Introduction
Castration-resistant prostate cancer (CRPC) pertains
to a prostate malignancy that is not controlled medically or via
surgical castration. The treatment of CRPC mainly involves novel
pharmaceutical therapy and chemotherapy [abiraterone acetate,
enzalutamide, docetaxel (DOC) or cabazitaxel] (1-4).
Radium-223 chloride (Ra-223) has emerged as a treatment option for
bone metastasis from CRPC (BmCRPC) (5).
Ra-223 accumulates at sites of bone metastases and
other regions with a high bone metabolic activity. It emits alpha
rays, thereby exerting a tumor-controlling effect (6,7). In an
overseas phase III study (ALSYMPCA study, Alpharadin in Symptomatic
Prostate Cancer), Ra-223 achieved favorable therapeutic results. It
prolonged the overall survival (OS) of patients by 3.6 months and
time to bone-related events (SRE) by 5.8 months (5). Thus, Ra-223 was recognized as a viable
treatment option that improved the SRE and OS.
Previous studies have identified several clinical
factors associated with the poor prognosis of patients with CRPC
(8-10).
However, the effect of the timing of Ra-223 administration on the
survival of the patient remains unclear. Ra-223 is typically
administered to patients with progressive disease, despite novel
pharmaceutical therapy and chemotherapy. Recent studies have
demonstrated that the earlier Ra-223 administration affects the
post-treatment prognosis of patients with BmCRPC (11,12).
Those studies analyzed the prognostic factors at the time of Ra-223
administration. However, their methods were insufficient to
determine the optimal timing of Ra-223 administration as various
subsequent treatment options remain available for patients who
receive early Ra-223 administration, while the patients who receive
later Ra-223 administration have limited subsequent treatment
options (11,12). Thus, in the present study, in an aim
to address this issue, the association between the survival and
timing of Ra-223 administration was analyzed, considering the
potential prognosis upon initiating Ra-223 administration.
Patients and methods
Between October, 2016 and January, 2022, 56 patients
were treated with Ra-223 at the National Hospital Organization
Shikoku Cancer Center Hospital and Ehime University Hospital
(Ehime, Japan). Among these patients, those with small cell
carcinoma (n=1), those included in the phase II study of
personalized peptide vaccine for DOC-based chemotherapy-resistant
CRPC (n=1), and those without follow-up after completing the Ra-223
regimen (n=3) were excluded from the study. Finally, 51 patients
(aged 64-90 years; median age, 72 years) were included in the
present study. The present retrospective study was approved by the
Ethics Committee of Ehime University Hospital and the National
Hospital Organization Shikoku Cancer Center (registration no.
2211017). The need for informed consent was waived due to the
retrospective nature of the study.
CRPC is defined as prostate cancer with a
testosterone level within the castration range (≤50 ng/dl) and a
prostate-specific antigen (PSA) level ≥25% from the lowest value,
measured at least 4 weeks apart, with an increase of ≥2.0 ng/ml
(13).
A total of 48 patients were pathologically diagnosed
by needle biopsy, and 3 patients were clinically diagnosed with
prostate cancer prior to the initial treatment. Bone metastases
were detected via bone scintigraphy in all patients, and
osteoplastic bone metastasis was proven via computed tomography.
The timing of the Ra-223 administration was decided at the
discretion of the physician and institution. Ra-223 was
administered at a dose of 50-55 kBq/kg every 4 weeks for up to six
cycles.
Statistical analysis
The survival time of patients following the
administration of Ra-223 was calculated from the initiation of
Ra-223 administration. The Kaplan-Meier method was used to generate
the OS curve. Univariate and multivariate analyses of the survival
time after Ra-223 were performed using the Cox proportional hazard
model to determine the hazard ratios (HRs), 95% confidence
intervals (CIs) and P-values. To evaluate the prognosis upon the
initiation of Ra-223 administration, the time from the diagnosis of
BmCRPC to the initiation of Ra-223 administration (BmCRPC-Ra223
time) was included in the multivariate analysis. Statistical
analyses were performed using JMP software (JMP version 14.3.0; SAS
Institute).
