Introduction
As early as 2010, there were concerns about the
spread of carbapenemase-producing Enterobacterales (CPE) in
healthcare facilities in Europe; therefore, the European Union (EU)
member states proposed an assessment of the risks of spreading CPE
from one patient to another (1).
Although the prevalence of CPE within the EU
community is not well known, it is acknowledged that these strains
are endemic to certain countries, including Romania (1). Carbapenemases are β-lactamases that
efficiently hydrolyze most β-lactams, including carbapenems
(1). CPE and carbapenem-resistant
Enterobacterales (CRE) strains usually include Escherichia
coli and Klebsiella pneumoniae (1,2).
The European Center for Disease Prevention and
Control (ECDC) claims that CRE is associated with a high mortality
rate; thus, it is considered that patients infected with CPE also
have a high risk of mortality. The ECDC also affirms that the
causes of death are due to the limitations of treatment options or
due to delays (1).
The risk factors associated with CPE infection are
similar to those associated with other multidrug-resistant
organisms (MDRO) (1,2). The risk factors for acquiring CPE are
prior antimicrobial use, previous hospitalization, admission to the
intensive care unit (ICU), the severity of illness, a duration of
hospitalization >20 days, the use of antibiotics for >10
days, pneumonia or chronic pulmonary disease, and the previous use
of nasogastric tubes (1,3-5).
CPE infections represent a threat to patient safety
owing to antimicrobial resistance (AMR), increased morbidity and
mortality, and very high hospital costs (1). The ECDC claims that in the European
Union/European Economic Area (EU/EEA), 33,000 individuals succumb
each year as a direct consequence of MDRO infections, and
>670,000 infections are caused by these organisms (6). According to the Centers for Disease
Control and Prevention (CDC) in the USA, it has been estimated that
the direct healthcare costs associated with AMR is up to 20 billion
dollars (7). Otter et al
(8) published an article on 40
patients from five hospitals and found that CPE outbreaks were
associated with high costs. A study from 2003 to 2017 carried out
in Scotland, in which 290 CPE strains from clinical and long-term
healthcare surveillance cultures were detected, supported the fact
that an age >60 years, systemic infection or organ failure, and
the presence of non-fermenters were independently associated with
30-day mortality (9).
In another study published by Pintado et al
(10), patients with coronavirus
disease 2019 (COVID-19) were observed to have an increased risk of
CPE infections and were associated with a high risk of mortality.
Following a systematic literature review performed by searching the
EMBASE, PubMed, and Cochrane Library databases, Hu et al
(11) found that the risk factors
most frequently associated with CRE mortality were antibiotic use,
comorbidities and hospital-related factors (11).
The available data on the mortality of patients with
CPE and the associated risk factors in Romania are not sufficient.
International data identified through the European Antimicrobial
Resistance Surveillance Network (EARS Net) support the fact that in
