Introduction
Acquired reactive perforating collagenosis (ARPC) is
a rare form of dermatosis. In 1989, Rapini et al (1) proposed the umbrella term acquired
perforating dermatosis (APD), encompassing cases with an onset in
adult life, and associated with diabetes mellitus and chronic renal
failure, particularly in patients on dialysis. APD forms with
perforating folliculitis, Kyrle's disease and elastosis perforans
serpiginosa, which are forms of perforating dermatosis (2). The prevalence and incidence of ARPC
remain unknown, with only a limited number of reported cases
(3-6).
Karpouzis et al (7) once
reported the epidemiological features of ARPC by analyzing the
diagnostic data of 101 cases, indicating that the peak onset age
ranged from 50 to 59 years.
The diagnosis of ARPC is made according to a
histopathological analysis, the age at onset and typical skin
lesions. Faver et al (8)
stated the criteria for ARPC as follows: The onset of lesions after
the age of 18 years, umbilicated papules or nodules with a central
adherent keratotic plug, and the elimination of necrotic collagen
tissue within an epithelium-lined crater. Accordingly, the
treatment of ARPC continues to be challenging. It usually consists
of a wide range of treatment measures. Apart from the treatment of
any underlying disease, systemic steroids and retinoids, as well as
UVB phototherapy are well-established treatment options (9). However, in spite of various therapeutic
measures, the prognosis of patients with this condition is not
satisfactory. The symptoms of the majority of reported cases can
only be improved rather than cured. It is worth noting that
infection is a key factor affecting the efficacy of therapeutics
for ARPC. The present study describes the case of a patient with
ARPC combined with methicillin-resistant Staphylococcus
aureus (MRSA), which was effectively treated by the addition of
sensitive antibiotics.
Case report
On July, 2021, a 75-year-old female patient was
admitted to the Department of Dermatology, Affiliated Hospital of
Qingdao University of University, Qingdao, China. She had erythema
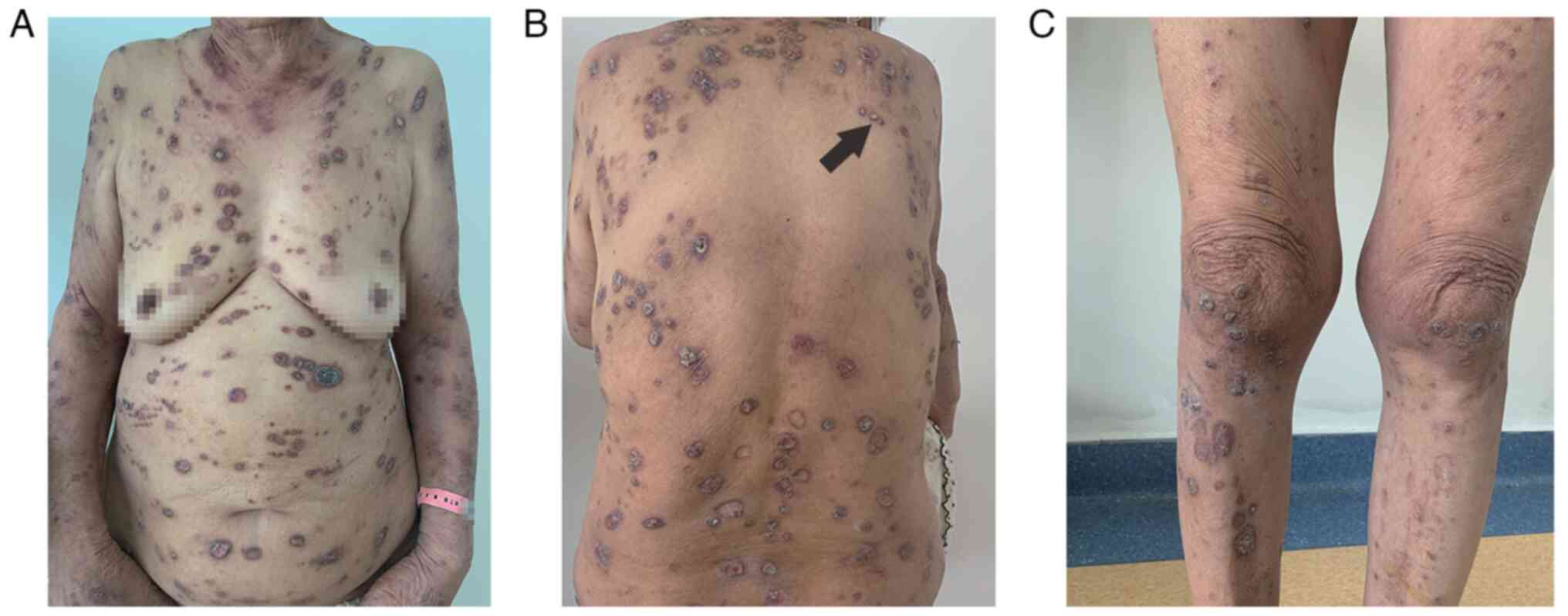