Results
A total of 51 patients with BmCRPC, treated with
Ra-223 at the authors' institutions between October, 2016 and
January, 2022, were retrospectively reviewed. Laboratory data prior
to Ra-223 administration (median, range) were as follows: i) PSA
(ng/ml; 15.66, 0.08-616.1); ii) hemoglobin (g/dl; 12.3, 8.1-14.8);
iii) platelets (x104/µl; 21.5, 14.3-38.3); iv) alkaline
phosphatase (U/l; 239, 57-6047); v) lactate dehydrogenase (U/l;
207, 148-554); vi) neutrophil-to-lymphocyte ratio (2.72,
0.91-17.19); and vii) platelet-to-lymphocyte ratio (0.88,
0.44-6.48). The patient characteristics are presented in Table I.
 | Table ICharacteristics of the patients in the
present study. |
Table I
Characteristics of the patients in the
present study.
| Variable | No. of patients | % |
|---|
| Age, years | | |
|
<70 | 20 | 39.2 |
|
≥70 | 31 | 60.8 |
| PS | | |
|
0 | 31 | 60.8 |
|
≥1 | 20 | 39.2 |
| No. of bone
metastases prior to Ra-223 therapy | | |
|
1-5 | 17 | 33.3 |
|
6-19 | 17 | 33.3 |
|
≥20 | 17 | 33.3 |
| Lymph node metastases
prior to Ra-223 therapy | | |
|
Yes | 22 | 43.1 |
|
No | 29 | 56.9 |
| Use of bone-modifying
agentsa | | |
|
Yes | 46 | 90.2 |
|
No | 5 | 9.8 |
| EBRT for bone
metastatic sites | | |
|
Yes | 9 | 17.6 |
|
No | 42 | 82.4 |
| Pre-treatment | | |
|
Abiraterone | 16 | 31.3 |
|
Enzalutamide | 26 | 51.0 |
|
Docetaxel | 17 | 33.3 |
|
Cabazitaxel | 5 | 9.8 |
| Post-treatment | | |
|
Abiraterone | 11 | 21.6 |
|
Enzalutamide | 19 | 37.3 |
|
Docetaxel | 18 | 35.3 |
|
Cabazitaxel | 14 | 27.5 |
| Line of Ra-223 in
BmCRPC | | |
|
1st | 15 | 29.4 |
|
2nd | 17 | 33.3 |
|
3rd | 12 | 23.5 |
|
4th | 4 | 7.8 |
|
5th | 3 | 5.9 |
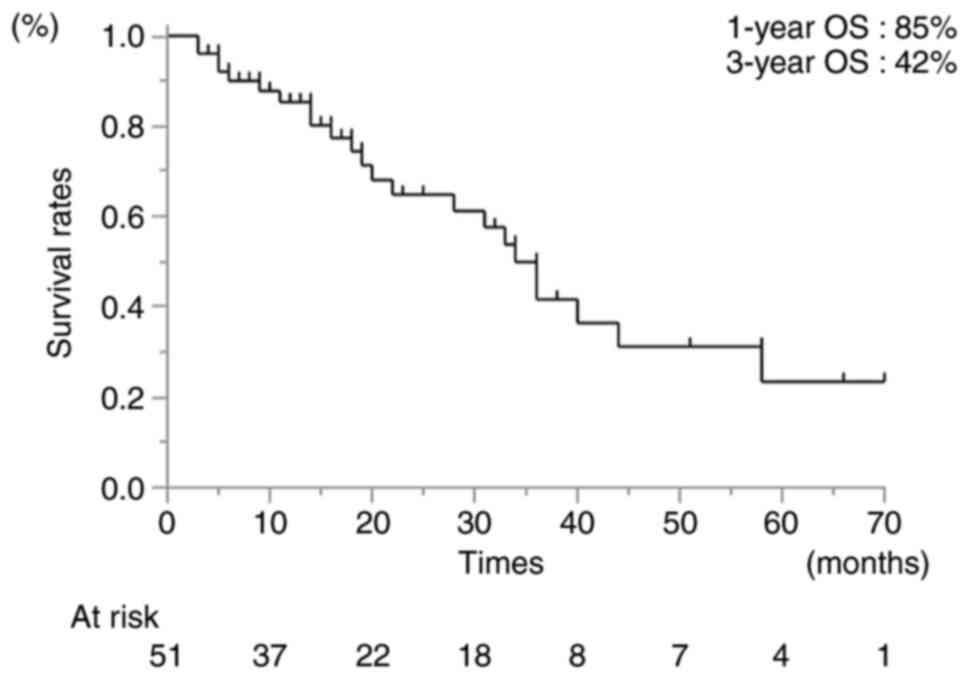
The median follow-up time was 18 months (range, 3-70
months). The 1- and 3-year OS rates following Ra-223 administration
were 85 and 42%, respectively (Fig.