Romania, the rates of CRE are increased, and the levels of AMR in
Romania are a matter of concern (6,12).
The present study aimed to identify CPE strains and
assess the risk factors for mortality in patients with CPE strains
who were hospitalized in the ICU.
Patients and methods
Study design
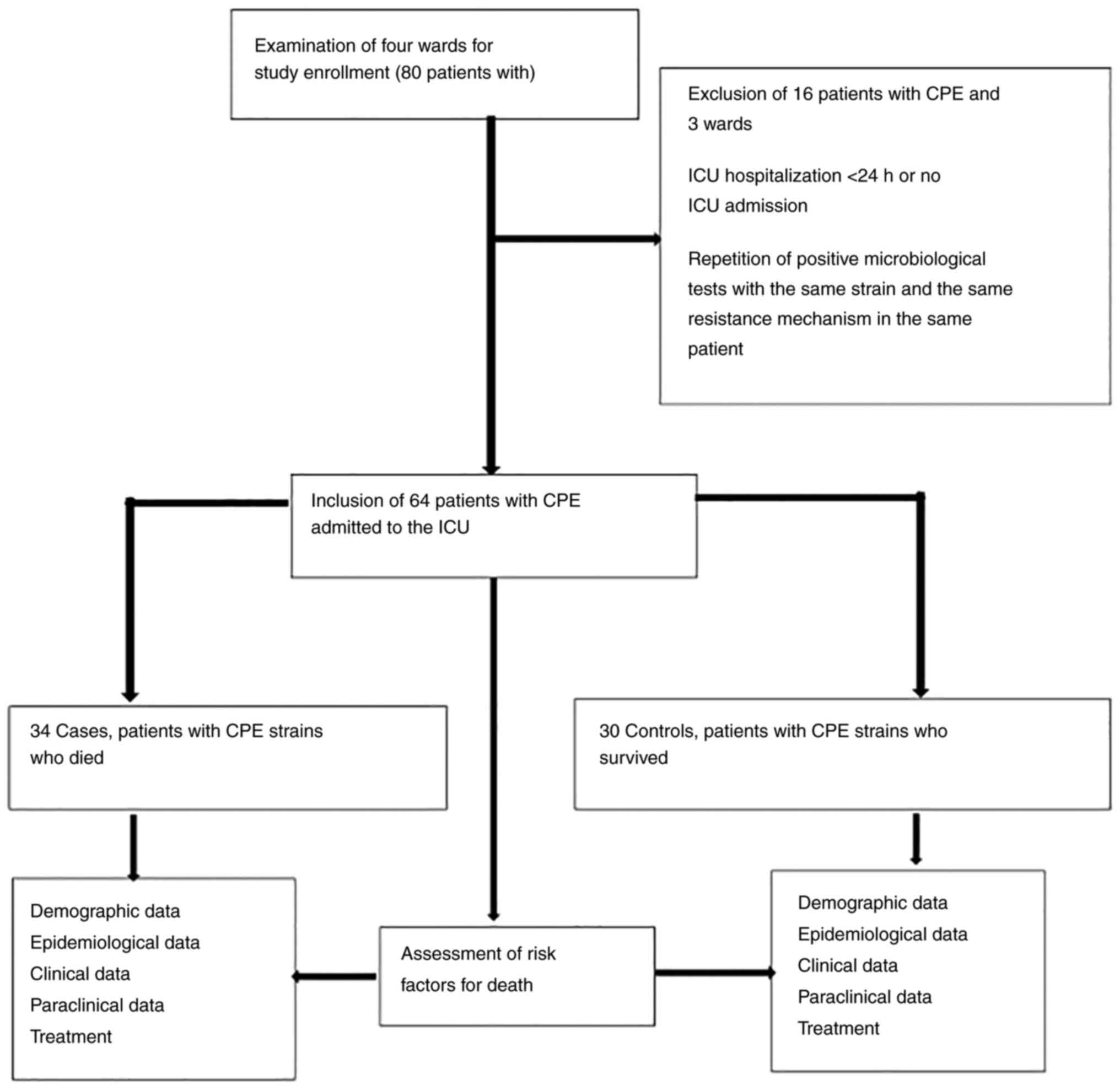
A retrospective, case-control study was conducted in
a single center, which included patients confirmed to be infected
wiht CPE between September, 2017 and October, 2021, who were
admitted to the ICU of the Infectious Diseases Hospital of
Constanta, Romania. The inclusion criterion was an ICU admission
>24 h associated with CPE infection or colonization. The
exclusion criteria were non-admission to the ICU and an ICU
admission of <24 h. CPE strains were detected upon admission to
the ICU or throughout the course of admission. Bacteriological
screening for bacterial colonization with CPE was performed upon
admission to the ICU and every 7 days after hospitalization. CPE
strains were found in rectal swabs, urine cultures, sputum, blood
cultures and skin swabs. Demographic, epidemiological, clinical,
paraclinical and treatment data were analyzed. The cause of
hospitalization in the ICU was predominantly the severity of
COVID-19 infection, and to a lesser extent, the CPE infection. It
should be reported that CPE colonization was detected only in the
ICU, which is the only department in the hospital where
bacteriological screening was performed to detect these
organisms.
The study population was divided into cases
(patients with CPE who succumbed) and controls (patients with CPE
who survived). The ICU of the Infectious Diseases Hospital of
Constanta included 10 beds for critical patients with infections.
The data were collected from the IT system of the hospital.
Demographic, epidemiological, clinical and paraclinical data, and
patient treatment data were analyzed. These data included variables
such as sex, age, the Charlson Comorbidity Index, admission with
COVID-19 or bacterial infection, previous hospitalization or
previous antibiotic therapy, leukopenia upon admission, C-reactive
protein (CRP) levels >5 mg/l upon admission, invasive mechanical
ventilation, CPE colonization or CPE infection, and length of stay
(LOS) in the ICU. The inclusion and exclusion criteria, and the
data used for analysis are presented in Fig. 1.
Microbiology
In the Infectious Diseases Hospital of Constanta
ICU, 73 CPE strains were detected in patients hospitalized for
>24 h. There were 52 cases of CPE colonization and 21 cases of
CPE infection in 64 patients. CPE colonization strains were
detected by bacteriological screening using carbapenem-resistant
Enterobacteriaceae chromogenic media and the Modified Hodge test.
CPE strains were detected using the VITEK 2 system (bioMérieux) or
matrix-assisted laser desorption/ionization time-of-flight mass
spectrometry. Rosco discs and the Modified Hodge test confirmed
carbapenemase production. Infection with severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) was detected using the RT-PCR
SARS-CoV-2 test. The strains were identified in the blood, sputum,
urine and rectal swab tests or skin swabs. CPE strains, including
Klebsiella spp. and Escherichia coli were identified.
