Introduction
Wellens syndrome is a medical pattern characterized
by a biphasic or symmetrical inversion of the T-wave and the
absence of pathological Q waves in the right precordial leads on an
ECG. This syndrome mainly interrelates to the coronary artery
stenosis of the proximal LAD artery. This can be observed during
the pain-free interval in those patients that have unstable angina
(1). It consists of two types: Type
A, which comprises ~25% of the cases, and is characterized by a
biphasic T wave with positive initials and negative terminals in
the precordial leads. Type B includes the majority of the cases
(75%) of Wellens syndrome, and it has deep and symmetrical inverted
T waves, particularly in leads V2 and V3(2). Wellens syndrome is an unfavorable
pattern that requires critical attention due to the risk of
myocardial infarction (1).
Pseudo-Wellens syndrome refers to any ECG pattern that mimics
Wellens syndrome but with no critical LAD artery-associated
coronary artery disease (2). To
date, to the best of our knowledge, there are a few studies on
pseudo-Wellens' syndrome available in the literature (2,3). Its
occurrence in association with pulmonary embolism has rarely been
reported (3).
The present study describes a rare case of
pseudo-Wellens syndrome associated with pulmonary embolism in a
63-year-old female.
Case report
Patient information
A 63-year-old female patient was admitted to the
Smart Cardiology Department, Smart Health Tower, Sulaimani, Iraq,
complaining of chest tightness for a duration of 72 h. This was
associated with dyspnea on exertion, intermittent local chest pain
and a dry cough. The chest pain restricted the daily activities of
the patient. She was neither an alcoholic nor a smoker, and she did
not experience orthopnea, hemoptysis, fever, or vomiting. The
patient had hypertension for 3 years and hypothyroidism for the
past 5 years. She had used amlodipine (5 mg once per day),
lisinopril (10 mg once per day) and levothyroxine (150 mcg once per
day). The past surgical history of the patient included
thyroidectomy, dilatation, and curettage. Her family history was
positive for hypertension and diabetes mellitus.
Clinical findings
The vital signs of the patient were as follows:
Respiratory rate (25 breaths/min), heart rate (95 beats/min),
peripheral capillary oxygen saturation (90-93%), blood pressure
(130/90 mmHg), and a temperature of 37˚C. Upon a general
examination, the patient was conscious, alert, and oriented.
Thyroid enlargement, pallor, cyanosis, lymphadenopathy, anemia, and
jaundice were not observed. The precordial examination and heart
sounds (S1 and S2) were normal, with no additional sounds or
murmurs. A mild bilateral lower-pitting leg edema (superficial
varicose vein) was observed, with no calf pain on palpation. The
