Introduction
The expansion of the prefrontal cortex (PFC) in the
human brain is considered to be responsible for the subjective
experience of anxiety and the ability to regulate anxiety-related
responses (1,2). While these abilities are advantageous
for adapting to the environment, they also contribute to developing
anxiety disorders (3). The PFC
gathers information from various cortical and subcortical
structures to plan adaptation actions. Understanding the function
of the PFC has been aided by organizing principles that describe
function gradients across the PFC, such as the configuration of the
lateral PFC (LPFC) along the rostral/caudal axis based on the level
of cognitive control (4). Research
using neuroimaging and lesion analysis has suggested that rostral
areas of the LPFC are involved in higher levels of cognitive
control, processing more abstract representations (5).
The hippocampus, known for its role in memory
formation, is also crucial in regulating emotions during fear
conditioning (6). Unlike the
amygdala, which processes cues anticipating a threat, the dorsal
hippocampus provides contextual information about the specific
threat cue through interactions with the amygdala and ventromedial
PFC (7). The dorsal hippocampus
encodes contextual information about the threat or cues learned
when presented with a stimulus, allowing the organism to
differentiate between threat and safety cues, and to evaluate the
threat level associated with the stimulus (8,9). This
ability to distinguish between threatening and safe features may be
essential for automatic cognitive appraisal and reappraisal,
contributing to emotion regulation. In animal studies, mice exposed
to a tone predicting a shock learn to fear both the tone and the
context in which the tone-shock pairs occur (10). Understanding the involvement of the
hippocampus in emotional regulation can provide insight into the
mechanisms through which emotions are processed and controlled.
Understanding the role of the PFC in anxiety
disorders has been challenging due to the complex nature of neural
processes associated with anxiety. The PFC is responsible for
assessing the likelihood of a threat in the environment by
gathering information from different brain regions (11). Distorted threat estimations can
create a cycle of overreactions to perceived dangers, leading to
anxiety and avoidance behaviors. The frontal lobe, specifically the
PFC, has significantly evolved in primates, and abnormalities in
prefrontal function have been linked to anxiety disorders and their
symptoms (2). Therefore, the present
study aimed to determine the effectiveness of increasing levels of
exposure therapy, in reducing anxiety levels and combating
maladaptive behaviors. Participants were first assessed according
to Spielberger's State-Trait Anxiety Inventory and were included in
the study according to whether they were anxious (score >17).
Participants were randomly divided into the experimental and
control groups, with a total of 30 participants.
Subjects and methods
Study design and ethics approval
The author initiated the data collection process
after obtaining permission from the Ethics Committee of Sakarya
University (registration no. 22.11.1122); written informed consent
was obtained from all subjects. Subjects were under no obligation
to participate in the study and were assured of the confidentiality
of all information.
The present study was an experimental research study
based on cause-and-effect relationships, using a pre-test and
post-test control group design. Experimental studies examine the
change caused by one or more dependent variables on an independent
variable, providing accurate results using comparable methods.
However, the present study was designed as a quasi-experimental
model. The pre-test and post-test control group design is commonly
employed in comparison studies conducted using experimental models.
The present study specifically employed the pre-test post-test
control group design (https://quantifyinghealth.com).
Within the scope of the research, two groups, one
experimental and one control, were formed. Prior to the
application, all participants in the experimental and control
groups were tested for their anxiety levels. In order to strengthen
the internal validity of the study, a pre-test comparison was made
between the experimental and control groups. In the present study,