Colonization was distinguished from infection by an infectious
disease doctor according to the patient's clinical and paraclinical
criteria. A total of 27 CPE infections were detected according to
the clinical and paraclinical data interpreted by the infectious
disease physician. All strains detected in the rectal swab through
the bacteriological screening program carried out in the ICU of the
hospital were bacterial colonization and not infections. Other
cultures performed at the time of admission or during
hospitalization at the indication of the attending physician were
interpreted as colonization or infection. Patients with
colonization did not meet the criteria for inflammation and had no
signs or symptoms, whereas patients with an infection had multiple
signs and symptoms that were specific to an infectious disease. The
antibiogram was performed only for the isolates detected in the
urinary tract, sputum and blood samples, and it was not performed
for the rectal swab cultures, as recommended by the hospital in
terms of costs. The antibiogram was interpreted according to the
guidelines of the European Committee for Antimicrobial
Susceptibility Testing (EUCAST).
Ethical review and approval
The present study was conducted in accordance with
the principles of the Declaration of Helsinki (13). All patients provided written informed
consent for the use of their personal data upon hospital admission.
Patient anonymity was guaranteed during the whole process of data
analysis and reporting of results. The Infectious Diseases Hospital
of Constanta considered ethical review and approval unnecessary due
to the retrospective nature of the study (NR 1/20/01.2023; CODE
F.05.PO.17.00-ACFOCG). This study conformed to the Strengthening
the Reporting of Observational Studies in Epidemiology (STROBE)
guidelines (14).
Statistical analysis
Statistical analysis was performed using IBM SPSS
Statistics, version 20.0 (IBM Corp.), and the data collected were
imported into Microsoft Excel. The study was divided into two
groups as follows: Cases (patients with CPE strains who succumbed)
and controls (patients with CPE strains who survived). The
variables associated with mortality with values of P≤0.05 in the
univariate analysis were evaluated using the χ2 test.
Variables with values of P≤0.05 in the univariate analysis were
selected for logistic regression analysis as a multivariate
statistical method. The hypothesis testing was two-tailed, and a
value of P≤0.05 was considered to indicate a statistically
significant difference.
Results
According to the inclusion and exclusion criteria,
64 patients with CPE were admitted to the ICU, including 34 cases
(patients with CPE who succumbed) and 30 controls (patients with
CPE who survived). As demonstrated in to Table I, it was found that Klebsiella
spp. strains were predominant, totaling 53 strains of which
31 were identified in patients who succumbed (with a 91.2%
prevalence, out of 34 strains). Escherichia coli was
detected in 3 patients (8.8%) who succumbed and in 8 patients
(26.7%) who survived. In the univariate analysis, this strain in
the patients did not exhibit a statistically significant difference
(P>0.05). As regards the isolates detected in patients with CPE
who succumbed, 27 strains (79.4%) were identified in rectal swab
samples, two strains (5.9%) were identified in urine culture, eight
strains (23.5%) were identified in sputum and three strains (8.8%)
were identified in blood samples. Univariate analysis revealed that
the source of the CPE strains was not statistically significant
(P>0.05) (Table I).
 | Table IThe CPE strains in patients in the
ICU. |
Table I
The CPE strains in patients in the
ICU.
| Microorganism, n
(%) | Cases group (CPE
strains and mortality) (n=34) (%) | Control group (CPE
strains and survival) (n=30) (%) | P-valuea |
|---|
| CPE strains | | | |
| Klebsiella
spp. | 31 (91.2) | 22 (73.4) | 0.38 |
| Escherichia
coli | 3 (8.8) | 8 (26.7) | 0.059 |
| Source of CPE
strains | | | |
|
Rectal
swab | 27 (79.4) | 22 (73.4) | 0.56 |
|
Urine
culture | 2 (5.9) | 4 (13.4) | 0.08 |
|
Sputum | 8 (23.5) | 3(10) | 0.15 |
|
Blood
culture | 3 (8.8) | 0 (0) | 0.09 |
|
Skin
swab | 0 (0) | 1 (3.4) | 0.28 |
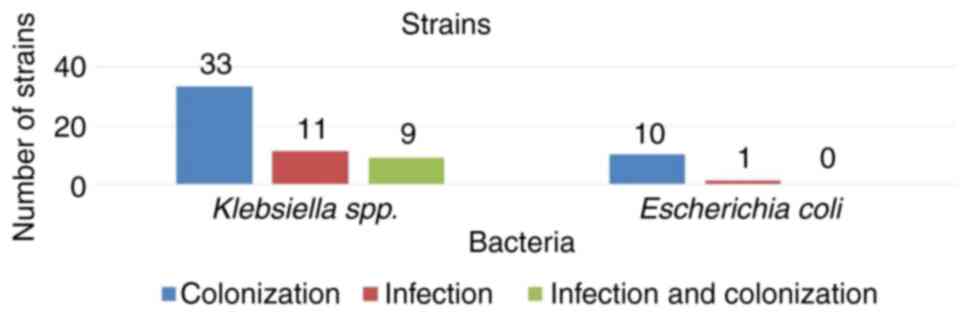
As demonstrated in Fig.
