Introduction
Coronavirus disease 2019 (COVID-19), caused by the
new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),
has had a marked impact on the worldwide civilization since
December 2019. Globally, >760 million confirmed COVID-19
infections and 2.9 million related deaths have been reported as of
May 7, 2023 (https://covid19.who.int/).
Apart from recurrent respiratory symptoms,
neurologic manifestations occur in ~80% of hospitalized patients
with COVID-19 at any point during the disease (1). Neurological entities, such as
encephalitis, stroke, muscle injury, or anosmia have been linked to
an increased risk of mortality in patients with COVID-19 (2,3).
Furthermore, it is currently being debated whether pre-existing
neurological diseases can become more severe following infection
with COVID-19.
The COVID-19 pandemic has had an indirect and direct
impact on individuals with Parkinson's disease (PD). Indirect
impacts include a significant influence on healthcare provisions,
such as a reduction in overall hospitalizations. Chronic diseases,
on the other hand, require substantial delivery of care, including
hospitalizations in situations of worsening of the main
neurological disease or increased comorbidities. The direct effects
of COVID-19 on PD were those that resulted in a substantial
deterioration of motor and non-motor symptoms, which were
attributable to both infection-related processes and poor
dopaminergic pharmacokinetics (4-6).
PD has been shown to be associated with poor
outcomes in patients with COVID-19-associated pneumonia (7). Considering the significant frequency of
age-related comorbidities in patients with PD (8,9), it was
determined that a putatively greater risk of mortality among
patients with PD and COVID-19 is associated with frailty in older
affected patients rather than with PD itself (10). Data on risk factors for mortality
among patients with PD with COVID-19-associated pneumonia are
limited. In addition, the studies that have been conducted to date
aiming to evaluate the characteristics of patients with PD
hospitalized for COVID-19-associated pneumonia and possible risk
factors for adverse outcomes only refer to the first pandemic
waves. Thus, the aim of the present study was to evaluate the
clinical characteristics and outcomes of elderly patients with PD
hospitalized due to COVID-19-assciated pneumonia over a long period
of time during the pandemic, including the period of omicron
variant predominance.
Patients and methods
Study design
The present study was a prospective study on elderly
patients (≥65 years old) with PD and COVID-19-associated pneumonia
hospitalized in the Department of Infectious Diseases and COVID-19
Unit of Laiko General Hospital (Athens, Greece) between February
15, 2021, and July 15, 2022. The research was conducted in line
with the Declaration of Helsinki and obtained approval by the
Institutional Review Board of Laiko General Hospital. Written
informed was obtained from the patients for publication of their
data. The following criteria for inclusion were applied: i) An age
≥65 years; ii) prior diagnosis of PD; iii) polymerase chain
reaction confirmation of COVID-19 infection; iv) severe or critical
disease according to the clinical spectrum of SARS-CoV-2 infection
(https://www.covid19treatmentguidelines.nih.gov/).
Data collection
Patient data regarding demographic characteristics,
history, vaccination status against COVID-19, and the Charlson
comorbidity index (CCI) were recorded. Hemoglobin and hematocrit
levels, white blood cell, neutrophil, lymphocyte, platelet (PLT)
and immature granulocyte counts, as well as the levels of
C-reactive protein, serum albumin, lactate dehydrogenase, d-dimer,
ferritin, liver and cholestatic enzyme were recorded upon
admission.
Statistical analysis
The Shapiro-Wilk test was used to assess the normal
distribution of the parameters. Continuous parameters exhibiting a
normal distribution are displayed as the mean (standard deviation),
and those with a non-normal distribution are displayed as the
median (range). The normally distributed continuous variables were
compared using an unpaired t-test, and the comparison of
non-normally distributed continuous variables was conducted using
an unpaired non-parametric two-tailed Mann-Whitney U test. The
examination of categorical variables was conducted using Fisher's
exact or Chi-squared tests and these variables are presented as
absolute numbers (frequency and percentage). The discriminative
ability of significant parameters was assessed using receiver
operating characteristic (ROC) curve analysis and the area under
the ROC curve (AUC) was calculated. Statistical analyses were
conducted using IBM SPSS-Statistics version 26.0 (IBM Corp.).
Values of P<0.05 were considered to indicate statistically
significant differences.