1). The 1-year OS rate differed significantly between the
patients who received early (first- to second-line) and late
(third- to fifth-line) Ra-223 therapy (87 and 74%, respectively;
HR, 5.90; 95% CI, 19.7-17.63; P<0.01). In addition, the 1-year
OS rates differed significantly between patients who completed or
failed to complete the Ra-233 regimen (97 and 37%, respectively;
HR, 42.88; 95% CI, 7.91-232.55; P<0.01) (Table II). The reasons for uncompleted
Ra-223 regimens were the following: i) Adverse events
(myelosuppression, n=1; febrile neutropenia, n=1); ii) the
appearance of metastases (liver, n=5; adrenal gland, n=1); iii)
other diseases (pneumonia, n=1; sudden death from aortic stenosis,
n=1); iv) refusal to undergo Ra-223 treatment (n=1). In patients
with a PSA level <15.66 ng/ml and ≥15.66 ng/ml, the 1-year OS
rate also differed significantly (100 and 72%, respectively; HR,
6.79; 95% CI, 2.47-18.66; P<0.01). The difference in the 1-year
OS rate was also significant between patients with a PS=0 and ≥1
(90 and 78%, respectively; HR, 2.79; 95% CI, 1.21-6.46; P=0.02)
(Table II). By contrast, there was
no statistically significant difference between patients with a
BmCRPC-Ra-223 time <1 year and ≥1 year (HR, 1.78; 95% CI,
0.76-4.18; P=0.19) (Table II).
 | Table IISurvival rates of the patients
following radium-223 chloride administration, and the results of
univariate and multivariate analyses including the bone metastasis
from the time of castration-resistant prostate cancer radium-223
chloride therapy. |
Table II
Survival rates of the patients
following radium-223 chloride administration, and the results of
univariate and multivariate analyses including the bone metastasis
from the time of castration-resistant prostate cancer radium-223
chloride therapy.
| | Survival | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | 1-year (%) | 3-year (%) | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | | | 1.47 | 0.60-3.59 | 0.40 | - | - | - |
|
<70 | 90 | 57 | | | | | | |
|
≥70 | 83 | 35 | | | | | | |
| PS | | | 2.79 | 1.21-6.46 | 0.02 | 1.22 | 0.40-3.73 | 0.73 |
|
0 | 90 | 54 | | | | | | |
|
≥1 | 78 | 15 | | | | | | |
| No. of bone
metastases prior to Ra-223 therapy | | | 2.51 | 0.99-6.27 | 0.06 | - | - | - |
|
1-5 | 94 | 58 | | | | | | |
|
≥6 | 81 | 32 | | | | | | |
| Lymph node
metastases prior to Ra-223 therapy | | | 1.060 | 0.44-2.55 | 0.90 | - | - | - |
|
Yes | 86 | 42 | | | | | | |
|
No | 85 | 41 | | | | | | |
| Use of
bone-modifying agentsa | | | 1.69 | 0.38-7.55 | 0.49 | - | - | - |
|
Yes | 86 | 38 | | | | | | |
|
No | 75 | 75 | | | | | | |
| EBRT for bone
metastatic sites | | | 0.57 | 0.19-1.70 | 0.31 | - | - | |
|
Yes | 60 | 30 | | | | | | |
|