2, it was observed that the majority of patients with bacterial
colonies were detected in the ICU. Of the 64 patients, 53 (82.8%)
were with Klebsiella spp. Of the 53 patients with
Klebsiella spp., 44 (83.01%) had only
carbapenemase-producing Klebsiella spp., of which 33 (75%)
had colonization and 11 (25%) had bacterial infections. Of the 53
patients with Klebsiella spp., 9 patients (17%) had
bacterial colonization and infection with the same
carbapenenase-producing Klebsiella spp. strain.
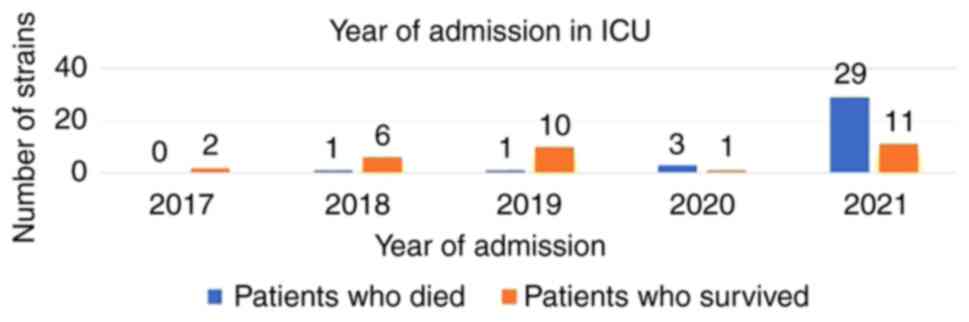
It was also observed that the majority of patients
passed away in 2021, while in 2017, all the patients with CPE
strains survived (Fig. 3).
The medical history and demographic, clinical and
paraclinical data of the patients are presented in Table II. Of the total number of patients
who succumbed in the ICU, 24 were male (70.6%), while of those who
survived, 21 were male (70%). The median age of the deceased
patients was 63.88, while the median age of those who survived was
64.67. Among those who succumbed, 19 patients (55.9%) were >65
years of age. As regards comorbidities, considering a Charlson
Comorbidity Index (CCI) score ≥4, the findings were similar in both
groups, with 18 patients (52.9%) in the case group and 16 (53.3%)
in the control group.
 | Table IICharacteristics of patients with CPE
strains admitted to the ICU. |
Table II
Characteristics of patients with CPE
strains admitted to the ICU.
| Characteristics of
patients with CPE strains, n (%) | Cases group (CPE
strains and mortality) (n=34) | Control group (CPE
strains and survival) (n=30) |
P-valuea | Univariate analysis
OR (95% CI) |
|---|
| Sex, male | 24 (70.6%) | 21 (70%) | 0.95 | 1.02
(0.35-3.01) |
| Sex, female | 10 (29.4%) | 9 (30%) | - | - |
| Average age,
years | 63.88 | 64.67 | - | - |
| ≥65 Years old | 19 (55.9%) | 17 (56.7%) | 0.95 | 0.96
(0.36-2.60) |
| Charlson
Comorbidity Index (CCI) ≥4 | 18 (52.9%) | 16 (53.3%) | 0.97 | 0.98
(0.36-2.63) |
| Admission with
COVID-19 | 30 (88.2%) | 8 (26.7%) | 0.001 | 20.62
(5.50-77.23) |
| Admission with a
bacterial infection Previous | 5 (14.7%) | 17 (56.7%) | 0.001 | 0.13
(0.04-0.43) |
|
hospitalization | 7 (20.6%) | 13 (43.3%) | 0.003 | 0.15
(0.04-0.56) |
| Previous antibiotic
therapy | 22 (64.7%) | 14 (46.7%) | 0.52 | 1.57
(0.38-6.43) |
| Leukopenia on
admission | 26 (76.5%) | 20 (66.7%) | 0.38 | 1.62
(0.54-4.86) |
| C-reactive protein
(CRP) level >5 mg/l at admission | 33 (97.1%) | 26 (86.7%) | 0.12 | 5.07
(0.53-48.20) |
| Invasive mechanical
ventilation | 13 (38.2%) | 1 (3.3%) | 0.001 | 17.95
(2.17-148.08) |
| CPE
colonization | 27 (79.4%) | 25 (83.3%) | 0.68 | 0.77
(0.21-2.74) |
| CPE infection | 13 (38.2%) | 8 (26.7%) | 0.32 | 1.70
(0.58-4.93) |
| Length of stay in
ICU >3 days after CPE diagnosis | 29 (85.3%) | 24 (80%) | 0.57 | 1.45
(0.39-5.34) |
| Length of stay in
ICU, median (IQR) | 9.61 | 9.46 | - | - |
Other possible predictors for mortality may be
admission with COVID-19 or admission with a bacterial infection. It
was determined that the number of patients with COVID-19 was 30
(88.2%) in the deceased group, and 8 (26.7%) in the control group.
