Introduction
Chemotherapy and radiotherapy have contributed to
improvements in the clinical outcomes of cancer patients. However,
secondary malignancy is one of the most critical late complications
associated with cytotoxic treatment. Although myeloid neoplasms
post-cytotoxic therapy (MN-pCT), such as acute myeloid leukemia
(AML) and myelodysplastic syndrome (MDS) are well-known
complications in the sphere of hematology (1), they have rarely been reported in
patients with thymoma.
The present study describes the case of a patient
with AML post-cytotoxic therapy (AML-pCT) that developed following
chemotherapy involving carboplatin/etoposide,
carboplatin/paclitaxel and amrubicin monotherapy for thymoma.
Case report
A 64-year-old female patient was referred to the
Osaka Metropolitan University Hospital (Osaka, Japan) due to an
abnormal shadow on a chest X-ray. A computed tomography scan
revealed an anterior mediastinal mass measuring 6x7 cm. The patient
underwent surgical resection, and a pathological examination of a
resected specimen revealed thymoma, type B1. Pleural metastases and
malignant pleural effusion were detected during surgery; therefore,
the patient was diagnosed with stage IVa disease (T3N0M1a,
according to the Union for International Cancer Control
classification) (2). The patient
underwent post-operative surveillance, and computed tomography
performed at 16 months post-surgery revealed disease recurrence.
Thereafter, the patient received four cycles of chemotherapy
involving carboplatin and etoposide. Although the recurrent tumor
yielded a partial response, pleural metastases indicated regrowth
at 25 months following surgery. At this time, widespread multiple
pleural metastases were detected; hence, it was considered that
surgical treatment and radiotherapy were not suitable for this case
due to the impossibility of achieving complete surgical treatment
and tumor control by radiotherapy on the basis of the pre-operative
evaluation. A total of four cycles of second-line chemotherapy,
including carboplatin and paclitaxel resulted in progressive
disease. Therefore, third-line chemotherapy (amrubicin monotherapy)
was administered. Although the patient achieved a partial response,
blasts in peripheral blood were detected in the 27th cycle. The
blasts in peripheral blood gradually increased to >20% and,
thus, the patient was referred to the Department of Hematology
(Osaka General Hospital of West Japan Railway Company). A bone
marrow examination was performed, which revealed an excess of
blasts. Based on this finding, the patient was diagnosed with
AML-pCT at 56 months following surgery.
A chromosomal analysis of the bone marrow cells