No | 90 | 44 | | | | | | |
| Line of Ra-223 in
BmCRPC | | | 5.90 | 1.97-17.63 | <0.01 | 2.67 | 0.79-9.07 | 0.11 |
|
1-2 | 87 | 49 | | | | | | |
|
3-5 | 74 | 0 | | | | | | |
| Completion of
Ra-223 | | | 42.88 | 7.91-232.55 | <0.01 | 128.03 | 10.59-1548.42 | <0.01 |
|
Yes | 97 | 49 | | | | | | |
|
No | 37 | 0 | | | | | | |
| PSA levels prior to
Ra-223 therapy | | | 6.79 | 2.47-18.66 | <0.01 | 7.86 | 2.70-27.24 | <0.01 |
|
<15.66
ng/ml | 100 | 72 | | | | | | |
|
≥15.66
ng/ml | 72 | 14 | | | | | | |
| Hemoglobin levels
prior to Ra-223 therapy | | | 0.46 | 0.20-1.08 | 0.08 | - | - | - |
|
<12.3
g/dl | 72 | 31 | | | | | | |
|
≥12.3
g/dl | 96 | 51 | | | | | | |
| Platelet levels
prior to Ra-223 therapy | | | 0.84 | 0.37-1.94 | 0.69 | - | - | |
|
<21.5x104/µl | 84 | 31 | | | | | | |
|
≥21.5x104/µl | 87 | 53 | | | | | | |
| ALP levels prior to
Ra-223 therapy | | | 1.38 | 0.59-3.20 | 0.45 | | - | |
|
<239
U/l | 86 | 52 | | | | | | |
|
≥239
U/l | 84 | 35 | | | | | | |
| LDH levels prior to
Ra-223 therapy | | | 0.64 | 0.28-1.48 | 0.30 | - | - | - |
|
<207
U/l | 82 | 37 | | | | | | |
|
≥207
U/l | 88 | 44 | | | | | | |
| NLR levels prior to
Ra-223 therapy | | | 1.72 | 0.75-3.96 | 0.20 | - | - | - |
|
<2.72 | 91 | 50 | | | | | | |
|
≥2.72 | 80 | 33 | | | | | | |
| PLR levels prior to
Ra-223 therapy | | | 1.54 | 0.67-3.55 | 0.31 | - | - | - |
|
<0.88 | 90 | 45 | | | | | | |
|
≥0.88 | 80 | 42 | | | | | | |
| BmCRPC-Ra-223
time | | | 1.78 | 0.76-4.18 | 0.19 | 1.29 | 0.43-3.92 | 0.65 |
|
<1
year | 81 | 51 | | | | | | |
|
≥1 year | 89 | 32 | | | | | | |
Although the BmCRPC-Ra-223 time was not a
significant factor in the univariate analysis, it was included as a
factor in the multivariate analysis as the present study aimed to
examine the effects of the potential prognosis following the
initiation of Ra-223 administration. Based on the multivariate
analysis, including the BmCRPC-Ra-223 time, late Ra-223 therapy
(third- to fifth-line) was not significantly associated with an
unfavorable prognosis (HR, 2.67; 95% CI, 0.79-9.07; P=0.11)
(Table II). By contrast, the
incompletion of Ra-223 therapy (HR, 128.03; 95% CI, 10.59-1548.42;
P<0.01) and a higher PSA level (≥15.66; HR, 7.86; 95% CI,
2.70-27.24; P<0.01) were independent factors that were
significantly associated with a poorer prognosis (Table II). Based on the multivariate
analysis excluding the BmCRPC-Ra-223 time, the incompletion of
Ra-223 (HR, 154.18; 95% CI, 13.40-1773.89; P<0.01), later-line
Ra-233 therapy (third- to fifth-line) (HR, 2.96; 95% CI, 1.00-9.27;
P=0.05) and a higher PSA level (≥15.66; HR, 8.23; 95% CI,
2.40-28.36; P<0.01) (Table SI)
were significant independent factors, associated with an
unfavorable post-treatment prognosis.