examination of the abdomen and the respiratory system was generally
normal, although there were right lower basal fine chest crackles
without wheezing.
Diagnostic assessment
A blood examination was conducted and revealed a
normal blood composition. The troponin I test (0.03 ng/ml), renal
function and liver function tests were within the normal ranges,
while the C-reactive protein (98.80 mg/l) (normal range, ≤5 mg/l)
and D-dimer (7599.9 ng/ml) (normal range, ≤500 ng/ml) levels were
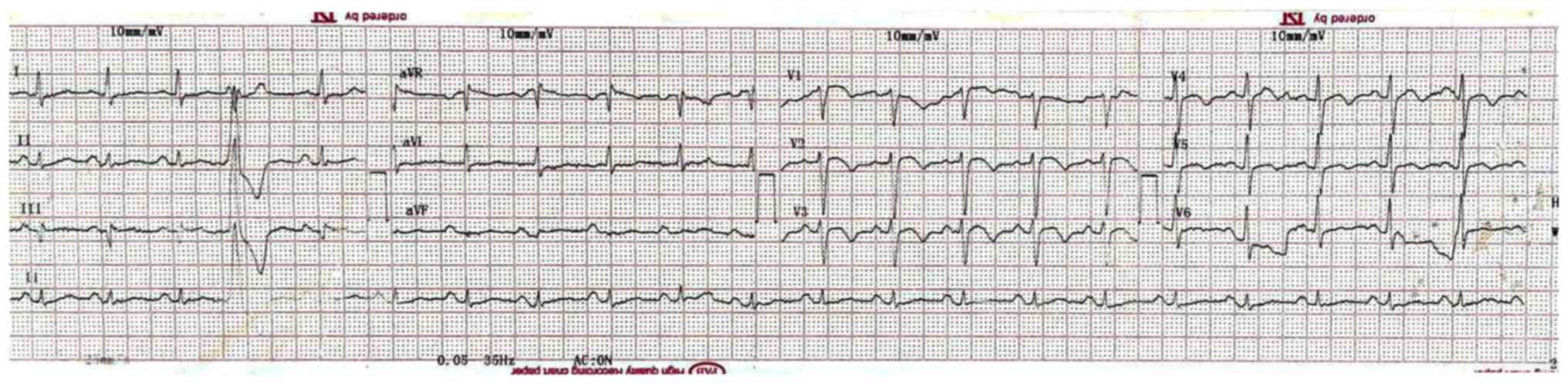
abnormal. An ECG revealed a biphasic inversion of the T wave in
precordial leads in the pain-free interval. A minimal elevation of
the ST segment, no precordial Q waves, and one ventricular
extrasystole were also observed in the ECG (Fig. 1). The echocardiography revealed a
moderate dilatation of the right ventricle, moderate tricuspid
regurgitation, inter-ventricular septum flattening (D shape), and
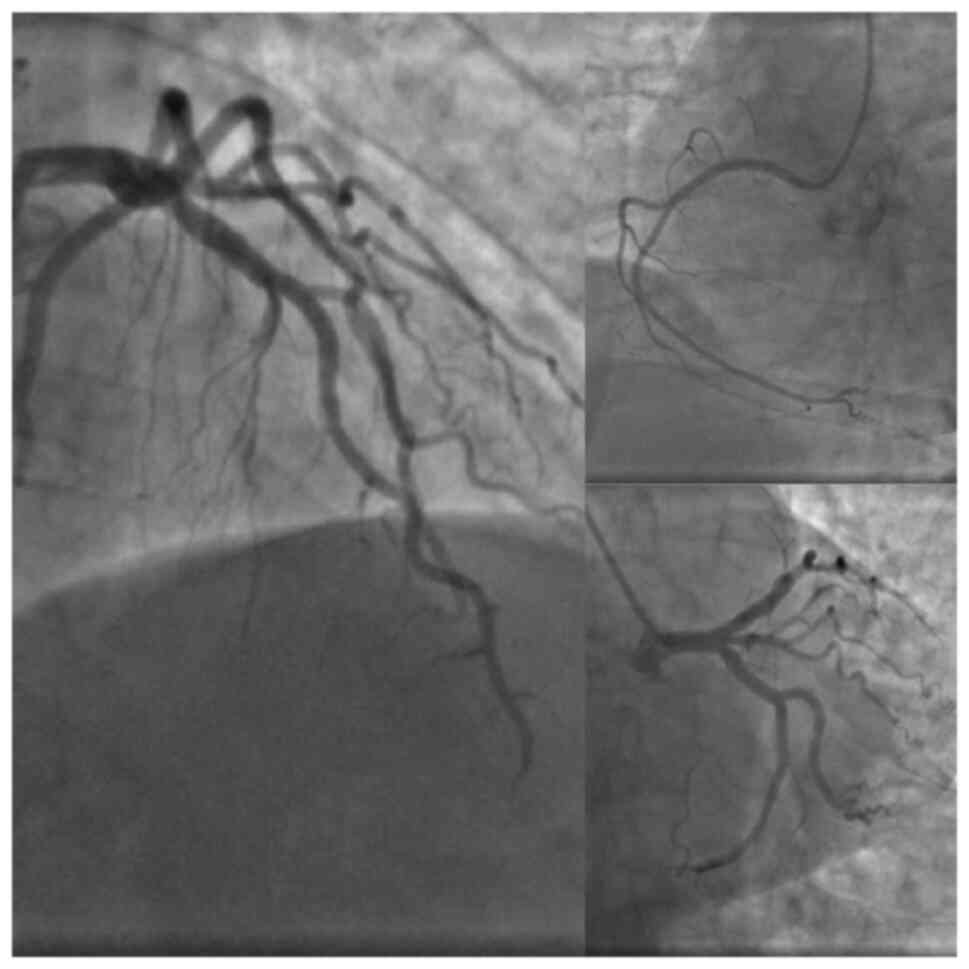
positive McConnell's sign. A coronary angiography was performed and
this did not reveal any notable findings (Fig. 2). There was no critical stenosis of
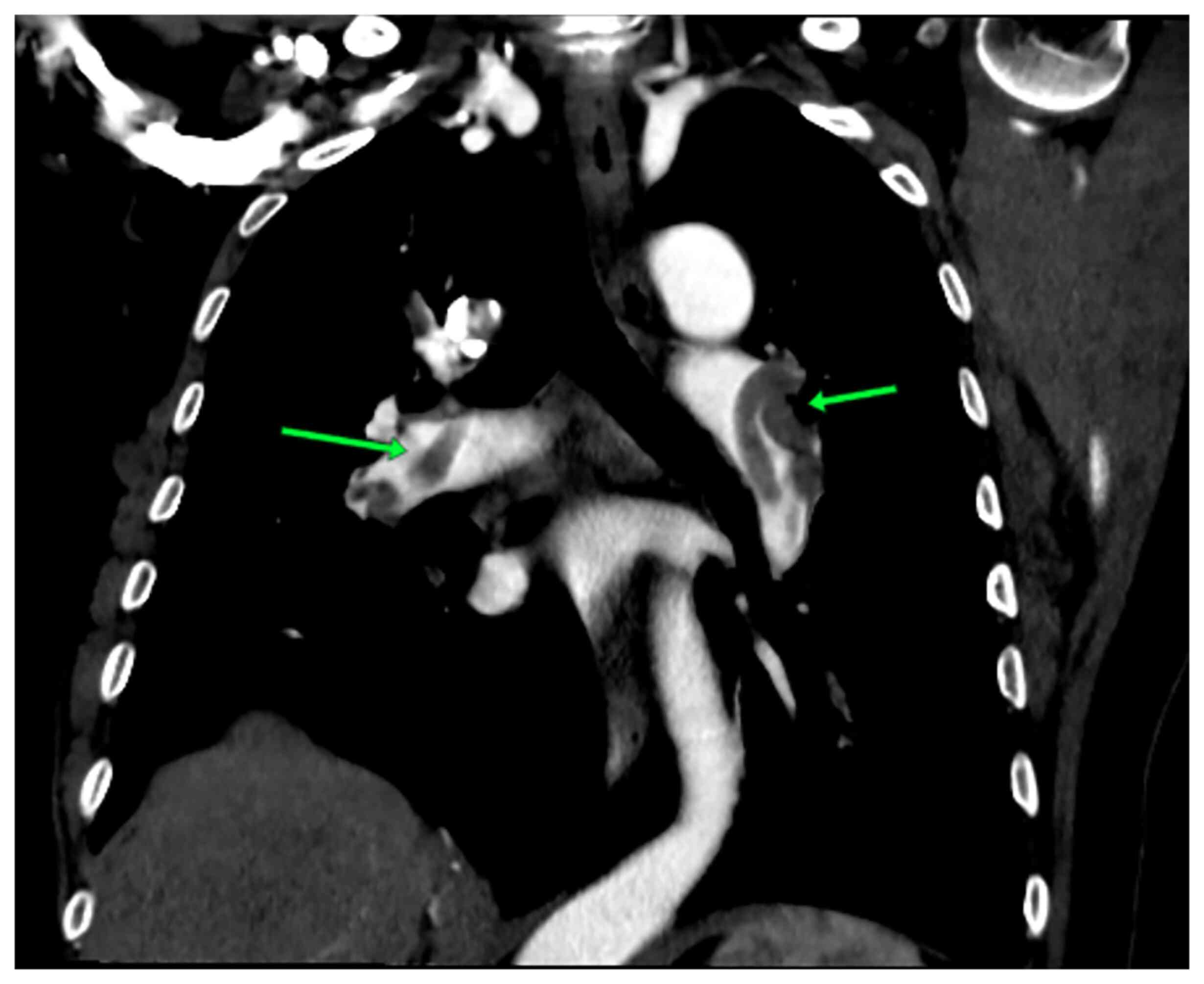
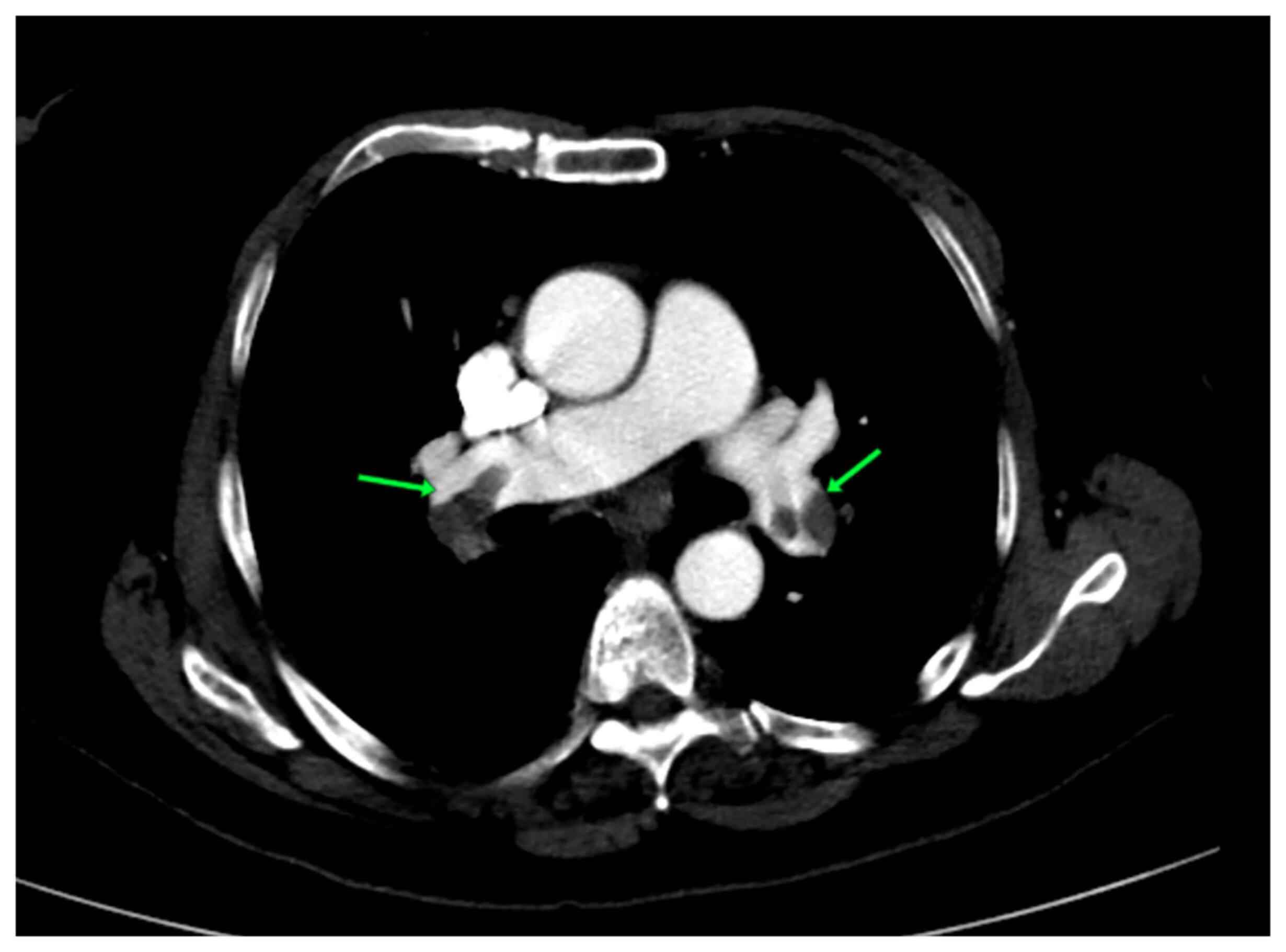
the proximal LAD coronary artery. A computed tomography pulmonary
angiography revealed an acute pulmonary embolism (Figs. 3 and 4). Therefore, all the results supported the
occurrence of pseudo-Wellens syndrome. The Thrombolysis in