Admission with bacterial infection was predominant in the control
group, with 17 cases (56.7%) among the patients who survived,
compared to 5 cases (14.7%) among the patients who succumbed. Upon
univariate analysis, a statistically significant positive
association was noted between admission with COVID-19 and mortality
[odds ratio (OR), 20.62; 95% confidence interval (CI), 5.50-77.23;
P=0.001].
As regards the epidemiological data, there were 7
patients (20.6%) with previous hospitalization who succumbed and 13
patients with previous hospitalization (43.3%) in the group of
patients who survived. Another observation was that there were 22
patients (64.7%) who had received previous antibiotic therapy among
the group of patients who succumbed during hospitalization in the
ICU and 14 patients (46.7%) who had received previous antibiotic
treatment among those who survived.
For the paraclinical data, 26 patients (76.5%) with
leukopenia upon admission to the ICU succumbed, whereas 20 patients
(66.7%) had leukopenia in the group of patients who survived. A CRP
level >5 mg/l upon admission to the ICU was identified in 33
patients (97.1%) who succumbed compared to 26 patients (86.7%) who
survived. It should be specified that the patients who were
mechanically ventilated had a high mortality rate, compared to the
patients who were not mechanically ventilated. Specifically, 13
patients (38.2%) who were mechanically ventilated succumbed,
whereas among the surviving patients, only 1 patient (3.3%) was
mechanically ventilated. Furthermore, univariate analysis confirmed
a statistically significant positive association between invasive
mechanical ventilation and mortality (OR,17.95; 95% CI,
2.17-148.08; P=0.001).
As regard the LOS in the ICU, as shown in Table II, it was found that there were 29
patients (85.3%) who had a LOS in the ICU >3 days after the CPE
diagnosis among those who succumbed, while the number of patients
with a LOS >3 days in the ICU among the group of patients who
survived was 24 (80%). The univariate analysis of LOS >3 days
after the diagnosis of CPE revealed no significant association with
mortality (P>0.05).
No statistically significant differences were
observed for the patient mortality and survival rates in patients
with CPE colonization on the one hand, and patients with CPE
infection on the other hand.
As regards the treatment prescribed to patients who
were admitted to the ICU, it was observed that all patients who
succumbed had received antibiotic treatment, and 26 (76.5%) of them
had received reserve antibiotics. Of the 6 patients who received
ceftazidime-avibactam treatment, 2 (5.9%) were in the group of
patients who succumbed, and 4 (13.3%) were in the group of patients
who survived. For treatment with other new antibiotics, 1 patient
from the group who succumbed (2.94%) received
ceftolozane-tazobactam, and 1 patient from the control group (3.3%)
received imipenem-cilastatin-relebactam. Colistin was administered
to 5 patients (14.7%) who succumbed and to 2 patients (6.7%) who
survived. Carbapenem treatment was administered to 17 patients
(50%) who died and eight patients (26.7%) survived. Other
treatments, such as oral vancomycin were predominantly administered
to patients who survived (OR, 0.02; 95% CI, 0.12, 0.01-0.97;
P=0.025), while corticosteroid treatment was administered
predominantly to patients who succumbed (OR, 4.66; 95% CI,
1.49-14.52; P=0.006). The treatment data of the patients are
presented in Table III.
 | Table IIITreatment of patients admitted to the
ICU. |
Table III
Treatment of patients admitted to the
ICU.
| Treatment of
patients with CPE strains, n (%) | Cases group (CPE
strains and mortality) (n=34) (%) | Control group (CPE
strains and survival) (n=30) (%) |
P-valuea | Univariate analysis
OR (95% CI) |
|---|
| Antibiotic
treatment | 34(100) | 26 (86.7) | - | - |
| Reserve antibiotic
treatment | 26 (76.5) | 16 (53.3) | 0.052 | 2.84
(0.97-8.28) |
|
Ceftazidim-avibactam | 2 (5.9) | 4 (13.3) | 0.30 | 0.40
(0.06-2.39) |
|
Ceftolozane-tazobactam | 1 (2.94) | 0 (0) | - | - |
|
Imipenem-cilastatin-relebactam | 0 (0) | 1 (3.3) | - | - |
| Colistin | 5 (14.7) | 2 (6.7) | 0.30 | 2.41
(0.43-13.48) |
| Carbapenem | 17(50) | 8 (26.7) | 0.056 | 2.75
(0.96-7.87) |
| Cephalosporins | 10 (29.4) | 4 (13.3) | 0.12 | 2.70
(0.74-9.79) |
|
Piperacillin-tazobactam | 1 (2.9) | 1 (3.3) | 0.92 | 0.87
(0.05-14.68) |
| Quinolones | 12 (35.3) | 7 (23.3) | 0.19 | 0.38
(0.08-1.70) |
| Oral
vancomycin | 1 (2.9) | 6(20) | 0.025 | 0.12
(0.01-0.97) |
| Linezolid | 8 (23.5) | 2 (6.7) | 0.06 | 4.30
(0.83-22.18) |
| Amikacin | 5 (14.7) | 5 (16.7) | 0.82 | 0.86
(0.22-3.32) |
| Doxycycline | 4 (11.8) | 2 (6.7) | 0.48 | 1.86
(0.31-11.00) |
|
Corticosteroids | 28 (82.4) | 15(50) | 0.006 | 4.66
(1.49-14.52) |
Multivariate analysis revealed that admission with
COVID-19 and invasive mechanical ventilation were independent risk
factors for mortality in patients with CPE. The statistical data
are presented in Table IV.