Results
During the study period, 1,144 elderly patients with
COVID-19-associated pneumonia were hospitalized in the Department
of Infectious Diseases and COVID-19 Unit of Laiko General Hospital.
A total of 36 (3.1%) patients with PD were hospitalized due to
COVID-19-associated pneumonia (18 males, 50%).
The mean age of the patients was 82.72±8.18 years.
In total, 8 (22.2%) patients were hospitalized during the period of
alpha variant predominance, 3 (8.3%) patients during the period of
delta variant predominance and 25 (69.4%) patients during the
omicron variant predominance period. Of note, 16 patients (44.4%)
were vaccinated with at least two doses. The most common
comorbidity was dementia (15 patients, 41.7%), while 4 (11.1%)
patients were nursing home residents. The demographics and clinical
characteristics of the patients are presented in Table I.
 | Table IDemographics and clinical
characteristics of the study population. |
Table I
Demographics and clinical
characteristics of the study population.
| Variable | Frequency |
|---|
| Sex, n (%) | |
|
Female | 18(50) |
|
Male | 18(50) |
| Variant, n (%) | |
|
Alpha | 8 (22.2) |
|
Delta | 3 (8.3) |
|
Omicron | 25 (69.4) |
| Comorbidities, n
(%) | |
|
Chronic lung
disease (COPD/asthma/pulmonary fibrosis) | 3 (8.3) |
|
Cardiovascular
disease (ischemic stroke, arrhythmia, coronary artery disease,
myocardial infarction) | 13 (36.1) |
|
Arterial
hypertension | 9(25) |
|
Diabetes
mellitus | 9(25) |
|
Obesity | 1 (2.8) |
|
Chronic
kidney disease | 3 (8.3) |
|
Dementia | 15 (41.7) |
|
Nursing home
residency | 4 (11.1) |
|
Solid tumor
malignancy | 1 (2.8) |
|
Hematological
malignancy | 2 (5.6) |
| Vaccination status, n
(%) | |
|
Fully
vaccinated | 16 (44.4) |
|
Unvaccinated
or partially vaccinated | 20 (55.6) |
| In-hospital
mortality, n (%) | |
|
No | 19 (52.8) |
|
Yes | 17 (47.2) |
| Age (years), mean
(SD) | 82.72 (8.18) |
| CCI, median
(range) | 5 (3-9) |
Of the patients in the present study, 17 (47.2%)
patients succumbed during their hospitalization. Between the
patients who survived and those who succumbed, a statistically
significant difference was only found in the mean value of albumin
(37.48±6.02 vs. 31.97±5.34 g/l, P=0.019) (Tables II and III).
 | Table IIUnivariate analysis of continuous
variables (outcome: Mortality). |
Table II
Univariate analysis of continuous
variables (outcome: Mortality).
| Variable | Survivors | Non-survivors | P-value |
|---|
| Age (years), mean
(SD) | 81.05 (8.05) | 84.59 (8.14) | 0.200 |
| Hb (g/dl), mean
(SD) | 12.27 (1.87) | 11.23 (2.58) | 0.173 |
| Hct (%), mean
(SD) | 37.34 (5.49) | 33.97 (7.62) | 0.135 |