the therapeutic application was applied to the participants in the
experimental group for a total 36 sessions, three times a week for
10 weeks. No intervention was applied to the participants in the
control group.
Participants
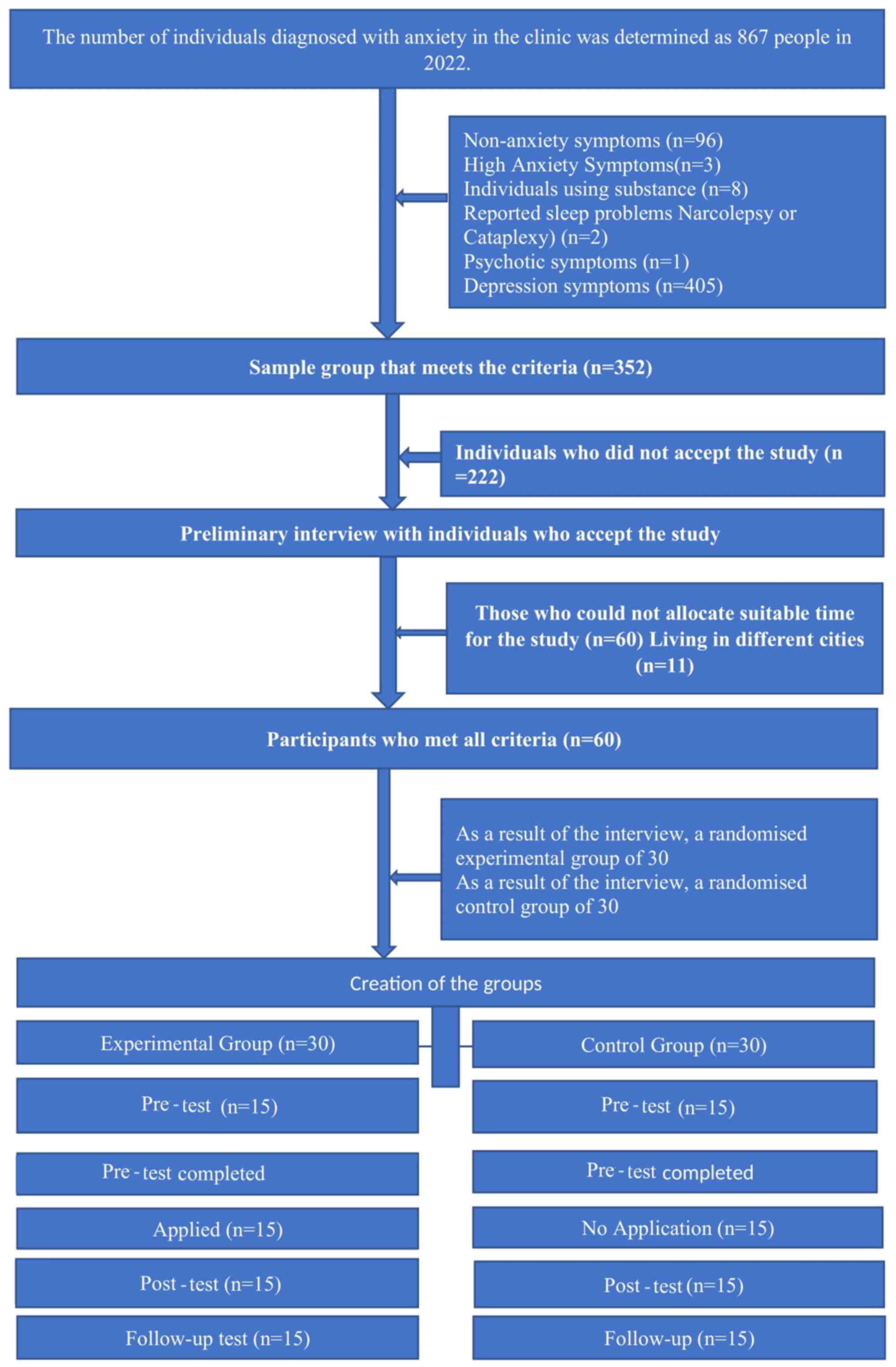
The study group comprised 60 individuals with
anxiety disorders who applied to a private health clinic in
Istanbul, Turkey. The selection of the working group and the flow
chart are presented in Fig. 1.
The inclusion and exclusion criteria for the study
were as follows: Participants were recruited from the clinical
population and were reviewed separately by the referring
psychiatrist. However, patients who ≥18 years of age and judged by
the referring clinician to meet the criteria for Generalized
Anxiety Disorder in the Diagnostic and Statistical Manual of Mental
Disorders, Fourth Edition (DSM-V-TR) (12) were included. Participants were
excluded if they expressed acute suicidal or homicidal ideation or
showed acute psychotic symptoms. In addition, the exclusion
criteria were as follows: The primary or secondary diagnosis met
the criteria for obsessive-compulsive disorder, post-traumatic
stress disorder, panic disorder, agoraphobia or acute stress
disorder, the primary diagnosis was not an anxiety disorder, mental
retardation, pervasive developmental disorder, psychosis,
oppositional defiant disorder, conduct disorder or substance use
was a co- or secondary diagnosis, the presence of a chronic
disorder of organic origin and a recent trauma with ongoing
forensic process.
Prior to commencing the research, all necessary
units of the private health institution were informed about the
research and the process, and the necessary permissions were
obtained. All persons, units and institutions participating in the
process were informed, and their application and research
permissions were obtained.
Study procedure
At this stage, the participants in the study group
were informed about the increasing levels of exposure therapy and
cognitive behavioral therapy.
Sessions
After meeting with the client, a detailed
conversation about anxiety, the treatment process for the client,
and short and long-term treatment processes were determined. A form
was prepared by discussing anxiety-related issues with each client
and creating a list. Brain-based explanations for anxiety were
given to each client. Short psychoeducational presentations were
made for questions, such as how the brain works and what it needs.
After the presentations, a form was created that included a list of
clients who would participate in the exposure therapy and cognitive
behavioral therapy. The goals selected from the list were divided
into achievable tasks to gradually relieve the clients' anxiety.
Behavioral changes were made by changing each client's behaviors
towards different goals in this way and dividing them into small,
easy practices. Clients were exposed to situations that aroused the
sensation that they had difficulty in doing with increasing
frequency, and the brain was reprogrammed. Any tasks that the
clients could not carry out during the session were given to them
as a home assignment. Unfulfilled home assignments were requested
again, and support was provided for carrying out the home
assignments. In this manner, the clients were provided with their
comfort zones with measured and frequent steps, and the control of
their home assignments was ensured. It was explained to the clients
that moderate and reasonable anxiety strengthens memory. Thus,
increasing levels of adrenaline in the brain play a role in
encoding and reinforcing the implicit memory of the amygdala. This
information enabled the clients to perform their home assignments
meticulously. At the end of the application, the clients were
informed that the research results would be shared and were thanked
for their participation.
Assessment measurements
Anxiety levels for the experimental group were
measured using Spielberger's State-Trait Anxiety Inventory
(pre-test and post-test) immediately before and after using the
therapeutic method. After applying this therapeutic method, the
anxiety levels were measured again. The demographic characteristics
of the participants and Spielberger's State-Trait Anxiety Inventory
were filled in the first session for the control group; the
Spielberger's State-Trait Anxiety Inventory was then completed
again. The measurement tools used are described below.