 | Table IVIndependent risk factors for
mortality. |
Table IV
Independent risk factors for
mortality.
| | 95% CIa |
|---|
| Mortality
predictors, multivariate analysis | P-value | OR | Lower | Upper |
|---|
| Admission with
COVID-19 | 0.001 | 16.26 | 3.56 | 74.14 |
| Invasive mechanical
ventilation | 0.002 | 14.98 | 1.35 | 166.22 |
|
Corticosteroids | 0.56 | 1.56 | 0.34 | 7.16 |
Discussion
In the present study, as in other studies,
SARS-CoV-2 infection and invasive mechanical ventilation were
determined to increase the risk of mortality in patients admitted
to the ICU (10,15,16).
Contou et al (17) claimed
that patients with COVID-19 had a high mortality rate, particularly
if they were intubated. In their study, they referred to a large
multicenter study in which it was reported that 14% of patients
with SARS-CoV-2 infection who were in critical condition
experienced cardiac arrest (17). In
another study, multivariate analysis revealed that the APACHE II
score, age, the need for invasive mechanical ventilation, increased
creatinine levels, and decreased serum albumin levels were
independent risk factors for mortality (18). In their study, Zhao et al
(9) reported that an age >60
years, the presence of non-fermenters and systemic infection or
organ failure were independently associated with 30-day mortality
in patients with CPE infection.
Although there are studies that have reported an
increased risk of mortality in patients with CPE infection
(19,20), this was not demonstrated in the
present study. In previous research, multivariate analysis revealed
that mechanical ventilation and the presence of indwelling medical
devices were significant risk factors for mortality in patients
with CPE infection (21).
As regards LOS in the ICU, some studies have
supported the fact that the majority of in-hospital deaths occurred
during the first days of ICU admission, while a greater risk of
mortality at a later stage was associated with discharge from the
hospital, despite the need for mechanical ventilation (22,23). In
the present study, it was not demonstrated that LOS in the ICU was
a risk factor for mortality.
In a previous study that compared the mortality and
survival rates of patients with COVID-19, paraclinical data such as
lymphopenia, thrombocytopenia and neutrophilia on admission were
the most frequent laboratory findings in the deceased group
(24). In the present study,
infection with SARS-CoV-2 was positively associated with mortality
in the univariate and multivariate analysis.
As regards the prescription of antibiotics, some
studies have confirmed that hospitalized patients are administered
irrational amounts of antibiotics, and in these cases, a higher
mortality rate was documented (25,26).
Despite this, in the present study, there was no association
between antibiotic prescription and the mortality or survival rates
of patients in the ICU, although all patients received antibiotic
treatment. The only observation was that orally administered
vancomycin was negatively associated with mortality in patients
with CPE infection; this treatment was also administered to
patients who had an associated Clostridioides difficile
infection. Nevertheless, from a statistical point of view, further
investigations are necessary to determine whether this treatment is
a protective factor in the present study group.
Consistent with the results of other studies, it was
observed that the predominant CPE strains included the
Klebsiella spp. group and not Escherichia coli
(27,28). The ECDC states that In Europe, on
average, 1.3 patients per 10.000 hospital admissions had a
carbapenemase-producing Klebsiella pneumoniae or
Escherichia coli infection, with the highest incidence found
in Southern and Southeastern Europe (29), where Romania is also located.
In Romania, only a limited number of studies have
been conducted on CPE strains (5,30). In a
previous study conducted in the authors' hospital, the predominance
of Klebsiella pneumoniae carbapenemase was documented
(5) and a multicenter study
conducted in eight hospitals in Romania revealed that OXA-48
carbapenemase was the most prevalent carbapenemase during the study
period (30).
The present study has some limitations, in that not
all the risk factors for mortality in hospitalized patients with
CPE infection were investigated, and the fact that although the
data were gathered from records spanning over a period of over a
period of 4 years (September, 2017 to October, 2021), the number of
patients was low.