| Neu (K/µl), mean
(SD) | 6.99 (4.18) | 6.95 (4.49) | 0.977 |
| PLTs (K/µl), mean
(SD) | 234.73 (85.77) | 216.52 (89.60) | 0.539 |
| LDH (U/l), mean
(SD) | 237.68 (116.92) | 328.29 (157.07) | 0.056 |
| Albumin (g/l), mean
(SD) | 37.48 (6.02 | 31.97 (5.34) | 0.019 |
| CCI, median
(range) | 5 (3-9) | 5 (3-9) | 0.925 |
| WBC (K/µl), median
(range) | 7.86 (2.1-21.45) | 7.52
(3.06-19.37) | 0.754 |
| Lym (K/µl), median
(range) | 0.91 (0.26-3.21) | 0.97 (0.23-1.53) | 0.573 |
| IGs (109/l), median
(range) | 0.05 (0.02-0.63) | 0.05 (0.01-0.87) | 0.639 |
| Creatinine (mg/dl),
median (range) | 1.33 (0.48-7.4) | 0.86 (0.44-5.36) | 0.573 |
| AST (U/l), median
(range) | 19.50 (11-42) | 33 (13-67) | 0.066 |
| ALT (U/l), median
(range) | 11 (6-43) | 10 (5-37) | 0.639 |
| ALP (U/l), median
(range) | 95.50 (30-175) | 80 (41-220) | 0.510 |
| GGT (U/l), median
(range) | 19 (10-209) | 36 (11-148) | 0.232 |
| CRP (mg/l), median
(range) | 27.59
(2.07-196.57) | 46.25 (1.83-446) | 0.639 |
| Fer (ng/ml), median
(range) | 313 (32.6-1501) | 537
(30.8-2340) | 0.346 |
| d-dimer (µg/ml),
median (range) | 1.48 (0.23-20) | 2.45
(0.24-8.47) | 0.292 |
| FIB (mg/dl), median
(range) | 471.5 (90-666) | 420 (255-957) | 0.851 |
| NLR, median
(range) | 7.75 (1-36) | 9.30 (2-44) | 0.802 |
| PLR, median
(range) | 290.29
(81-816) | 231.40
(76-1203) | 0.999 |
| CAR, median
(range) | 0.66 (0-9) | 1.27 (2-21) | 0.519 |
 | Table IIIUnivariate analysis of categorical
variables (outcome: Mortality). |
Table III
Univariate analysis of categorical
variables (outcome: Mortality).
| | No. of
patients | |
|---|
| Variable | Survivors | Non-survivors | P-value |
|---|
| Sex | | | 0.999 |
|
Female | 10 | 8 | |
|
Male | 9 | 9 | |
| Vaccination
status | | | 0.756 |
|
Unvaccinated
or partially vaccinated | 10 | 10 | |
|
Fully
vaccinated | 9 | 7 | |
|
Variant | | | 0.274 |
|
Alpha | 6 | 2 | |
|
Delta | 2 | 1 | |
|
Omicron | 11 | 14 | |
| Omicron
variant | | | 0.156 |
|
Yes | 11 | 14 | |
|
No | 8 | 3 | |
| Chronic lung
disease (COPD/asthma/pulmonary fibrosis) | | | 0.087 |
|
Yes | 3 | 0 | |
|
No | 16 | 17 | |
| Cardiovascular
disease (ischemic stroke, arrhythmia, coronary artery disease,
myocardial infarction) | | | 0.502 |
|
Yes | 8 | 5 | |
|
No | 11 | 12 | |
| Arterial
hypertension | | | 0.847 |
|
Yes | 5 | 4 | |
|
No | 14 | 13 | |
| Diabetes
mellitus | | | 0.563 |
|
Yes | 4 | 5 | |
|
No | 15 | 12 | |
| Obesity | | | 0.337 |
|
Yes | 1 | 0 | |
|
No | 18 | 17 | |
| Chronic kidney
disease | | | 0.481 |
|
Yes | 1 | 2 | |
|
No | 18 | 15 | |
| Dementia | | | 0.736 |
|
Yes | 7 | 8 | |
|
No | 12 | 9 | |
| Nursing home