Personal information form
This form included eight questions about sex,
economic status, employment status, educational status and personal
health.
Spielberger's State-Trait Anxiety
Inventory
The scale measures normal and abnormal traits and
state anxiety levels of individuals (3). There are a total of 40 short statements
on the scale. The first 20 items measure the level of anxiety
related to the situation, and each item is graded as ‘Not at all’
(1 point), ‘Somewhat’ (2 points), ‘Very’ (3 points) and ‘Totally’
(4 points). A number of items were reverse scored (items 1, 2, 5,
8, 10, 11, 15, 16, 19 and 20). State anxiety scores are obtained by
adding the constant v0, the constant value of the state anxiety
scale, to the value obtained by subtracting the total score of the
reverse-coded items from the total score of the directly coded
items. Items 21 to 40 of the scale measure the trait anxiety level
of the individual, and each statement is scored as ‘Not at all’ (1
point), ‘A little’ (2 points), ‘Very’ (3 points) and ‘Totally’ (4
points). In this section, seven items are reverse-coded (articles
21, 26, 27, 33, 36 and 39 contain these items). The trait anxiety
level of the individual is obtained by subtracting the total score
of the reverse-coded items from the total score of the directly
coded items and adding 35, which is the constant value of the trait
anxiety scale. It states that 0-19 points obtained from the scale
do not indicate anxiety, 20-39 points indicate mild anxiety, 40-59
points indicate moderate anxiety, and 60-79 points indicate severe
anxiety; individuals with a score ≥60 required professional
assistance (4).
Electroencephalography (EEG)
measurements
Electrodes for EEG measurements were recorded using
the International 10/20 Electrode Placement System. EEG data were
visually scored for artifacts from blinking, eye movements and
other motor movements when amplitudes exceeded ±50 V using the EEG
Analysis Program software (WinEEG 256 EEG channels for QEEG/ERP
processing and analysis) All artifact-free EEG data were analyzed
using a 1-second-wide discrete Fourier transform (DFT). Power
(square microvolts) was derived from the DFT output in three alpha
frequency bands (alpha-I: 8-10 Hz; alpha-II: 10-13 Hz; full alpha:
8-13 Hz).
Statistical analyses
After the data collection phase was over, all data
were entered into the cells on an item basis using the Statistical
Program for Social Sciences 25 00 package program (IBM Corp.), and
the total scores obtained from the scales were obtained.
The data obtained from the personal information form
were analyzed using the descriptive analysis method, and the
frequency and percentage distributions of the sociodemographic
information of the study group were obtained. It was examined
whether the data obtained exhibited a significant difference before
and after the therapeutic application in the experimental and
control groups. The data collected in the experimental and control
groups were analyzed using descriptive and inferential statistical
tests, such as an independent t-test (to compare the average
depression level in the two groups) and a paired t-test (to compare
the average depression level) using the SPSS-25 program to
determine the anxiety level before and after the intervention.
Moreover, the Chi-squared test was used to investigate the
demographic characteristics of the study participants.
Participants' Spielberger's Trait-State Anxiety Inventory, pretest,
posttest, and follow-up test scores were analyzed by ANOVA and
ANCOVA and multiple comparisons were analyzed using Tukey's HSD
test. Finally, reliability analyses were conducted for the
reliability of the scale used in the study. The results were
evaluated at 95% confidence intervals. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic characteristics of the
study participants
All the demographic characteristics of the study
participants are presented in Table
I. The Chi-squared test was used to analyze demographic
variables to determine whether the participants in the study were
equally distributed in the experimental and control groups.
Considering the sex variable of the experimental group in the
study, the number of males was 46.7% (n=7) and that of females was
53.3% (n=8). The sex variable in the control group was considered;
the number of males was 53.3.7% (n=8) and that of females was 46.7%
(n=7). Considering the age variable of the experimental group, the
number of participants between the ages of 18-25 years was 40.0%
(n=6), that between 26-45 years was 20.0% (n=3), and the number of
participants ≥46 years of age was 40.0% (n=6). Considering the age
variable of the control group, the number of participants between
the ages of 18-25 years was 40.0% (n=6) and the number of
participants between the ages of 26-45 years was 20.0% (n=3). The
number of participants over the age of 46 was 40% (n=6).
Considering the educational variable of the experimental group, the
number of primary school graduates was 40.0% (n=6), the number of
secondary school graduates was 20.0% (n=3), the number of high
school graduates was 13.3% (n=3), and that of university graduates
was 26.7% (n=4). When the education variable of the control group
was considered, the number of primary school graduates was 40.0%
(n=6), the number of secondary school graduates was 20.0% (n=3),
the number of high school graduates was 13% (n=3), and the number
of university graduates was 26.7% (n=4). Considering the drug use
variable in the experimental group, the number of participants
using drugs was 40.0% (n=6), and the number of participants not
using drugs was 60.0% (n=9). The number of participants using drugs
(anti-depressants, benzodiazepine or others) was 53.3% (n=9) when
looking at the variable of drug use in the control group. =8), and
the number of participants who did not use drugs was 46.7% (n=9).