In conclusion, in the present study, it was observed
that the majority of CPE strains were detected in 2021, with most
patients presenting bacterial colonization, with no difference in
mortality regarding CPE colonization and CPE infection. The LOS in
patients who acquired CPE did not influence mortality, while
infection with SARS-CoV-2 increased the risk of mortality
16.16-fold, and invasive mechanical ventilation increased the risk
of mortality by 14.98-fold.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data that support the findings of this study are
available from the authors, but restrictions apply to the
availability of these data, which were used under license for the
current study, and so are not publicly available. Data are however
available from the authors upon reasonable request and with
permission from the Clinical Infection Diseases Hospital, Constanta
(NR 1/20/01.2023. CODE F.05.PO.17.00-ACFOCG).
Authors' contributions
NDV and IMD conceptualized the study. ED, CSC and AD
were involved in the study methodology. NDV was involved in the
formal analysis, in the preparation of the original draft of the
manuscript, and in the reviewing and editing of the manuscript. CGP
was involved in the investigative aspects of the study. NDV BA, CI
and RVM were involved in data curation. IMD and ED supervised the
study. NDV and IMD confirm the authenticity of all the raw data.
All authors have read and agreed to the published version of the
manuscript.
Ethics approval and consent to
participate
The ethical review and approval were waived for the
present study as per the requirements of the Ethics Committee of
the Clinical Infectious Diseases Hospital. Patients provided
written consent to use their personal data upon admission to the
hospital. Patient anonymity was guaranteed during the whole process
of data analysis and reporting of results.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
European Centre for Disease Prevention and
Control (ECDC). Risk assessment on the spread of
carbapenemase-producing Enterobacteriaceae (CPE) through patient
transfer between healthcare facilities, with special emphasis on
cross-border transfer. Stockholm, 2011.
|
|
2
|
European Centre for Disease Prevention and
Control (ECDC). Carbapenem-resistant Enterobacteriaceae, second
update-26 September 2019. Stockholm, 2019.
|
|
3
|
Segagni Lusignani L, Presterl E, Zatorska
B, Van den Nest M and Diab-Elschahawi M: Infection control and risk
factors for acquisition of carbapenemase-producing
Enterobacteriaceae. A 5 year (2011-2016) case-control study.
Antimicrob Resist Infect Control. 9(18)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim YA, Lee SJ, Park YS, Lee YJ, Yeon JH,
Seo YH and Lee K: Risk factors for carbapenemase-producing
enterobacterales infection or colonization in a korean intensive
care unit: A case-control study. Antibiotics (Basel).
9(680)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dumitru IM, Dumitrascu M, Vlad ND, Cernat
RC, Ilie-Serban C, Hangan A, Slujitoru RE, Gherghina A,
Mitroi-Maxim C, Curtali L, et al: Carbapenem-Resistant
Klebsiella pneumoniae Associated with COVID-19. Antibiotics
(Basel). 10(561)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
WHO Regional Office for Europe/European
Centre for Disease Prevention and Control. Antimicrobial resistance
surveillance in Europe 2022-2020 data. Copenhagen, 2022.
|
|
7
|
van Duin D and Doi Y: The global
epidemiology of carbapenemase-producing Enterobacteriaceae.
Virulence. 8:460–469. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Otter JA, Burgess P, Davies F, Mookerjee
S, Singleton J, Gilchrist M, Parsons D, Brannigan ET, Robotham J
and Holmes AH: Counting the cost of an outbreak of
carbapenemase-producing Enterobacteriaceae: An economic evaluation
from a hospital perspective. Clin Microbiol Infect. 23:188–196.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao S, Kennedy S, Perry MR, Wilson J,
Chase-Topping M, Anderson E, Woolhouse MEJ and Lockhart M:
Epidemiology of and risk factors for mortality due to
carbapenemase-producing organisms (CPO) in healthcare facilities. J
Hosp Infect. 110:184–193. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pintado V, Ruiz-Garbajosa P,
Escudero-Sanchez R, Gioia F, Herrera S, Vizcarra P, Fortún J, Cobo
J, Martín-Dávila P, Morosini MI, et al: Carbapenemase-producing
enterobacterales infections in COVID-19 patients. Infect Dis
(Lond). 54:36–45. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu Q, Chen J, Sun S and Deng S:
Mortality-related risk factors and novel antimicrobial regimens for
carbapenem-resistant Enterobacteriaceae infections: A systematic
review. Infect Drug Resist. 15:6907–6926. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
European Centre for Disease Prevention and
Control (ECDC). ECDC country visit to Romania to discuss
antimicrobial resistance issues. Stockholm, 2018.
|
|
13
|
World Medical Association. World medical
association. World medical association declaration of Helsinki:
Ethical principles for medical research involving human subjects.