revealed the following unbalanced complex karyotype:
46,XX,add(6)(q23)[1]/46,idem,-19,+mar[1]/47,XX,+mar1[6]/46,XX[11].
The patient received induction chemotherapy involving daunorubicin
and cytosine arabinoside, and achieved complete remission.
Thereafter, three cycles of consolidative chemotherapy consisting
of high-dose cytosine arabinoside were administered. However, the
regrowth of pleural metastases of thymoma was observed. A total of
ten cycles of amrubicin monotherapy were attempted; however, these
resulted in progressive disease. AML-pCT also recurred at 55 months
following surgery. Although the patient received six cycles of
azacitidine monotherapy, pancytopenia gradually progressed. At this
time, a bone marrow examination revealed an excess of blasts
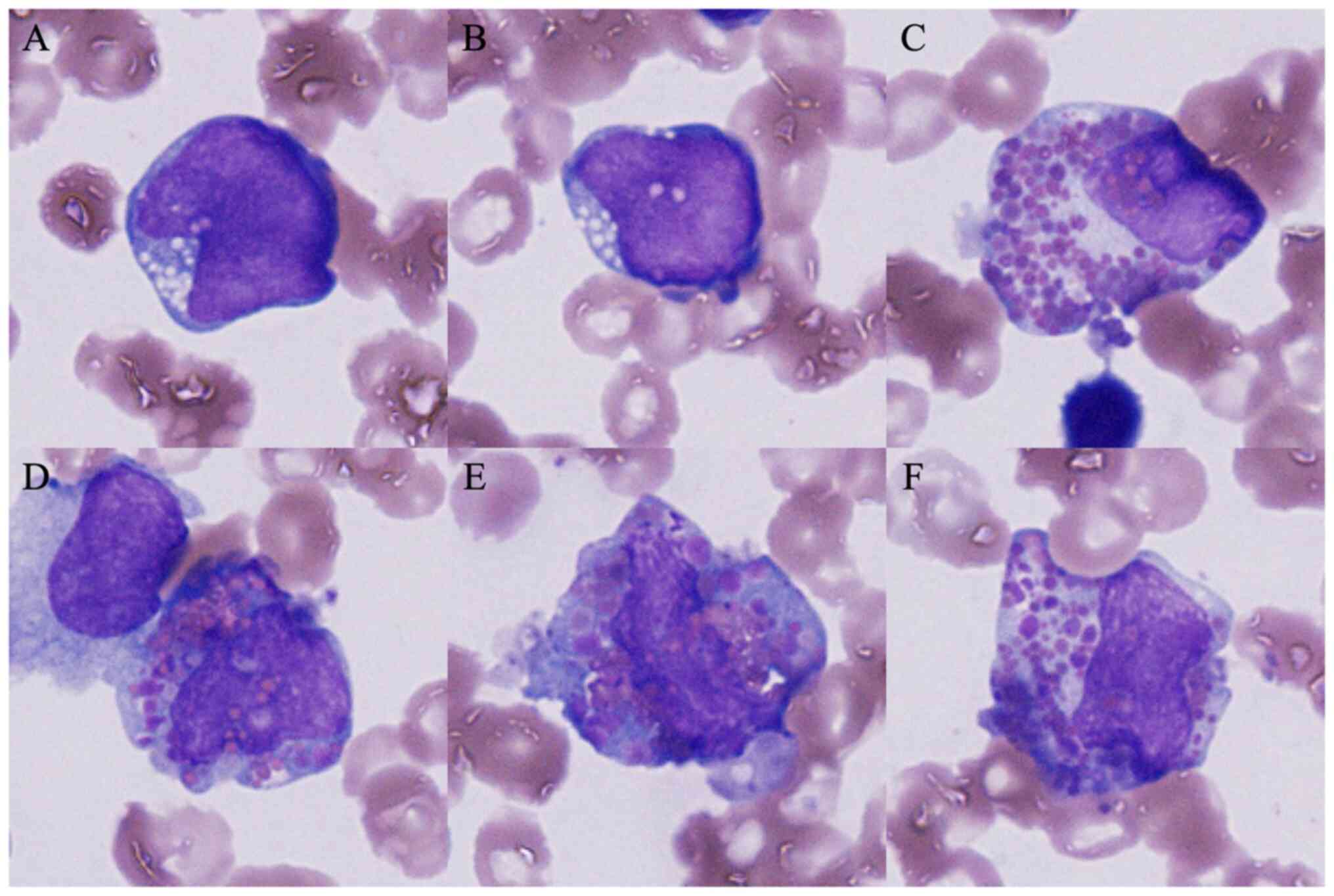
(31.6%). In addition, some blasts harboring both eosinophilic and
basophilic granules in their cytoplasm, so-called harlequin cells,
were detected (Fig. 1). The cells
were fixed with absolute methanol for 10 min, stained with May
Grünwald solution (cat. no. 954-0791-7; Sysmex Corporation) for 5
min, stained with Giemsa solution (cat. no. 954-0801-7; Sysmex
Corporation) for 5 min and then in buffer (pH 6.8) for 2 min, all
at room temperature. Images were captured using a light microscope
(BX43, Olympus Corporation) equipped with a DP73 camera (Olympus
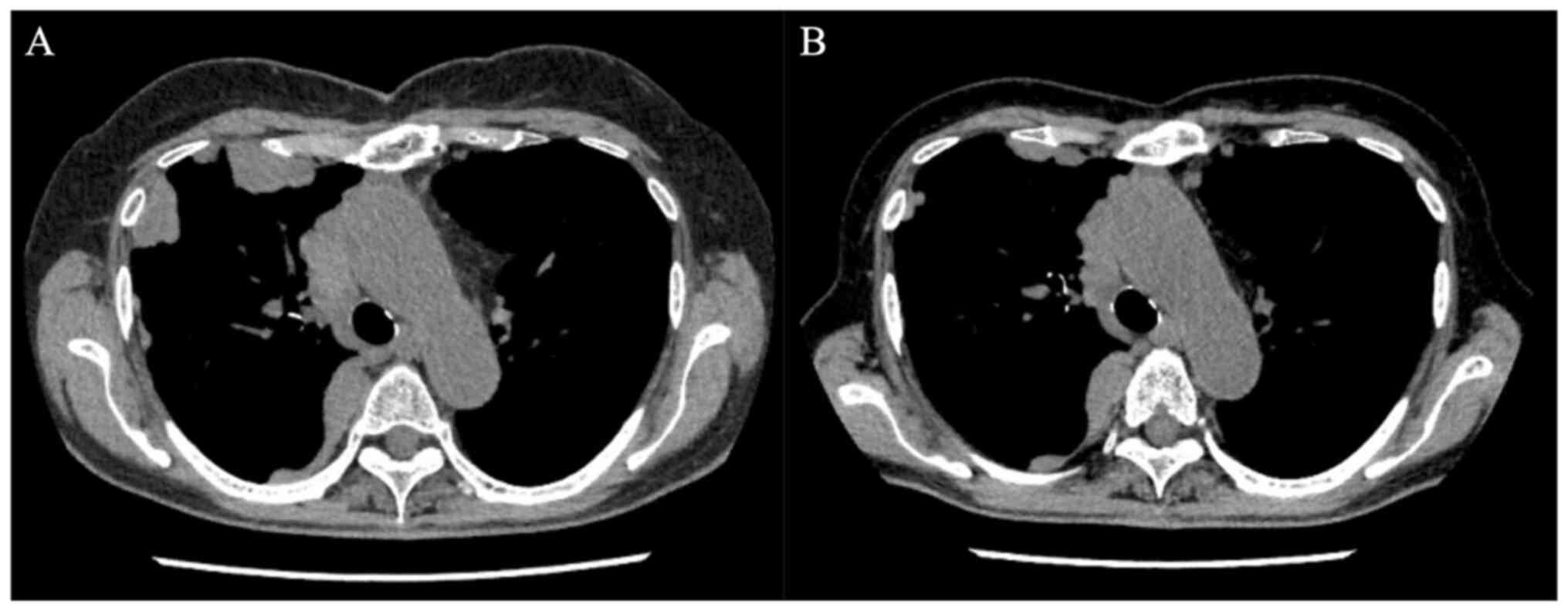
Corporation). The patient began induction chemotherapy with
idarubicin and cytosine arabinoside. Although the residual lesions
of metastatic thymoma exhibited improvement (Fig. 2), the remission of leukemia was not
achieved. A total of three cycles of chemotherapy involving
azacitidine and venetoclax, and re-induction chemotherapy with
etoposide, cytosine arabinoside and mitoxantrone were attempted;
however, this resulted in leukemic progression. The patient opted
to discontinue chemotherapy and was discharged from the hospital at
102 months following surgery.
Discussion
MN-pCT are some of the most common secondary
malignancies occurring as late complications of cytotoxic therapy.
MN-pCT may be caused by conventional chemotherapy, as well as
large-field radiation therapy. AML-pCT accounts for 7-8% of all AML
cases (3-5).
The association between the causative treatment and leukemogenesis
differs for alkylating agents and topoisomerase II inhibitors.
Alkylating drugs frequently cause deletion-type chromosomal
abnormalities, such as monosomy and/or the deletion of chromosomes
5 and 7, and AML develops through MDS 5 to 7 years after their
administration (6). Furthermore,
platinum agents, such as carboplatin, which was administered to the