Considering the marital status variable of the experimental group,
the number of single participants was 46.7% (n=7), and the number
of married participants was 53.3% (n=8). When the marital status
variable of the control group was examined, the number of single
participants was 40.0% (n=6), and the number of married
participants was 60.0% (n=9) (Table
I). As shown by these demographic variables, there were no
statistically significant differences in demographic
characteristics such as sex, education level, age, and drug use
between the two groups and the characteristics were homogeneous
(P>0.05).
 | Table IDescriptive analysis comparison of the
sociodemographic characteristics of the participants in the
experimental and control groups. |
Table I
Descriptive analysis comparison of the
sociodemographic characteristics of the participants in the
experimental and control groups.
| | Analyses |
|---|
| Variable | Experimental group, n
(%) | Control group, n
(%) | χ² value | df | P-value |
|---|
| Sex | | | | | |
|
Male | 7 (46.7) | 8 (53.3) | 0.67 | 1 | 0.796 |
|
Female | 8 (53.3) | 7 (46.7) | | | |
|
Total | 15 (100.0) | 15 (100.0) | | | |
| Age, years | | | | | |
|
18-25 | 6 (40,0) | 6 (40.0) | 1.200 | 2 | 0.549 |
|
26-45 | 3 (20,0) | 3 (20.0) | | | |
|
≥46 | 6 (40,0) | 6 (40.0) | | | |
|
Total | 15 (100.0) | 15 (100.0) | | | |
| Level of
education | | | | | |
|
Primary
school | 6 (40.0) | 6 (40.0) | 2.300 | 3 | 0.506 |
|
Secondary
school | 3 (20.0) | 3 (20.0) | | | |
|
High
school | 2 (13.3) | 2 (13.3) | | | |
|
University | 4 (26.7) | 4 (26.7) | | | |
|
Total | 15 (100.0) | 15 (100.0) | 0.067 | 1 | 0.736 |
| Drug use | | | | | |
|
Yes | 6 (40,0) | 8 (53.3) | | | |
|
No | 9 (60,0) | 7 (46.7) | | | |
|
Total | 15 (100.0) | 15 (100.0) | | | |
| Marital status | | | | | |
|
Single | 7 (46.,7) | 6 (40.0) | 0.600 | 1 | 0.439 |
|
Married | 8 (53.3) | 9 (60.0) | | | |
|
Total | 15 (100.0) | 15 (100.0) | | | |
Pre-test, post-test and follow-up test
scores
As shown in Table
II, in the experimental group, there was a significant
difference between the participants' Spielberger's State-Trait
Anxiety Inventory pre-test, post-test and follow-up test scores
(Wilk's λ, 800=F (1.14)=29.122, P<0.001 ɳ2=0.530) and the effect
size was calculated to be high. The post-test mean score
(x̄=4.6667) and follow-up test mean score (x̄=4.4667) were lower
than the pre-test mean score(x̄=8.0667) in Table III. On the other hand, in the
control group the difference between the post-test and follow-up
test scores was not significant. This finding shows that the
participants' anxiety levels significantly decreased after the
application and in the measurements made after the application, and
the results did not change in the follow-up measurements made
afterward. This finding shows that the effect of the application
made in the research continues. The descriptive analysis results of
the pre-test, post-test and follow-up test scores of the
experimental and control groups are presented in Table III.
 | Table IIResults of repeated measures ANOVA of
the pre-test, post-test and follow-up test scores of the study
participants of the experimental group. |
Table II
Results of repeated measures ANOVA of
the pre-test, post-test and follow-up test scores of the study
participants of the experimental group.
| Source | Sum of squares | s.d | Mean squares | F value | P-value | Effect size | Difference |
|---|
| Intercept | 56.711 | 28 | 2.025 | 29.122 | 0.001 | 0.530 | 2>1, 3>1
3=2 |
| Measurement | 67.489 | 2 | 33.744 | | | | |
| Error | 64.889 | 56 | 1.159 | | | | |
| Total | 189.089 | 86 | | | | | |
 | Table IIIResults of descriptive analysis of
the pre-test, post-test and follow-up test scores of the
experimental and control groups. |
Table III
Results of descriptive analysis of
the pre-test, post-test and follow-up test scores of the
experimental and control groups.