and itchy papules on her back 5 years prior. The lesions gradually
became exacerbated with severe pruritus, spreading all over the
trunk of her body, right upper and left lower limb, part of which
was covered with crust (Fig. 1). The
lesions then improved by the topical administration of fluticasone;
however, the disease relapsed several times. She presented with a
12-year-history of diabetes and well-controlled blood glucose level
by the daily subcutaneous insulin injection and oral metformin and
acarbose.
A cutaneous examination revealed diffusely
distributed multiple keratotic, hyperpigmented papules on the
limbs, back, chest and abdomen, and a linear distribution of the
skin lesions. The diameter of the papules ranged from 2 to 8 mm,
with keratotic plugs and a crust in the center.
Laboratory examinations revealed elevated eosinophil
levels (1.24x109/l). The urine analysis indicated
positive leucocyte, protein and occult blood. The liver function
test revealed decreased total protein (63.3 g/l) and prealbumin
(150 mg/l) levels. Thyroid dysfunction was also observed in this
patient. Fasting blood glucose levels reached 6.43 mmol/l. MRSA was
identified from the bacterial culture.
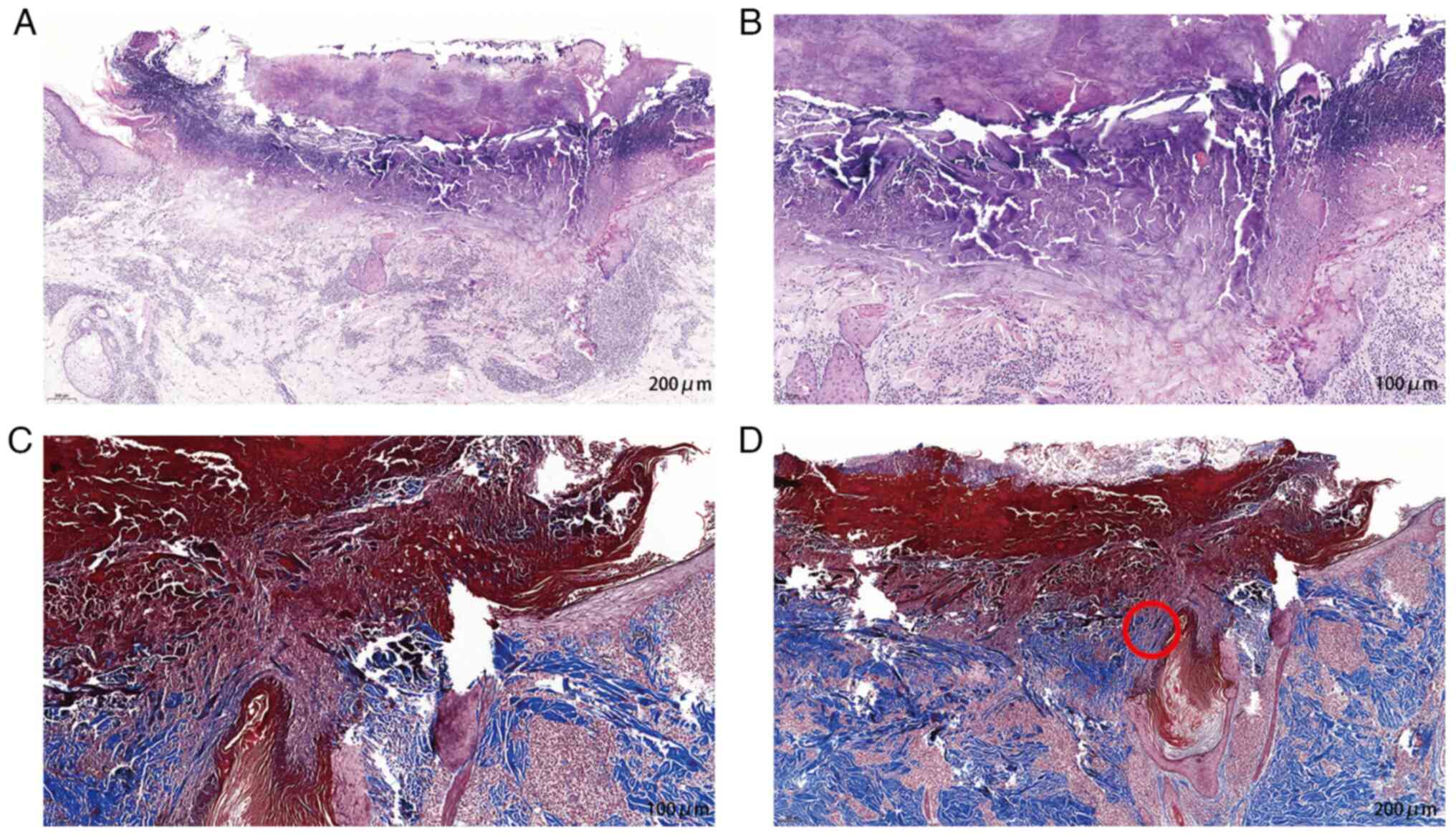
Lesion skin biopsy samples examined using
hematoxylin and eosin staining (as described below) revealed a
cup-shaped depression plugged with necrotic inflammatory debris.
The majority of the collagen was found in the lower part of plug,
and the surrounding epidermal spinous layer was thickened.
Lymphocytes, neutrophils and eosinophils infiltrated the
superficial dermis vessels. Masson's staining(as described below)
revealed collagen fibers penetrating the surface (Fig. 2). Masson's staining for elastin
fibers was negative.
The patient was treated with topical
corticosteroids, antihistamine tablets (Ebastine, oral, 10 mg, once
a day) for the skin lesion and pruritus, glycyrrhizin (60 mg,
intravenous, once a day) to regulate immunity, and acitretin (20
mg, oral, once a day) to regulate epithelial cell keratinization.
In addition, an insulin injection (the unit was adjusted according