patient described in the present study, act as alkylators. On the
other hand, topoisomerase II inhibitors, including anthracycline,
cause AML to develop within 2 years of their administration without
undergoing MDS, and are often accompanied by balanced chromosomal
translocations (6). Amrubicin and
etoposide administered to the patient in the present study were
applicable to this category, and a platinum agent harboring an
alkylating effect appeared to be the causative drug as AML occurred
>2 years following the initiation of chemotherapy for thymoma,
in addition to the presence of unbalanced chromosomal abnormalities
at its diagnosis.
The most common cause of AML-pCT is breast cancer,
followed by hematological malignancies, and thyroid,
gastrointestinal, prostate and testicular cancer (3). However, the incidence of MN-pCT that
develop after other malignancies, such as ovarian cancer and skin
cancers, is low. Furthermore, cases of MN-pCT in which thymoma is
the primary disease are even rarer. As regards the disease
incidence, Kayser et al (3)
detected only 2 patients (1%) who received cytotoxic therapy for
mediastinal cancers among 200 patients with AML-pCT. Although there
have been cases of AML-pCT occurring in patients with thymic cancer
receiving long-term chemotherapy (7), such as case described herein, they are
extremely rare. Apart from therapy-related complications, thymoma
is associated with secondary malignancies, and leukemia has also
been reported (8), although at a low
incidence rate. Although it is unclear whether the scarcity of
MN-pCT cases caused by treatment for thymoma is due to these cases
truly being rare or misdiagnosed (i.e., as de novo myeloid
neoplasm or secondary malignancy associated with thymoma), the
accumulation of further cases is required in order to obtain
accurate data on the frequency of MN-pCT following treatment for
thymoma.
As regards clinical outcomes, the prognosis of
MN-pCT, including overall survival, is poorer than that of de
novo AML; i.e., the estimated median overall survival is 10-14
months (3,5,9).
However, the therapeutic outcomes of patients with thymoma have
recently improved due to advances in surgery and chemotherapy. In
addition, the median latency period between the diagnosis of the
primary malignancy and the occurrence of AML-pCT was 4.04 years
(3). As the incidence of MN-pCT in
patients with thymoma may increase in the future as treatment
advances prolong their survival, as has been observed in all cancer
patients in the USA (10), it is
critical for clinicians in thoracic surgery/clinical oncology
departments to be aware that MN-pCT may occur as a late
complication of treatment for thymoma.