| | Experimental
group | Control group |
|---|
| Dependent
variable | Pre-test | Post-test | Follow-up | Pre-test | Post-test | Follow-up |
|---|
| SSDKE | Mean | Sd | Mean | SS | Mean | Sd | Mean | SS | Mean | Sd | Mean | SS |
|---|
| | 8.0667 | 1.53375 | 4.6667 | 1.58865 | 4.4667 | 1.84649 | 8.0667 | 0.45774 | 7.7333 | 0.25820 | 7.8667 | 0.35187 |
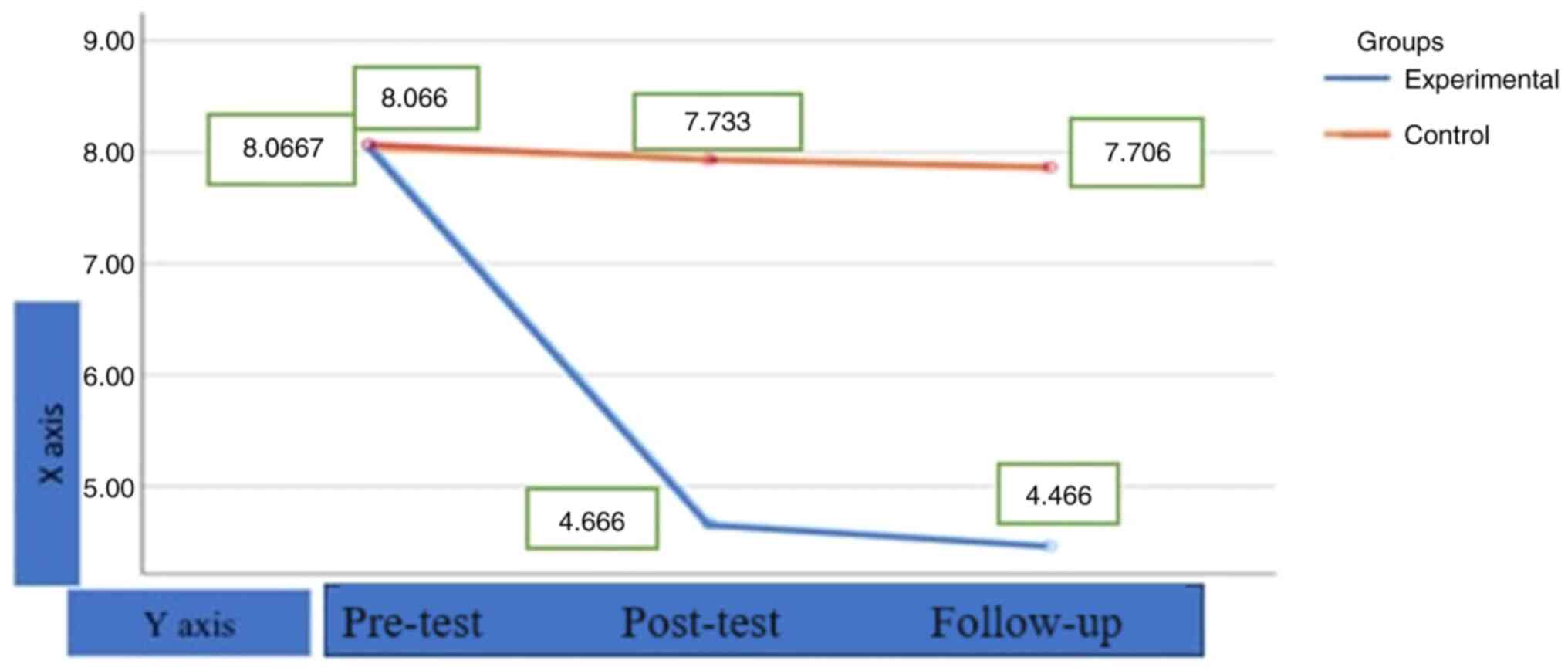
As shown in Fig. 2,
the pre-test Trait-State Anxiety mean score of the experimental
group was 8.0667, the post-test mean score was 4.6667, and the
follow-up mean score was 4.4667. The pre-test Trait-State Anxiety
mean score of the control group was 8.0667, the post-test mean
score was 7.733, and the follow-up mean score was 7.706. This
finding indicates that the participants' anxiety levels markedly
decreased after the application and in the measurements made after
the application, and the results did not change in the follow-up
measurements made afterwards. This finding indicates that the
effect of the therapeutic application made in the research
continues. On the other hand, it can be seen that there is no
change in the control group. As shown in Fig. 2, it is clear that the change is due
to the therapeutic application.
In addition, preliminary checks were made to confirm
that the variances were not violated, reliable measurement of
normality, linearity, homogeneity of variances, and homogeneity of
regression trends. According to the results of ANCOVA, no
significant difference was found between the post-test mean scores
adjusted for Spielberger's State-Trait Anxiety Inventory according
to sex (P>0.05) (Table IV). In
other words, the results indicate that females and males do not
react differently to the therapeutic approach used in the
study.
 | Table IVResults of ANCOVA for Spielberger's
State-Trait Anxiety Inventory post-test scores according to
sex. |
Table IV
Results of ANCOVA for Spielberger's
State-Trait Anxiety Inventory post-test scores according to
sex.