Discussion
In the present study, when BmCRPC-Ra-223 time was
analyzed as a prognostic factor, an unfavorable prognosis was
associated with an incomplete Ra-223 administration (five cycles or
less) and a higher PSA level (≥15.66). By contrast, the timing of
Ra-223 administration did not affect the survival of patients with
BmCRPC. However, when the BmCRPC-Ra-223 time was not analyzed as a
prognostic factor, an unfavorable prognosis was associated with an
incomplete Ra-223 administration (five cycles or less), later
Ra-233 therapy (third- to fifth-line) and a higher PSA level
(≥15.66).
Ra-223 treatment has been shown to prolong the OS
rate of patients and improved their quality of life (5). Although the European Medicines Agency
(EMA) recommends the administration of Ra-223 to patients who have
already received at least two previous treatments (14), the optimal timing of Ra-223
administration in patients with BmCRPC remains controversial.
Previous studies have suggested that an early Ra-223 administration
in patients with BmCRPC positively affects their survival (7,8,10). However, these studies may have been
biased as the timing of Ra-223 administration likely affected the
prognosis. Patients who received Ra-223 earlier had longer life
expectancies, while those who received Ra-223 later likely had
shorter life expectancies. In the present study, when the
BmCRPC-Ra-223 time was not evaluated as a prognostic factor, early
Ra-223 administration significantly prolonged survival. However,
when the BmCRPC-Ra-223 time was assessed as a prognostic factor, it
had a smaller impact on survival. Based on these results, the
timing of Ra-223 administration appeared to have a minimal effect
on the survival of patients.
Herein, the number of Ra-223 cycles and PSA level
were critical prognostic factors from the initiation of Ra-223
administration. A number of studies have demonstrated that these
are significant prognostic factors for patients with BmCRPC
(5,8-11,15,16).
Ra-223 should be initiated before the PSA levels increase, but
other systemic therapies for CRPC should also be initiated during
this time. Therefore, the indications for the optimal timing of
Ra-223 administration remain unclear. Some studies have suggested
that the number of metastatic bone lesions prior to Ra-223
administration are significantly associated with the completion of
Ra-223 administration (10,16,17). In
the present study, in the univariate analysis, this tended to be
associated with a more favorable prognosis. The timing of Ra-223
administration may be considered, depending on the number of bone
metastases prior to Ra-223 therapy.
The present study had some limitations associated
with its retrospective nature. First, the sample size was small.
However, it is a notable finding that the BmCRPC-Ra-223 time, which
is a factor not examined in previous studies, reduced the impact of
earlier Ra-223 administration on prognosis. Although the present
study demonstrated that the impact of an earlier Ra-223
administration was minimal, further studies with greater
statistical power are warranted. Second, the completion or
non-completion of the Ra-223 regimen affected prognosis in this
study, but this had potential risk of immortal time bias (18). Further prospective studies are
therefore warranted. Third, half of the cases in the present study
had lymph node metastasis, but this may have been missed as our
institutions (Ehime University Hospital and the National Hospital
Organization Shikoku Cancer Center) did not have prostate-specific
membrane antigen (PSMA)-positron emission tomography (PET), which
detects where the prostate cancer cells are located in the body.
This may have affected treatment outcomes in the present study.
Finally, SRE, a critical factor associated with the administration
of Ra-223, was not evaluated in the present study as information on
SRE was challenging to obtain from the pre-existing medical
records. Despite these limitations, the present study provides a
novel perspective regarding the timing of Ra-223 administration.
However, further large-scale studies are required to confirm the
results obtained herein.
In conclusion, the timing of Ra-223 administration
did not significantly affect the survival of patients from the
initiation of treatment in the present study. Further studies are
required to determine the optimal timing for Ra-223
administration.