Myocardial Infarction (TIMI) score was equal to one and revealed a
low risk of adverse cardiac issues (Table I). The score was determined by the
occurrence of only a marked change in her ECG scan.
 | Table IThrombolysis in Myocardial Infarction
(TIMI) risk score. |
Table I
Thrombolysis in Myocardial Infarction
(TIMI) risk score.
| Predicting
factors | Score |
|---|
| Age over 65
years | One point |
| Three or more
atherosclerosis risk factors | One point |
| Coronary artery
disease | One point |
| Two or more episodes
of unstable angina in the last 24 h | One point |
| Using acetylsalicylic
acid in the 7 days prior to hospitalization | One point |
| Elevated cardiac
markers | One point |
| Marked changes in the
electrocardiogram results | One point |
Therapeutic intervention
The patient was initially treated as a case of
non-ST-elevation myocardial infarction. On the first day of
admission, she was administered the following drugs: Metoclopramide
[10 mg once per day; intravenously (i.v.)], tramadol (once per day;
intravenously), plavix (300 mg tab), aspirin (300 mg once per day;
tab), atorvastatin (40 mg once per day), unfractionated heparin
(UFH) 1 cubic centimeter (5,000 IU; intravenously) and metoprolol
(50 mg once per day). Following the coronary angiography, the
patient received UFH (20,000 IU) by intravenous infusion in a
manner of 2 ml/h for 5 days with 24 h monitoring of vital signs.