| Dependent variable:
Post-test |
|---|
| Source of
variance | Sum of squares | df | Mean squares | F value | P-value | Effect size |
|---|
| Corrected
model | 10.057 | 3 | 3,352 | 1.459 | 0.279 | 0.285 |
| Sex | 1.936 | 1 | 1,936 | | | |
| Pre-test | 1.028 | 1 | 1,028 | | | |
| re-test of sex | 1.028 | 1 | 1,028 | | | |
| Error | 25.276 | 11 | 2,298 | | | |
| Total | 362.,000 | 15 | | | | |
| Corrected
total | 35.333 | 14 | | | | |
EEG asymmetry changes at the cortical
level
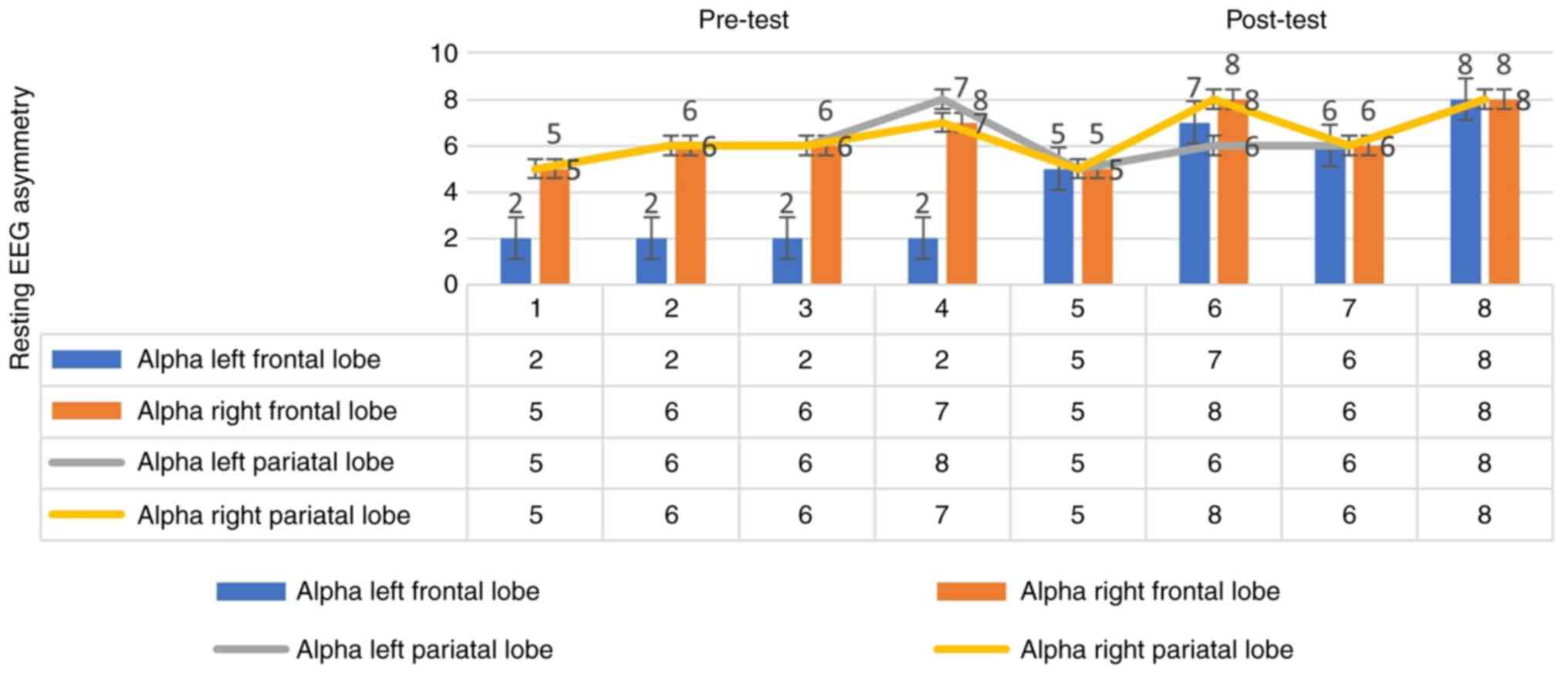
As shown in Fig. 3,
in the pre-test, in the first measurement, the pre-test alpha
frequency of the right frontal lobe was 5 Hz and that of the left
frontal lobe was 2 Hz; in the second measurement, the alpha
frequency of the right frontal lobe was 6 Hz and that of the left
frontal lobe was 2 Hz; in the third measurement, the alpha
frequency of the right frontal lobe was 6 Hz and that of the left
frontal lobe was 2 Hz; in the fourth measurement, the alpha
frequency of the right frontal lobe was 7 Hz and that of the left
frontal lobe was 2 Hz. In the post-test, in the fifth measurement,
the alpha frequency of the right frontal lobe was 5 Hz and that of
the left frontal lobe was 5 Hz; in the sixth measurement, the alpha
frequency of the right frontal lobe was 8 Hz and that of the left
frontal lobe was 7 Hz; in the 7th measurement, the alpha frequency
of the right frontal lobe was 6 Hz and that of the left frontal
lobe was 6 Hz; in the 8th measurement, the alpha frequency of the
right frontal lobe was 8 Hz and that of the left frontal lobe was 8
Hz. As can be seen by the graph in Fig.
3, a change was observed in the pre-test. There was no change
in the post-test. These findings indicate that there was a marked
interaction of the application according to the frontal region.
Therefore, this indicates that the application affects EEG
asymmetry changes at the cortical level.
The changes in the frontal and parietal lobe
frequencies were examined. In the experimental group, the analysis
was performed separately for the resting EEG asymmetry alpha right
and left before and after the therapeutic application. It was
determined that there was a significant interaction at 10-13 Hz for
alpha right and left relative to the frontal region [t (14)=1.344, P<0.05]. This indicates a
change in EEG asymmetry (please also see the aforementioned data;
Fig. 3). In the experimental group,
the analysis was performed separately for the resting EEG asymmetry
alpha right and left before and after the application. It was
determined that there was a significant interaction to the parietal
region at 10-13 Hz for alpha right and left [(t (14)=3.111, P<0.05] (Table V) This indicates a change in EEG
asymmetry asymmetry (please also see the aforementioned data;
Fig. 3). These findings demonstrated
that the therapeutic application affected the EEG asymmetry changes
at the cortical level.
 | Table VThe changes in the right and left
frontal and parietal lobe alpha frequencies (10-13 Hz) between the
pre-test and post-test. |
Table V
The changes in the right and left
frontal and parietal lobe alpha frequencies (10-13 Hz) between the
pre-test and post-test.