residency | | | 0.238 |
|
Yes | 1 | 3 | |
|
No | 18 | 14 | |
| Solid tumor
malignancy | | | 0.337 |
|
Yes | 1 | 0 | |
|
No | 18 | 17 | |
| Hematological
malignancy | | | 0.124 |
|
Yes | 0 | 2 | |
|
No | 19 | 15 | |
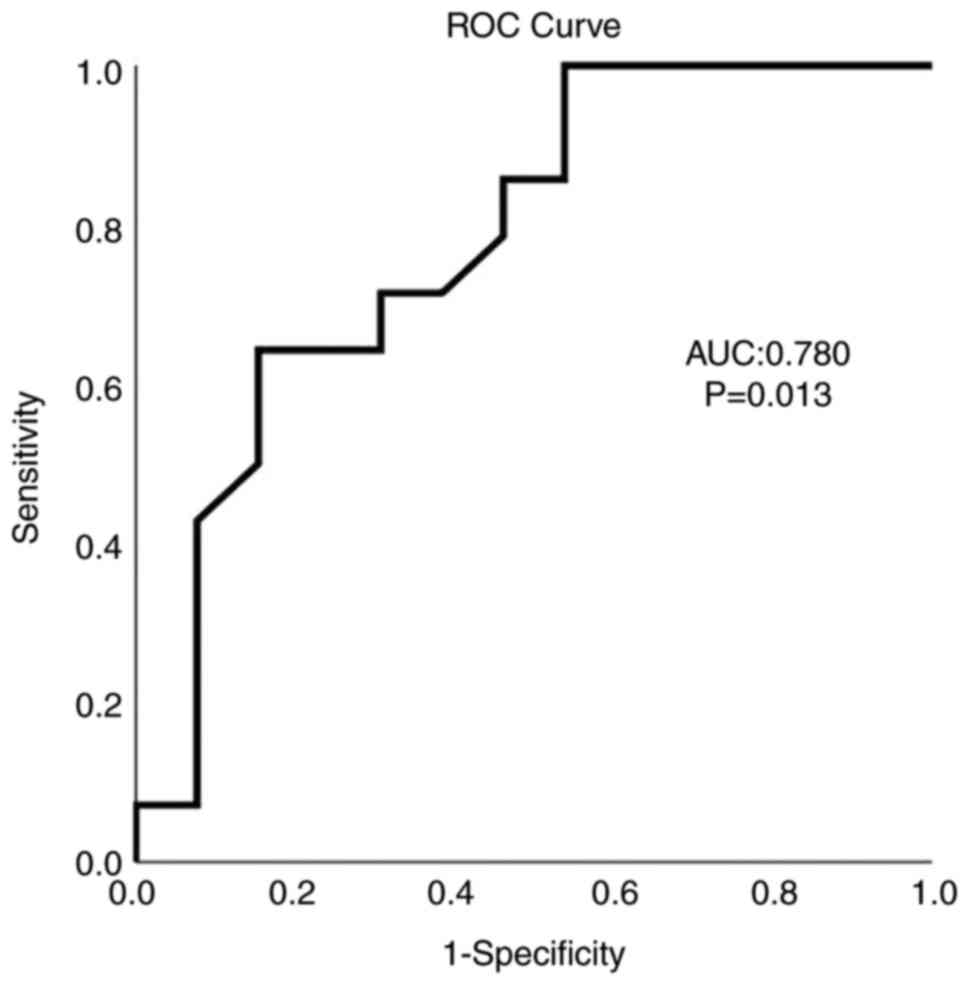
As determined using ROC curve analysis, albumin
exhibited a satisfactory predictive ability for mortality (AUC,
0.780; P=0.013) with an albumin value ≤37.7 g/l being able to
predict mortality with 85.7% sensitivity and 54.8% specificity
(Fig. 1).
Discussion
As aforementioned, the available studies that have
evaluated the mortality rates and the risk factors of adverse
outcomes in patients PD are limited, mostly referring to the first
periods of the pandemic. Scherbaum et al (11), in their cross-sectional study on the
clinical profiles and mortality of inpatients with PD and COVID-19
in Germany, reported a mortality rate of 35.4%. Parihar et
al (12) compared the outcomes
of patients with PD with or without COVID-19 and found that
patients with PD with SARS-CoV-2 infection had a mortality rate of
35.8%. Fathi et al (13), in
their study, compared the prognosis of hospitalized patients with a
known diagnosis of Alzheimer's or PD who had COVID-19 infection
with that of other hospitalized patients with COVID-19 and reported
a mortality rate of 35.1%. In addition, Salari et al
(14), in their research exploring
the mortality rates among patients with PD with COVID-19 in Iran,
reported a mortality rate of 35.6% in these patients. In the
present study, the mortality rate was 47.2%; however, the present
study evaluated the clinical characteristics and outcomes of
elderly patients with PD hospitalized with COVID-19-associated
pneumonia over a long period of time during the pandemic.
Fortunately with the advancement of the vaccination
campaign and the succession of SARS-CoV-2 mutations, the percentage
of patients with COVID-19 with hospital admissions and
COVID-19-associated mortality has changed significantly during the
length of the pandemic, and the current trend in confirmed cases
and deaths continues to decrease globally (https://covid19.who.int/). In the present study, no
statistically significant differences were found in mortality rates
between the periods of different variant predominance. This
observation highlights the increased vulnerability of patients
suffering from PD.
PD is one of the most prevalent age-related
degenerative conditions, and it is frequently accompanied by
comorbidities, notably cardiovascular disease (8); as a result, patients with PD are in the
high-risk group for SARS CoV-2 infection-related mortality.
