopathic cause of granulomatous inflammation
that is usually multisystemic and involves the breast in <1% of
cases. Sarcoid granulomas are well-formed, typically
non-necrotizing and are composed mainly of epithelioid histiocytes
and Langhans giant cells (13,20,21). The
role of autoimmunity or immune dysregulation in the pathogenesis
and progression of CNGM is uncertain. Corticosteroids and other
anti-inflammatory agents have been used in the management of CNGM;
however, there is a scarcity of available evidence of their
efficacy alone or in combination with antibiotic therapy (1).
Despite the wealth of evidence associating
Corynebacterium with CNGM, a causal link has not yet been
established. A proposed alternative hypothesis is that
Corynebacterium colonizes necrotic fat parenchyma following
granulomatous inflammation and is not the causative agent. However,
the detection of Corynebacterium associated with a host
immune response in deep breast parenchyma early in the course of
the disease, as well as the therapeutic response to antibiotics in
some cases favors an etiological link (2,3,22).
An antecedent initiating factor is not always
described in cases of CNGM. However, the organism was isolated in
lactating women with mastitis in one case series. In the same
series, a history of trauma was documented in two non-lactating
persons with CNGM from whom Corynebacterium was isolated
(22). These findings suggest that
the breach of the skin barrier secondary to trauma or breast
feeding is a possible route of infection. Colonization of
lactiferous ducts will allow spread to the terminal duct lobular
unit, resulting in lobulocentric inflammation.
Emerging evidence suggests a potential role of other
bacterial organisms in the pathogenesis of CNGM. A series of 40
cases from the Shenzhen Traditional Chinese Medicine Hospital with
a CNGM-like pattern of inflammation all had negative Gram stains
(16). Notably,
Corynebacterium species was not the most common organism
detected using the next-generation sequencing of paraffin-embedded
tissue. Other bacterial species, including Pseudomonas
aeruginosa were associated in these cases (16). That study, although small, provided
intriguing evidence suggesting the association of organisms other
than Corynebacterium and Mycobacterium with
granulomatous mastitis. This finding may explain the poor response
to antibiotics directed against Corynebacterium species in
cases diagnosed only based on a Gram stain. Neither microbial
culture nor molecular testing was performed in the case described
herein, precluding definitive proof that Corynebacterium was
the associated organism.
A major obstacle to the collation of data on CNGM is
a lack of standardized nomenclature of the entity. Case series on
idiopathic granulomatous mastitis and granulomatous mastitis
include patients that would meet the diagnostic criteria for CNGM
(11,23,24). A
standardization of the diagnostic nomenclature will enable more
comprehensive research to develop protocols for the diagnosis and
treatment of this entity. The application of the term CNGM should
thus perhaps only be used in cases that exhibit the
histomorphological pattern and evidence of infection with
Gram-positive rods morphologically consistent with
Corynebacterium species (24). An effort should be made to culture
the organism or conduct ancillary molecular tests for confirmation,
if possible.
The present study describes a rare classic case of
CNGM occurring in a woman of Afro-Caribbean descent. The
clinicopathological features were characteristic, and Gram-positive
bacilli were identified on a Gram stain. This case contributes to
the increasing evidence that CNGM is a histomorphologically
distinct entity associated with bacterial infection. The
pathologist can play a critical role in patient management by
recognizing the pattern of inflammation and requesting the
appropriate histochemical stains. The finding of an infectious
organism can direct antimicrobial therapy.
Corynebacterium species have been confirmed
in numerous reported cases (13,22,24) with
the pattern of neutrophilic granulomatous inflammation and the
cuneiform configuration of Gram-positive bacilli demonstrated in
the case described herein. In light of these histomorphological
findings, Corynebacterium may be considered to be the most
probable infectious etiology. Ideally, the authors of the present
study would have liked to obtain culture or molecular testing as
supportive evidence that the Gram-positive rods identified in this
case were indeed Corynebacterium. However, these more
specific tests were costly and were not locally available.
To the best of our knowledge, the present study
reports the first case of CNGM in the English-speaking Caribbean.
The identification of bacterial organisms in the present case
underscores the widely reported role of bacterial infection in the
etiopathogenesis of this entity. The treatment of this disease
remains a significant challenge due to the scarcity of available
data for effective treatment protocols. This report encourages the
consideration of this entity in the differential diagnoses of
mastitis among Afro-Caribbeans.
Acknowledgements
The authors would like to acknowledge Mr. Justin
Ward of Integrated Pathology Services for technical support,
including assistance with the editing and formatting of the
manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DG was responsible for the conception and design of
the study, the interpretation of the patient's data and in the
critical revision of the manuscript. DS and KL were responsible for
acquisition of the patient's sample, and revised the manuscript
critically for intellectual content. PSG was responsible for the
design of the study and revised the manuscript critically for
intellectual content. AR was responsible for designing the study,
in the drafting of the manuscript, and revised the manuscript
critically for intellectual content. KL and AR confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Approval for the study was granted by the Ethics
Committee of Queen Elizabeth Hospital (Ref: 462023-DG), Bridgetown,
Barbados. The patient provided signed informed consent for
participation in the study.
Patient consent for publication
The patient provided signed informed consent for the
publication of her data and any related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu JM and Turashvili G: Cystic
neutrophilic granulomatous mastitis: An update. J Clin Pathol.