| Group | 10-13 Hz | Mean | n | Std. deviation | df | t | P-value |
|---|
| Experimental
group | Frontal lobe | Pre-test right | 2.0000 | 15 | 0.00000 | 14 | 1.344 | 0.05 |
| | | Post-test left | 6.0000 | 15 | 0.81650 | | | |
| | Parietal lobe | Pre-test right | 2.5000 | 15 | 0.29099 | 14 | 3.111 | 0.05 |
| | | Post-test left | 6.7500 | 15 | 1.50000 | | | |
Discussion
In the present study, when the data obtained from
the analyses were examined, the effectiveness of the gradually
increasing levels of exposure therapy and cognitive behavioral
therapy for maladaptive behavior and anxiety was examined. To date,
to the best of our knowledge, there are no studies available in
Turkey on the application of such methods, namely a brain-based
psychological intervention program for anxiety. Therefore, the
study is the first of its kind in Turkey. Herein, it was found that
the methods used for maladaptive behavior and anxiety were
effective and reduced the levels of anxiety in the study
participants. This finding can be interpreted as an effective
application method for anxiety.
According to the follow-up evaluations at the end of
the therapy, it was stated by all clients that they benefited
greatly from the therapeutic application and that their quality of
life increased compared to that before the therapy. A previous
study on cognitive behavioral therapy demonstrated that the
participants experienced a significant shift in their resting
forebrain activity from greater relative right to greater relative
left after receiving treatment (13). That study also found that individuals
with higher levels of left frontal brain activity before treatment
had larger reductions in anxiety symptoms after treatment and lower
levels of anxiety post-treatment (11). These associations were specifically
related to the frontal alpha EEG asymmetry metric. These findings
suggest that resting frontal EEG asymmetry may indicate symptom
improvement and overall functioning in anxious patients who receive
effective psychological treatment (11). The results of the present study are
supported by other international research that has shown
alterations in neural circuits related to anxiety disorders, such
as exaggerated amygdala responses and impaired regulation by the
PFC and hippocampus (14,15). Exposure to chronic stress can also
affect the brain's fear circuitry; however, pharmacological and
non-pharmacological interventions can help reverse this damage
(16,17).
The frontal EEG asymmetry-emotion model suggests
that individuals with greater right frontal asymmetry exhibit
higher anxiety symptoms (13).
However, other researchers have found that the association between
EEG asymmetry, withdrawal behavior and negative effects is more
complex (18). Anxiety disorders are
characterized by difficulties regulating emotional responses to
perceived threats (19). This may be
due to increased activity in the amygdala and other
limbic/subcortical regions, decreased activity in the PFC and
hippocampus, or a failure of top-down processes to regulate the
ventral nervous system (20).
Individuals with anxiety disorders often display a heightened
sensitivity to threats or negative information in their
environment, selectively attending to and experiencing increased
amygdala activity in response to threats. There is evidence to
indicate an association between anxiety disorders and heightened
reactivity to threats and negative stimuli (21).
The hippocampus has provided valuable insight into
understanding the impact of stress on the brain. Research has
expanded to include interconnected regions, such as the amygdala
and PFC (22). In rodents, chronic
stress has been shown to lead to the degeneration of the PFC,
specifically dendritic and spine loss in pyramidal cells,
associated with impaired working memory (23). Notably, chronic stress has
differential effects on different brain circuits. While dendritic
growth increases in the amygdala, leading to an imbalance between
the amygdala and prefrontal cortex function, the PFC neurons that
form connections with other cortical areas undergo dendritic loss
(23). However, neurons in the
orbital prefrontal cortex and those that activate the amygdala do
not atrophy during chronic stress. These findings highlight the
complex effects of stress on brain structures and the
interconnectedness of different brain regions (24).
A previous study discussed the association between
reduced gray matter volume in the PFC and exposure to adverse
events in humans. It also highlighted the impact of chronic stress
on the functional connectivity and regulation of the PFC by the
amygdala (23). Another study
followed depressed adults over a period of 3 years and found that
those who went into remission had less volume reduction in certain
brain regions, such as the hippocampus and various regions of the
PFC, compared to those who did not go into remission (25). The present study points out that
internal and external factors were not fully controlled in the
study and mentions that there was no control group for EEG data,
which limits the certainty of attributing the changes observed to
the treatment alone. The text emphasizes the need for further
research to establish causality in the relationship between changes
in EEG asymmetry and brain regions. It acknowledges that the data
obtained from scales may suggest a relationship but recognizes
these limitations.
In line with the results obtained from the present
study, further studies need to be conducted in Turkey, to further
examine the effectiveness of increasing levels of exposure and
behavioral therapy for maladaptive behavior and anxiety. It is also
recommended that this therapy be used in clinics as a psychotherapy
method and presented as behavioral homework. It is also recommended
that brain-based methods be added as an additional protocol to all
psychotherapy methods, applied and recommended for other
psychological problems other than anxiety. It is recommended that
the findings in the present study should be confirmed by supported
other studies and repeated in future studies with different samples
or study groups.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author upon reasonable
request.
Author's contributions
The author FB was responsible for the study design,
the analyses and the literature search, and the writing of the
study. The author has read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were under the ethical standards of the institutional
or national research committee and with the 1964 Declaration of
Helsinki and its later amendments or comparable ethical standards.
The author initiated the data collection process after obtaining
permission from the Ethics Committee of Sakarya University
(registration no. 22.11.1122); written informed consent was
obtained from all subjects. Subjects were under no obligation to
participate in the study and were assured of the confidentiality of
all information.