Furthermore, there is an ‘indirect risk’ associated with the
documented association between PD, age and cardiovascular
comorbidities (15). Additionally,
there is a direct risk of an unfavorable outcome following COVID-19
infection in patients with PD who already have respiratory
impairment. Respiratory muscle weakness and aberrant posture,
dictating respiratory muscle stiffness and insufficient respiratory
excursions, contribute to ventilator failure in the advanced stage
of PD (16,17). Pneumonia has been found to be the
most prevalent reason for hospitalization and the leading cause of
mortality among patients with PD, according to previous studies
(16,18). In addition, 25-30% of patients with
PD develop dementia, which is another risk factor for increased
COVID-19-related mortality in these patients (19,20).
Several prognostic factors for mortality among
hospitalized patients with PD with COVID-19 have been identified,
such as age, race, an advanced PD stage, the reduction of PD
medications and the presence of dementia (7,12-14).
Serum albumin levels have been linked to poor outcomes in patients
with COVID-19. As a typical nutritional indicator, low albumin
levels in patients with COVID-19 may be a marker of excessive
consumption due to tissue damage and hypermetabolism (21). A recent study demonstrated that
higher serum albumin levels are significantly associated with
improved cognitive function and play a protective role in motor
impairment and PD-related mortality (22). To the best of our knowledge, the
present study is the first to identify a laboratory parameter,
serum albumin levels, as a prognostic factor for mortality among
hospitalized patients with PD with COVID-19-assocaited
pneumonia.
The present study has some limitations, however.
Although it is a study referring to a long period of the pandemic,
it is a single-center study with a relatively small number of
patients. In addition, no medications regarding COVID-19 or other
medications for the underlying disease were included in the
analysis. Moreover, the stage of the disease and scales assessing
the functional status of the patients were not included.
Furthermore, viral variants were not identified individually for
the participants. The assignment of variants was based on the
predominant variant at the time the patient was diagnosed with the
SARS-CoV2 infection.
In conclusion, the present study demonstrates that
the mortality rate of elderly patients with PD hospitalized with
COVID-19-associated pneumonia is high in all phases of the
pandemic. A low albumin value, as an indicator of the immune
status, as well as the nutritional status, is a predictor of
adverse outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AB and VEG conceived the study. VEG, AT, DB, DAS,
SM, AG, GK, EA and NVS made substantial contributions to data
interpretation and analysis, and wrote and prepared the draft of
the manuscript. VEG and AB analyzed the data and provided critical
revisions. VEG and NVS confirm the authenticity of all the data.
All authors contributed to manuscript revision, and have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in line with the
Declaration of Helsinki and obtained approval by the Institutional
Review Board of Laiko General Hospital (protocol no. 765/12-2021).
Written informed was obtained from the patients for publication of
their data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liotta EM, Batra A, Clark JR, Shlobin NA,
Hoffman SC, Orban ZS and Koralnik IJ: Frequent neurologic
manifestations and encephalopathy-associated morbidity in Covid-19
patients. Ann Clin Transl Neurol. 7:2221–2230. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pezzini A and Padovani A: Lifting the mask
on neurological manifestations of COVID-19. Nat Rev Neurol.
16:636–644. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Frontera JA, Sabadia S, Lalchan R, Fang T,
Flusty B, Millar-Vernetti P, Snyder T, Berger S, Yang D, Granger A,
et al: A prospective study of neurologic disorders in hospitalized
patients with COVID-19 in New York City. Neurology. 96:e575–e586.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cilia R, Bonvegna S, Straccia G, Andreasi
NG, Elia AE, Romito LM, Devigili G, Cereda E and Eleopra R: Effects
of COVID-19 on Parkinson's disease clinical features: A
Community-based case-control study. Mov Disord. 35:1287–1292.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Angelopoulou E, Karlafti E, Georgakopoulou
VE, Papalexis P, Papageorgiou SG, Tegos T and Savopoulos C:
Exploring the Role of ACE2 as a Connecting Link between COVID-19
and Parkinson's Disease. Life (Basel). 13(536)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bougea A, Georgakopoulou VE, Palkopoulou
M, Efthymiopoulou E, Angelopoulou E, Spandidos DA and Zikos P:
New-onset non-motor symptoms in patients with Parkinson's disease
and post-COVID-19 syndrome: A prospective cross-sectional study.