to the blood glucose levels) together with acarbose tablets (100
mg, oral, twice a day) were applied for glucose control. At 1 week
following admission, the treatment efficacy was found to be poor
and the lesions continued to progress. Considering the possibility
of local secondary infection, the patient was also treated with a
combination polymyxin B cream and a bacterial culture examination
was performed. Minocycline (100 mg, oral, once a day) was also
added to the treatment regimen according to the results of the
bacterial culture and drug sensitivity test, which indicated that
the strain cultured at the site of infection was MRSA and was
sensitive to minocycline. On the day of hospital discharge, the
keratotic plug had narrowed down slightly; however, the patient
noted a marked reduction in the itchiness of the lesions.
Subsequently, her outpatient follow-up records were examined and
her condition was found to be gradually in remission within 6
months.
Staining procedures
Under aseptic operation, two sections of skin lesion
were obtained. One was stained with hematoxylin and eosin (H&E)
and the other with Masson's stain. For H&E staining, the
tissues were then fixed in 10% neutral phosphate-buffered formalin
(ZheJiang Shitai Industrial Co., Ltd.) for 24 h at room
temperature. This was followed by dehydration with ethanol. The
tissue sections were then infiltrated with paraffin wax and cut
into sections of a thickness of 3 µm. The tissues were washed three
times with xylene for 10 min each and washing with anhydrous
alcohol and diluted alcohols (95 and 70%) before washing with
water. The sections were then stained with hematoxylin solution
(Roche Diagnostics) for 12 min at room temperature. The unbound
hematoxylin was then removed with water rinses followed by an
optional differentiation step using 1% acid alcohol and bluing for
25 min. Eosin (Roche Diagnostics) was then used to stain in the
cytoplasmic counterstain for 5 min at room temperature. Following
dehydration with 95 and 100% alcohol, the sections were sealed with
neutral gum and examined under a microscope (ECLIPSE Ci-L, Nikon
Corporation).