Patient consent for publication
Not applicable.
Competing interests
The author declares that he has no competing
interests.
References
|
1
|
Armstrong E, Zilles K, Curtis M and
Schleicher A: Cortical folding, the lunate sulcus and the evolution
of the human brain. J Hum Evolution. 20:341–348. 1991.
|
|
2
|
Coan JA and Allen JJB: Frontal EEG
asymmetry and the behavioral activation and inhibition systems.
Psychophysiology. 40:106–114. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kenwood MM, Kalin NH and Barbas H: The
prefrontal cortex, pathological anxiety, and anxiety disorders.
Neuropsychopharmacology. 47:260–275. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miller EK and Cohen JD: An integrative
theory of prefrontal cortex function. Ann Rev Neurosci. 24:167–202.
2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Badre D and D'Esposito M: Functional
magnetic resonance imaging evidence for a hierarchical organization
of the prefrontal cortex. J Cogn Neurosci. 19:2082–2099.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tanji J and Hoshi E: Behavioral planning
in the prefrontal cortex. Curr Opin Neurobiol. 11:164–170.
2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Thompson RF and Kim JJ: Memory systems in
the brain and localization of a memory. Proc Natl Acad Sci USA.
93:13438–13444. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fell J, Klaver P, Elfadil H, Schaller C,
Elger CE and Fernández G: Rhinal-hippocampal theta coherence during
declarative memory formation: Interaction with gamma
synchronization? Eur J Neurosci. 17:1082–1088. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Maren S, Phan KL and Liberzon I: The
contextual brain: Implications for fear conditioning, extinction,
and psychopathology. Nat Rev Neurosci. 14:417–428. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Curzon P, Rustay NR and Browman KE: Cued
and Contextual Fear Conditioning for Rodents. In: Methods of
Behavior Analysis in Neuroscience. 2nd edition. CRC Press/Taylor
& Francis, Boca Raton (FL), 2009.
|
|
11
|
Britton JC, Lissek S, Grillon C, Norcross
MA and Pine DS: Development of anxiety: The role of threat
appraisal and fear learning. Depress Anxiety. 28:5–17.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
American Psychiatric Association:
Diagnostic and Statistical Manual of Mental Disorders. Fifth
edition. Washington, DC, American Psychiatric Association, 2013.
See more at: http://www.adhdinstitute.com/assessment
diagnosis/diagnosis/dsm-5tm/#sthash.8YJw9SHo.dpuf.
|
|
13
|
Moscovitch DA, Santesso DL, Miskovic V,
McCabe RE, Antony MM and Schmidt LA: Frontal EEG asymmetry and
symptom response to cognitive behavioral therapy in patients with
social anxiety disorder. Biol Psychol. 87:379–385. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Herry C, Ferraguti F, Singewald N, Letzkus
JJ, Ehrlich I and Lüthi A: Neuronal circuits of fear extinction.
Eur J Neurosci. 31:599–612. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim MJ, Loucks RA, Palmer AL, Brown AC,
Solomon KM, Marchante AN and Whalen PJ: The structural and
functional connectivity of the amygdala: From normal emotion to
pathological anxiety. Behav Brain Res. 223:403–410. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jaggar M, Fanibunda SE, Ghosh S, Duman RS
and Vaidya VA: The neurotrophic hypothesis of depression revisited:
New insights and therapeutic implications in Neurobiology of
depression. Academic Press, 2019.
|
|
17
|
Mah L, Szabuniewicz C and Fiocco AJ: Can
anxiety damage the brain? Curr Opin Psychiatry. 29:56–63.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Thayer JF, Friedman BH, Borkovec TD,
Johnsen BH and Molina S: Phasic heart period reactions to cued
threat and nonthreat stimuli in generalized anxiety disorder.
Psychophysiology. 37:361–368. 2000.PubMed/NCBI
|
|
19
|
Siever LJ: Neurobiology of aggression and
violence. Am J Psychiatry. 165:429–442. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Streeter CC, Gerbarg PL, Saper RB, Ciraulo
DA and Brown RP: Effects of yoga on the autonomic nervous system,
gamma-aminobutyric acid, and allostasis in epilepsy, depression,
and post-traumatic stress disorder. Med Hypotheses. 78:571–579.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McEwen BS, Nasca C and Gray JD: Stress
effects on neuronal structure: Hippocampus, amygdala, and
prefrontal cortex. Neuropsychopharmacology. 41:3–23.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hains AB, Vu MAT, Maciejewski PK, van Dyck
CH, Gottron M and Arnsten AF: Inhibition of protein kinase C
signaling protects prefrontal cortex dendritic spines and cognition
from the effects of chronic stress. Proc Natl Acad Sci USA.
106:17957–17962. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Arnsten AF: Stress signaling pathways
impair prefrontal cortex structure and function. Nat Rev Neurosci.
10:410–422. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Sarter M, Givens B and Bruno JP: The
cognitive neuroscience of sustained attention: Where top-down meets
bottom-up. Brain Res Rev. 35:146–160. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Frodl TS, Koutsouleris N, Bottlender R,
Born C, Jäger M, Scupin I, Reiser M, Möller HJ and Meisenzahl EM:
Depression-related variation in brain morphology over 3 years:
Effects of stress? Arch Gen Psychiatry. 65:1156–1165.
2008.PubMed/NCBI View Article : Google Scholar
|