Supplementary Material
Survival rates of the patients
following radium-223 chloride administration, and the results of
univariate and multivariate analyses excluding bone metastasis from
the time of castration-resistant prostate cancer radium-223
chloride therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM and YH designed and conceived the study. KM, YH,
HK, KN, NY, KH, NM, TS and TK collected the patient data and
drafted the manuscript. KM, YH, HK, KN, NY, KH, NM, TS and TK
collaborated in the discussions regarding the study. KM and YH
prepared the manuscript and HK edited the manuscript. KM and YH
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures in the present study were performed
in accordance with the ethical standards of the institutional
research committee and with the 1964 Helsinki declaration and its
later amendments or comparable ethical standards. The present study
was approved by the Ethics Committee of Ehime University Hospital
and the National Hospital Organization Shikoku Cancer Center
(registration no. 2211017). The need for informed consent was
waived due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al: Alpha emitter radium-223 and survival in metastatic prostate
cancer. N Engl J Med. 369:213–223. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Humm JL, Sartor O, Parker C, Bruland OS
and Macklis R: Radium-223 in the treatment of osteoblastic
metastases: A critical clinical review. Int J Radiat Oncol Biol
Phys. 91:898–906. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dekempeneer Y, Keyaerts M, Krasniqi A,
Puttemans J, Muyldermans S, Lahoutte T, D'huyvetter M and Devoogdt
N: Targeted alpha therapy using short-lived alpha-particles and the
promise of nanobodies as targeting vehicle. Expert Opin Biol Ther.
16:1035–1047. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Saad F, Carles J, Gillessen S, Heidenreich
A, Heinrich D, Gratt J, Lévy J, Miller K, Nilsson S, Petrenciuc O,
et al: Radium-223 and concomitant therapies in patients with
metastatic castration-resistant prostate cancer: An international,
early access, open-label, single-arm phase 3b trial. Lancet Oncol.
17:1306–1316. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sartor O, Coleman RE, Nilsson S, Heinrich
D, Helle SI, O'Sullivan JM, Vogelzang NJ, Bruland Ø, Kobina S,
Wilhelm S, et al: An exploratory analysis of alkaline phosphatase,
lactate dehydrogenase, and prostate-specific antigen dynamics in
the phase 3 ALSYMPCA trial with radium-223. Ann Oncol.
28:1090–1097. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Naito M, Ukai R and Hashimoto K: Bone scan
index can be a useful biomarker of survival outcomes in patients
with metastatic castration-resistant prostate cancer treated with
radium-223. Cancer Rep (Hoboken). 2(e1203)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jarvis P, Ho A and Sundram F: Radium-223
therapy for metastatic castration-resistant prostate cancer:
Survival benefit when used earlier in the treatment pathway. Nucl
Med Commun. 42:332–336. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oguma Y, Hosono M, Okajima K, Inoue E,
Nakamatsu K, Doi H, Matsuura T, Inada M, Uehara T, Wada Y, et al:
Investigation into the optimal strategy of radium-223 therapy for
metastatic castration-resistant prostate cancer. Radiation.
2:273–284. 2022.
|
|
13
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing, and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Energy Market Authority of Singapore: EMA
restricts use of prostate cancer medicine Xofigo, London, 2018.
https://www.ema.europa.eu/en/medicines/human/referrals/xofigo.
|
|
15
|
McKay RR, Jacobus S, Fiorillo M, Ledet EM,
Cotogna PM, Steinberger AE, Jacene HA, Sartor O and Taplin ME:
Radium-223 use in clinical practice and variables associated with
completion of therapy. Clin Genitourin Cancer. 15:e289–e298.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sasaki D, Hatakeyama S, Kawaguchi H,
Hatayama Y, Ishibashi Y, Kusaka A, Noro D, Tanaka T, Ito H, Okuyama
Y, et al: Effects of six-cycle completion and earlier use of
radium-223 therapy on prognosis for metastatic castration-resistant
prostate cancer: A real-world multicenter retrospective study. Urol
Oncol. 40:64.e1–64.e8. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fizazi K, Massard C, Smith M, Rader M,
Brown J, Milecki P, Shore N, Oudard S, Karsh L, Carducci M, et al:
Bone-related parameters are the main prognostic factors for overall
survival in men with bone metastases from castration-resistant
prostate cancer. Eur Urol. 68:42–50. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yadav K and Lewis RJ: Immortal time bias
in observational studies. JAMA. 325:686–687. 2021.PubMed/NCBI View Article : Google Scholar
|