For Masson's staining, the procedure before staining
was the same as that described above H&E staining. The nuclei
were stained with Weigert's iron hematoxylin (Roche Diagnostics)
for 5 min at room temperature. This was followed by washing with
distilled water and differentiation with 1% hydrochloric acid
alcohol. Following rinsing running water for a few minutes, the
sections were stained with Masson's trichrome solution (Roche
Diagnostics) for 5 min at room temperature. The sections were then
soaked in 2% glacial acetic acid solution for 1 min followed by
differentiation with 1% phosphomolybdic acid solution for 5 min.
The sections were then directly stained with aniline blue or light
green liquid for 5 min at room temperature followed by soaking in
0.2% glacial acetic acid solution again for 20 sec. Subsequently,
95% alcohol was used for dehydration multiple times. Finally,
dehydration was performed with anhydrous alcohol, washing with
xylene and sealing with neutral gum. An ECLIPSE Ci-L microscope
(Nikon Corporation) was then used to examine the sections. All
stains used were supplied by Roche Diagnostics.
Discussion
Reactive perforating collagenosis (RPC) was first
described in 1967 by Mehregan et al (10). There are two types of RPC, ARPC and
inherited RPC (IRPC). IRPC, hereditary with autosomal dominant
transmission, is more common in infants and children, whereas ARPC
usually develops in adults. The patient in the present study,
without a family history of RPC, had presented with ARPC and
diabetes, which already confirmed a cause-effect association. The
pathogenesis of ARPC remains unknown. It has been suggested that
mild superficial trauma, sometimes in association with diabetic
vasculopathy and a hypoxic state, in genetically susceptible
individuals, leads to necrobiosis of papillary dermis collagen that
is subsequently eliminated by a trans epidermal route (7). This hypothesis has been supported by
the findings of a positive stain with periodic acid-Schiff (PAS),
and the thickening of the vessel walls in the upper dermis of
diabetic patients with APD (11).
PAS-positive and thickened vessel walls were detected in all 8
diabetic patients in the study by Kawakami and Saito (12). Depending on all these observations,
diabetic vasculopathy appears to be an underlying factor in this
eruption. The condition of the patient in the present study has
been speculated to develop from the chronic irritation that occurs
secondary to the subcutaneous insulin injection. In addition, this
patient was affected by MRSA.
Subsequent infection can be caused by colonizing
bacteria or other pathogens. As regards bacteria, the skin is
dominated by members of the genera Staphylococcus aureus,
Corynebacterium, Streptococcus and
Propionibacterium (13).
Notably, it has been reported that Staphylococcus aureus
skin colonization rates are relatively low, and abundance levels
compared to other bacterial skin colonizers are barely detectable
(14). The patient described herein
was affected by MRSA, a variant of Staphylococcus aureus. In
recent decades, with the application of antibiotics, some bacteria
exhibit resistance, among which MRSA is resistant to all
β-lactamase antibiotics, the majority of macrolides and
aminoglycoside antibiotics (15).
MRSA infection has become an epidemic, occurring most frequently on
the skin and soft tissue (16).
The mortality rates for patients diagnosed with ARPC
may be higher in the case of a secondary infection. The study by
Weiss et al (17) described a
patient with ARPC who developed extensive cutaneous mucormycosis.
The patient in that study succumbed due to progressive disease
after 3 weeks of hospitalization (17). Accordingly, physicians need to be
warned of the possibility and potential complications of fungal,
mycobacterial, or bacterial opportunistic superinfection.
To the best of our knowledge, the present study is
the first to report a case of concurrent ARPC and MRSA infection.
Early treatment can be administered by conducting timely
examinations to confirm the pathogen and select sensitive drugs for
treatment. Apart from infection, ARPC is frequently accompanied by
several systemic diseases, including malignant conditions. Thus, a
thorough paraclinical exploration is necessary to reveal a possible
underlying extracutaneous disease and to avoid secondary
infections.