The case was discharged on rivaroxaban (15 mg) twice daily for 21
days.
Follow-up
The post-treatment period was uneventful, and the
patient's condition was relieved.
Discussion
Wellens syndrome, an abnormal pattern on an ECG, was
first reported in 1982. It is identified by T wave changes in the
precordial leads, which are an indicator of critical stenosis in
the proximal LAD coronary artery (1). The abnormalities of T waves can be
presented in two critical patterns; the deep inversion of T waves
or biphasic T waves in several precordial leads. The changes in T
waves commonly occur in leads V2-V3, although they can extend to
other precordial leads and persist for hours and weeks (4).
Rhinehardt et al (4) proposed several criteria to help
differentiate Wellens syndrome from the other potential causes of T
wave inversion in the precordial leads. The criteria include the
following properties: i) Biphasic or deep inversion of T waves,
particularly in leads V2-V3 or in leads V1, V4, V5 and V6; ii) a
normal or minimal elevation of the ST-segment and cardiac enzymes;
iii) previous experience of angina i) normal Q waves and precordial
R-wave progression (4). However, the
identification of these criteria is critical in the presence of ECG
alternations; the specificity, sensitivity, and positive predictive
value of inverted T waves for LAD stenosis are 89, 69 and 86%,
respectively. This indicates that ECG alternations with the
properties of Wellens syndrome do not always guarantee its
occurrence, and in the presence of a normal coronary artery, the
condition is termed pseudo-Wellens syndrome (5). The case described herein matched the
criteria described in the study by Rhinehardt et al
(4), although the history of angina
was insignificant and the cardiac enzyme (troponin I) level was
normal. The angiography of the case exhibited a normal coronary
artery.
Pseudo-Wellens syndrome has been mentioned in
patients with coronary spasms, myocardial bridges, acute
cholecystitis, and in those using illicit drugs (5). The study by Batra et al
(6) revealed the drug effects on the
development of pseudo-Wellens syndrome. They claimed that it is
crucial to take the complete drug history of patients, particularly
the use of illicit drugs. This may help to evaluate the chances of
Wellens syndrome and interpret the cause of an abnormal ECG
pattern. A coronary angiography was conducted in the study of Batra
et al (6) and it showed no
LAD lesion. They stated that the major cause of the ECG changes in
their case was the coronary artery spasm due to heroin use
(6). The effects of illicit drugs,
such as cocaine on the occurrence of pseudo-Wellens syndrome have
been confirmed by others (7).
However, another study revealed that the condition can be resolved
after cocaine clearance from the body and the ECG pattern finally
returns to normal (8).
Disorders, such as alcohol-induced pancreatitis,
hemorrhagic stroke, subarachnoid hemorrhage, apical hypertrophic
cardiomyopathy, myocarditis, pericarditis and hypertension have
been reported to be associated with pseudo-Wellens syndrome
(2). In addition, Milne et al
(9) also reported a case of
pseudo-Wellens syndrome that was induced by a myocardial
bridge.
Several factors induce venous thromboembolism (VTE),
such as genetic factors, pregnancy, recent surgery, immobilization
and obesity. Furthermore, it has been found that antipsychotic
agents can elevate the risk of developing VTE. The mortality rate
associated with pulmonary embolism due to clozapine use has been
reported to be >44% (10).
Abrahim (2) conducted a literature
review in the PubMed electronic database for English-published
manuscripts with the keyword of pseudo-Wellens and found 17
articles. In almost half of the studies, biphasic T wave changes in
the precordial leads demonstrated type A Wellens syndrome, and one
case reported the association of pseudo-Wellens syndrome with
hypertension. However, they did not mention the identity of the
other seven studies (2). The
association of pulmonary embolism with pseudo-Wellens syndrome has
rarely been mentioned in the literature. Vanni et al
(11) reported a case of a right
ventricular strain pattern in an ECG associated with pulmonary
embolism. Sedhai et al (3)
reported a case of pseudo-Wellens syndrome associated with
pulmonary embolism. The patient was affected by the pulmonary
embolism after the first week of using risperidone to manage his
schizoaffective disorder (3). In the
present study, the patient was free from most of the conditions
associated with pseudo-Wellens syndrome. She had hypertension and
mild bilateral lower-pitting leg edema. The patient had used
amlodipine, lisinopril and levothyroxine. She had undergone
dilatation and curettage 1 month prior to the presentation of
pseudo-Wellens syndrome.