73:445–453. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Renshaw AA, Derhagopian RP and Gould EW:
Cystic neutrophilic granulomatous mastitis: An underappreciated
pattern strongly associated with Gram-positive bacilli. Am J Clin
Pathol. 136:424–427. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Taylor GB, Paviour SD, Musaad S, Jones WO
and Holland DJ: A clinicopathological review of 34 cases of
inflammatory breast disease showing an association between
corynebacteria infection and granulomatous mastitis. Pathology.
35:109–119. 2003.PubMed/NCBI
|
|
4
|
Funke G, von Graevenitz A, Clarridge J III
and Bernard KA: Clinical microbiology of coryneform bacteria. Clin
Microbiol Rev. 10:125–159. 1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alibi S, Ferjani A, Gaillot O, Marzouk M,
Courcol R and Boukadida J: Identification of clinically relevant
Corynebacterium strains by Api Coryne, MALDI-TOF-mass
spectrometry and molecular approaches. Pathol Biol (Paris).
63:153–157. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bacon DR, Ngeve SM and Jordan SG:
Granulomatous mastitis: An underdiagnosed inflammatory disease
afflicting minority women. Radiol Case Rep. 16:3990–3994.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Troxell ML, Gordon NT, Doggett JS, Ballard
M, Vetto JT, Pommier RF and Naik AM: Cystic neutrophilic
granulomatous mastitis: Association with Gram-positive bacilli and
Corynebacterium. Am J Clin Pathol. 145:635–645.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Metanat S, Jobaneh YS, Noori M, Sadeghi F,
Mirzapour A, Mashoori N, Mossahebi S, Kaviani A and Karbakhsh M:
Global distribution of idiopathic granulomatous mastitis: A scoping
review: IGM global distribution. Arch Breast Cancer. 9:261–271.
2022.
|
|
9
|
Tse GM, Poon CS, Ramachandram K, Ma TK,
Pang LM, Law BK, Chu WC, Tang AP and Cheung HS: Granulomatous
mastitis: A clinicopathological review of 26 cases. Pathology.
36:254–257. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kessler E and Wolloch Y: Granulomatous
mastitis: A lesion clinically simulating carcinoma. Am J Clin
Pathol. 58:642–646. 1972.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aljawder AAA, Li JJX, Ng JKM, Chan RCK,
Lui PCW, Poon IK, Tsang JYS and Tse GM: Idiopathic granulomatous
mastitis and cystic neutrophilic granulomatous mastitis: Two sides
of the same coin or distinct entities? Pathology. 55:335–341.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Altintoprak F, Kivilcim T and Ozkan OV:
Aetiology of idiopathic granulomatous mastitis. World J Clin Cases.
2:852–858. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
D'Alfonso TM, Moo TA, Arleo EK, Cheng E,
Antonio LB and Hoda SA: Cystic neutrophilic granulomatous mastitis:
Further characterization of a distinctive histopathologic entity
not always demonstrably attributable to Corynebacterium
infection. Am J Surg Pathol. 39:1440–1447. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Febery A and Bennett I: Sonographic
features of inflammatory conditions of the breast. Australas J
Ultrasound Med. 22:165–173. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sangoi AR: ‘Thick section’ Gram stain
yields improved detection of organisms in tissue sections of cystic
neutrophilic granulomatous mastitis. Am J Clin Pathol. 153:593–597.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang J, Xu H, Li Z, Li F, Yang Y, Yu X,
Jiang D, Xing L, Sun H and Shao M: Pathogens in patients with
granulomatous lobular mastitis. Int J Infect Dis. 81:123–127.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Basetti B, Periakaruppan G, Murali A, Dev
B, Radhakrishnan PR and Sai PMV: Breast involvement in
granulomatosis with polyangiitis: A case report. Egypt J Radiol
Nucl Med. 52(193)2021.
|
|
18
|
Iqbal FM, Ali H and Vidya R: Breast lumps:
A rare site for rheumatoid nodules. BMJ Case Rep.
2015(bcr2014208586)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baykan AH, Sayiner HS, Inan I, Aydin E and
Erturk SM: Primary breast tuberculosis: Imaging findings of a rare
disease. Insights Imaging. 12(19)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Baharoon S: Tuberculosis of the breast.
Ann Thorac Med. 3:110–114. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shoyele O, Vidhun R, Dodge J, Cheng Z,
Margules R, Nee P and Sieber S: Cystic neutrophilic granulomatous
mastitis: A clinicopathologic study of a distinct entity with
supporting evidence of a role for Corynebacterium-targeted
therapy. Ann Diagn Pathol. 37:51–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Paviour S, Musaad S, Roberts S, Taylor G,
Taylor S, Shore K, Lang S and Holland D: Corynebacterium
species isolated from patients with mastitis. Clin Infect Dis.
35:1434–1440. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Thomas VM, Alexander SA, Bindal P and
Vredenburgh J: Idiopathic granulomatous mastitis-a mystery yet to
be unraveled: A case series and review of literature. Cureus.
12(e6895)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nguyen MH, Molland JG, Kennedy S, Gray TJ
and Limaye S: Idiopathic granulomatous mastitis: Case series and
clinical review. Intern Med J. 51:1791–1797. 2021.PubMed/NCBI View Article : Google Scholar
|