JAMA. 310:2191–2194. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP: STROBE Initiative. The
strengthening the reporting of observational studies in
epidemiology (STROBE) statement: Guidelines for reporting
observational studies. Ann Intern Med. 147:573–577. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Serpa Neto A, Deliberato RO, Johnson AEW,
Bos LD, Amorim P, Pereira SM, Cazati DC, Cordioli RL, Correa TD,
Pollard TJ, et al: Mechanical power of ventilation is associated
with mortality in critically ill patients: An analysis of patients
in two observational cohorts. Intensive Care Med. 44:1914–1922.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pascale R, Bussini L, Gaibani P, Bovo F,
Fornaro G, Lombardo D, Ambretti S, Pensalfine G, Appolloni L,
Bartoletti M, et al: Carbapenem-resistant bacteria in an intensive
care unit during the coronavirus disease 2019 (COVID-19) pandemic:
A multicenter before-and-after cross-sectional study. Infect
Control Hosp Epidemiol. 43:461–466. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Contou D, Cally R, Sarfati F, Desaint P,
Fraissé M and Plantefève G: Causes and timing of death in
critically ill COVID-19 patients. Crit Care. 25(79)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kalın BS, Özçaylak S, Solmaz İ and Kılıç
J: Assessment of risk factors for mortality in patients in medical
intensive care unit of a tertiary hospital. Indian J Crit Care Med.
26:49–52. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hughes LD, Aljawadi A and Pillai A: An
overview of carbapenemase producing Enterobacteriaceae (CPE) in
trauma and orthopaedics. J Orthop. 16:455–458. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tzouvelekis LS, Markogiannakis A, Piperaki
E, Souli M and Daikos GL: Treating infections caused by
carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect.
20:862–872. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mariappan S, Sekar U and Kamalanathan A:
Carbapenemase-producing Enterobacteriaceae: Risk factors for
infection and impact of resistance on outcomes. Int J Appl Basic
Med Res. 7:32–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moitra VK, Guerra C, Linde-Zwirble WT and
Wunsch H: Relationship between ICU length of stay and long-term
mortality for elderly ICU survivors. Crit Care Med. 44:655–662.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Williams TA, Ho KM, Dobb GJ, Finn JC,
Knuiman M and Webb SA: Royal Perth Hospital ICU Data Linkage Group.
Effect of length of stay in intensive care unit on hospital and
long-term mortality of critically ill adult patients. Br J Anaesth.
104:459–464. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rokni M, Ahmadikia K, Asghari S, Mashaei S
and Hassanali F: Comparison of clinical, para-clinical and
laboratory findings in survived and deceased patients with
COVID-19: Diagnostic role of inflammatory indications in
determining the severity of illness. BMC Infect Dis.
20(869)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ali M, Naureen H, Tariq MH, Farrukh MJ,
Usman A, Khattak S and Ahsan H: Rational use of antibiotics in an
intensive care unit: A retrospective study of the impact on
clinical outcomes and mortality rate. Infect Drug Resist.
12:493–499. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pinte L, Ceasovschih A, Niculae CM,
Stoichitoiu LE, Ionescu RA, Balea MI, Cernat RC, Vlad N, Padureanu
V, Purcarea A, et al: Antibiotic prescription and in-hospital
mortality in COVID-19: A prospective multicentre cohort study. J
Pers Med. 12(877)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tzouvelekis LS, Markogiannakis A,
Psichogiou M, Tassios PT and Daikos GL: Carbapenemases in
Klebsiella pneumoniae and other Enterobacteriaceae: An
evolving crisis of global dimensions. Clin Microbiol Rev.
25:682–707. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sherry NL, Lane CR, Kwong JC, Schultz M,
Sait M, Stevens K, Ballard S, Gonçalves da Silva A, Seemann T,
Gorrie CL, et al: Genomics for molecular epidemiology and detecting
transmission of carbapenemase-producing Enterobacterales in
Victoria, Australia, 2012 to 2016. J Clin Microbiol. 57:e00573–19.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Grundmann H, Glasner C, Albiger B,
Aanensen DM, Tomlinson CT, Andrasević AT, Cantón R, Carmeli Y,
Friedrich AW, Giske CG, et al: Occurrence of
carbapenemase-producing Klebsiella pneumoniae and
Escherichia coli in the European survey of
carbapenemase-producing Enterobacteriaceae (EuSCAPE): A
prospective, multinational study. Lancet Infect Dis. 17:153–163.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lixandru BE, Cotar AI, Straut M, Usein CR,
Cristea D, Ciontea S, Tatu-Chitoiu D, Codita I, Rafila A, Nica M,
et al: Carbapenemase-producing Klebsiella pneumoniae in
Romania: A six-month survey. PLoS One. 10(e0143214)2015.PubMed/NCBI View Article : Google Scholar
|