In conclusion, the present study reports a rare case
of ARPC associated with MRSA. The present case report should alert
dermatologists to the possibility of secondary infection in
patients with chronic ARPC.
Acknowledgements
The authors would like to thank Professor Jun Wang
(Department of Dermatology, Affiliated Hospital of Qingdao
University, Qingdao, China) for providing helpful discussions and
suggestions.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (FH, WR, MW, XL and MP) were involved in
the acquisition, analysis, or interpretation of the data. MP was
involved in the conception and design of the study, and supervised
the study. FH and WR were involved in the drafting of the
manuscript. All authors had full access to all the study data and
are responsible for the integrity of the data and the accuracy of
the data analysis, confirm the authenticity of all the raw data,
and have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and approved by the
Ethics Committee of the Affiliated Hospital of Qingdao University
(Qingdao, China). Written informed consent was obtained from the
patient.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the data and images in the present case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rapini RP, Hebert AA and Drucker CR:
Acquired perforating dermatosis: Evidence for combined
transepidermal elimination of both collagen and elastic fibers.
Arch Dermatol. 125:1074–1078. 1989.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eljazouly M, Alj M, Chahboun F, Chahdi H
and Chiheb S: Acquired reactive perforating collagenosis: A case
report. Cureus. 13(e13583)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wagner G and Sachse MM: Acquired reactive
perforating dermatosis. J Dtsch Dermatol Ges. 11:723–729, 723-730.
2013.PubMed/NCBI View Article : Google Scholar : (In English,
German).
|
|
4
|
Pai VV, Naveen KN, Athanikar SB, Shastri
DU and Rai V: Familial reactive perforating collagenosis: A report
of two cases. Indian J Dermatol. 59:287–289. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fei C, Wang Y, Gong Y, Xu H, Yu Q and Shi
Y: Acquired reactive perforating collagenosis: A report of a
typical case. Medicine (Baltimore). 95(e4305)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ormerod E, Atwan A, Intzedy L and Stone N:
Dermoscopy features of acquired reactive perforating collagenosis:
A case series. Dermatol Pract Concept. 8:303–305. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Karpouzis A, Giatromanolaki A, Sivridis E
and Kouskoukis C: Acquired reactive perforating collagenosis:
Current status. J Dermatol. 37:585–592. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Faver IR, Daoud MS and Su WP: Acquired
reactive perforating collagenosis. Report of six cases and review
of the literature. J Am Acad Dermatol. 30:575–580. 1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lukács J, Schliemann S and Elsner P:
Treatment of acquired reactive perforating dermatosis-a systematic
review. J Dtsch Dermatol Ges. 16:825–842. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mehregan AH, Schwartz OD and Livingood CS:
Reactive perforating collagenosis. Arch Dermatol. 96:277–282.
1967.PubMed/NCBI
|
|
11
|
Kim SW, Kim MS, Lee JH, Son SJ, Park KY,
Li K, Seo SJ and Han TY: A clinicopathologic study of thirty cases
of acquired perforating dermatosis in Korea. Ann Dermatol.
26:162–171. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kawakami T and Saito R: Acquired reactive
perforating collagenosis associated with diabetes mellitus: Eight
cases that meet Faver's criteria. Br J Dermatol. 140:521–524.
1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Peetermans M, de Prost N, Eckmann C,
Norrby-Teglund A, Skrede S and De Waele JJ: Necrotizing skin and
soft-tissue infections in the intensive care unit. Clin Microbiol
Infect. 26:8–17. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Parlet CP, Brown MM and Horswill AR:
Commensal staphylococci influence Staphylococcus aureus skin
colonization and disease. Trends Microbiol. 27:497–507.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lakhundi S and Zhang K:
Methicillin-Resistant Staphylococcus aureus: Molecular
characterization, evolution, and epidemiology. Clin Microbiol Rev.
31:e00020–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stryjewski ME and Chambers HF: Skin and
soft-tissue infections caused by community-acquired
methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 46
(Suppl 5):S368–S377. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Weiss SC, Moschella SL, Kwan T and Craven
DE: Cutaneous mucormycosis secondary to acquired reactive
perforating collagenosis. Cutis. 72:119–123. 2003.PubMed/NCBI
|