Cardiac magnetic resonance imaging (MRI) is regarded
as an accurate diagnostic modality for the detection of myocardial
infarction in cases of abnormal ECG patterns and Wellens syndrome
(12). Cardiac MRI was not conducted
in the present study as the TIMI score was equal to one and
revealed a low risk of adverse cardiac issues.
Sedhai et al (3) initially treated their case using
heparin infusion, and the case later used rivaroxaban for a
duration of 3 months (3). The
present case, following the coronary angiography, was treated with
UFH (20,000 IU) by intravenous infusion for 5 days. She was then
discharged on rivaroxaban (15 mg) for 21 days.
In conclusion, patients with pulmonary embolism may
have the symptoms of Wellens syndrome in the electrocardiographic
examination without the occurrence of proximal LAD
artery-associated coronary artery disease. The present study also
suggests that conducting a coronary angiography is crucial for
those patients who have an association of pulmonary embolism with
an abnormal electrocardiographic pattern in order to prevent
unnecessary intervention.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHA was a major contributor to the conception of the
study, as well as in the literature search for related studies.
SFA, BAA and FHK were involved in the literature review, in the
writing of the manuscript, and in the examination and
interpretation of the patient's data. FHF, BJHA and DHMS were
involved in the literature review, the design of the study, the
revision of the manuscript and in the processing of the figures.
SFA and FHK confirm the authenticity of all the raw data. SHT was
the radiologist who performed the assessment of the subject's
pseudo Wellens syndrome. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent for
participation in the study.
Patient consent for publication
The patient provided written informed consent for
the publication of her data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Zwaan C, Bär FW and Wellens HJ:
Characteristic electrocardiographic pattern indicating a critical
stenosis high in left anterior descending coronary artery in
patients admitted because of impending myocardial infarction. Am
Heart J. 103:730–736. 1982.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abrahim M: Pseudo-Wellens' syndrome type A
in asymptomatic severe hypertension at a rural emergency
department. CJEM. 24:224–226. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sedhai YR, Basnyat S and Bhattacharya PT:
Pseudo-Wellens' syndrome in pulmonary embolism. BMJ Case Rep.
11(e227464)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rhinehardt J, Brady WJ, Perron AD and
Mattu A: Electrocardiographic manifestations of Wellens' syndrome.
Am J Emerg Med. 20:638–643. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ola O and Tak T: Pseudo-Wellens syndrome
in a patient with hypertension and left ventricular hypertrophy. Am
J Case Rep. 20:1231–1234. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Batra R, Mishra A and Ng K: Pseudo-Wellens
syndrome-a case report. Kardiol Pol. 66:340–342; discussion 342-3.
2008.PubMed/NCBI
|
|
7
|
Langston W and Pollack M: Pseudo-Wellens
syndrome in a cocaine user. Am J Emerg Med. 24:122–123.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miner B, Grigg WS and Hart EH: Wellens
syndrome. In: StatPearls.StatPearls Publishing,Treasure Island, FL,
2022.
|
|
9
|
Milne D, Ramadhin D, Seecheran R,
Seecheran V, Henry R and Seecheran NA: The curious case of
Pseudo-Wellens' Syndrome and Myocardial bridging. J Investig Med
High Impact Case Rep. 10(23247096211073255)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Paciullo CA: Evaluating the association
between clozapine and venous thromboembolism. Am J Health Syst
Pharm. 65:1825–1829. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vanni S, Polidori G, Vergara R, Pepe G,
Nazerian P, Moroni F, Garbelli E, Daviddi F and Grifoni S:
Prognostic value of ECG among patients with acute pulmonary
embolism and normal blood pressure. Am J Med. 122:257–264.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ricciardi MJ, Wu E, Davidson CJ, Choi KM,
Klocke FJ, Bonow RO, Judd RM and Kim RJ: Visualization of discrete
microinfarction after percutaneous coronary intervention associated
with mild creatine kinase-MB elevation. Circulation. 103:2780–2783.
2001.PubMed/NCBI View Article : Google Scholar
|