Med Int. 3(23)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Putri C, Hariyanto TI, Hananto JE,
Christian K, Situmeang RFV and Kurniawan A: Parkinson's disease may
worsen outcomes from coronavirus disease 2019 (COVID-19) pneumonia
in hospitalized patients: A systematic review, meta-analysis, and
meta-regression. Parkinsonism Relat Disord. 87:155–161.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McLean G, Hindle JV, Guthrie B and Mercer
SW: Co-morbidity and polypharmacy in Parkinson's disease: Insights
from a large Scottish primary care database. BMC Neurol.
17(126)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Richter D, Bartig D, Krogias C and Tönges
L: Letter to the editor: Risk comorbidities of COVID-19 in
Parkinson's disease patients in Germany. Neurol Res Pract.
2(22)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fasano A, Cereda E, Barichella M, Cassani
E, Ferri V, Zecchinelli AL and Pezzoli G: COVID-19 in Parkinson's
Disease Patients Living in Lombardy, Italy. Mov Disord.
35:1089–1093. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Scherbaum R, Kwon EH, Richter D, Bartig D,
Gold R, Krogias C and Tönges L: Clinical profiles and mortality of
COVID-19 inpatients with parkinson's disease in Germany. Mov
Disord. 36:1049–1057. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Parihar R, Ferastraoaru V, Galanopoulou
AS, Geyer HL and Kaufman DM: Outcome of hospitalized Parkinson's
disease patients with and without COVID-19. Mov Disord Clin Pract.
8:859–867. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fathi M, Taghizadeh F, Mojtahedi H, Zargar
Balaye Jame S and Markazi Moghaddam N: The effects of Alzheimer's
and Parkinson's disease on 28-day mortality of COVID-19. Rev Neurol
(Paris). 178:129–136. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Salari M, Etemadifar M, Ashrafi F, Ommi D,
Aminzade Z and Tehrani Fateh S: Parkinson's disease patients may
have higher rates of Covid-19 mortality in Iran. Parkinsonism Relat
Disord. 89:90–92. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Scorza FA, Fiorini AC, Scorza CA and
Finsterer J: Cardiac abnormalities in Parkinson's disease and
Parkinsonism. J Clin Neurosci. 53:1–5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baille G, Chenivesse C, Perez T, Machuron
F, Dujardin K, Devos D, Defebvre L and Moreau C: Dyspnea: An
underestimated symptom in Parkinson's disease. Parkinsonism Relat
Disord. 60:162–166. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bugalho P, Ladeira F, Barbosa R, Marto JP,
Borbinha C, Salavisa M, da Conceição L, Saraiva M, Fernandes M and
Meira B: Motor and non-motor function predictors of mortality in
Parkinson's disease. J Neural Transm (Vienna). 126:1409–1415.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Okunoye O, Kojima G, Marston L, Walters K
and Schrag A: Factors associated with hospitalisation among people
with Parkinson's disease-A systematic review and meta-analysis.
Parkinsonism Relat Disord. 71:66–72. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dementia in parkinsonism. Ann Indian Acad
Neurol. 14 (Suppl 1):S21–S14. 2011.PubMed/NCBI
|
|
20
|
Kostev K, Gessler N, Wohlmuth P, Arnold D,
Bein B, Bohlken J, Herrlinger K, Jacob L, Koyanagi A, Nowak L, et
al: Is Dementia associated with COVID-19 mortality? A multicenter
retrospective cohort study conducted in 50 hospitals in Germany. J
Alzheimers Dis. 91:719–726. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mathioudakis N, Zachiotis M, Papadakos S,
Triantafyllou M, Karapanou A, Samara S, Karamanakos G, Spandidos
DA, Papalexis P, Damaskos C, et al: Onodera's prognostic
nutritional index: Comparison of its role in the severity and
outcomes of patients with COVID-19 during the periods of alpha,
delta and omicron variant predominance. Exp Ther Med.
24(675)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun S, Wen Y and Li Y: Serum albumin,
cognitive function, motor impairment, and survival prognosis in
Parkinson disease. Medicine (Baltimore). 101(e30324)2022.PubMed/NCBI View Article : Google Scholar
|