Concerning the treatment outcome of the case
described in the present study, chemotherapy for AML resulted in a
refractory course. On the other hand, chemotherapy involving
idarubicin and cytosine arabinoside contributed to a reduction in
the residual pleural metastases of the thymoma. The treatment of
cancer patients with synchronous multiple primaries is generally
challenging and often a therapeutic dilemma. Additionally, in the
case of advanced disease, the selection of antitumor therapy is
often difficult and mostly not based on evidence from the
literature and clinical trials (11). Therefore, in cases involving
refractory co-existing malignancies, the continuation of definitive
chemotherapy requires careful consideration. Key points that need
to be considered with synchronous malignancies are patient fitness,
the prognosis of each cancer, the chance of a cure, whether one
tumor may be treated radically and the other sequentially,
anticipated complications, obstructive symptoms and if other
similar treatments may be used (12). In addition to the performance status
being good in the case present herein, cytopenia caused by AML
progressed; therefore, chemotherapy for AML was prioritized
following a multi-disciplinary consultation board. Although
idarubicin, which is categorized as an anthracycline similar to
amrubicin and was used as chemotherapy for thymoma, may have been
effective, there have been no reports of similar cases, at least to
the best of our knowledge. Therefore, the clinical course of the
present case is considered to be valuable as a reference for future
practice.
This case was morphologically characteristic in that
harlequin cells were noted. Harlequin cells are defined as
involving both basophilic and eosinophilic granules (13). As regards their morphological
significance, harlequin cells are often observed in leukemia,
particularly in AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22)
(13). On the other hand, harlequin
cells have also been ubiquitously detected and are not limited to
hematological malignancies (14),
and their evaluation remains controversial. Confirmation in
large-scale studies on a large number of patients is required in
order to establish whether this is common in AML-pCT, as in the
case described in the present study.
Lastly, as for therapeutic strategy for the
recurrence of thymoma in the patient described herein, systemic
chemotherapy rather than surgical treatment and/or radiotherapy was
selected. Although long-term survival was observed in a previous
study following the surgical treatment of thymoma recurrences, that
study examined patients with stage I-III disease and did not
include cases with advanced-stage IV disease, such as in the
present study (15). In addition,
surgical treatment was not considered to be curable due to the
large number of metastases in the case in the present study.
Furthermore, radiation therapy was avoided for similar reasons. As
the results of previous studies analyzing therapeutic approaches
for the treatment of recurrences of thymoma have been controversial
(16,17), further studies are warranted to
establish which modalities among chemotherapy, surgical treatment,
or radiotherapy, are appropriate for the treatment of recurrent
thymoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
MM and YT designed the study. MM was the principal
author responsible for the study and wrote the original manuscript.
NI, YH, NS and SN performed the laboratory analysis of the
specimens described in the study. NS, SN and KRK conducted a
critical literature review, contributed to the acquisition,
analysis, and interpretation of data and contributed to the
drafting of the Discussion section. MM and YT confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the study.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the ethical standards from the 1964 Declaration of Helsinki and its
later amendments. Written informed consent was obtained from the
patient for the case information.
Patient consent for publication
Written informed consent was obtained from the
patient for the case information and any related images to be
published in this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khoury JD, Solary E, Abla O, Akkari Y,
Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, et
al: The 5th edition of the World Health Organization Classification
of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic
Neoplasms. Leukemia. 36:1703–1719. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Union for International Cancer Control
(UICC): TNM Classification of Malignant Tumors. Brierley JD,
Gospodarowicz MK, Wittekind C (eds): 8th edition. Wiley-Blackwell,
Hoboken, NJ, 2017.
|
|
3
|
Kayser S, Döhner K, Krauter J, Köhne CH,
Horst HA, Held G, von Lilienfeld-Toal M, Wilhelm S, Kündgen A,
Götze K, et al: The impact of therapy-related acute myeloid
leukemia (AML) on outcome in 2853 adult patients with newly
diagnosed AML. Blood. 17:2137–2145. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hulegårdh E, Nilsson C, Lazarevic V,
Garelius H, Antunovic P, Rangert Derolf Å, Möllgård L, Uggla B,
Wennström L, Wahlin A, et al: Characterization and prognostic
features of secondary acute myeloid leukemia in a population-based
setting: A report from the Swedish Acute Leukemia Registry. Am J
Hematol. 90:208–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Granfeldt Østgård LS, Medeiros BC,
Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, Friis LS, Kjeldsen
E, Marcher CW, Preiss B, et al: Epidemiology and clinical
significance of secondary and therapy-related acute myeloid
leukemia: A national population-based cohort study. J Clin Oncol.
33:3641–3649. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Strickland SA and Vey N: Diagnosis and
treatment of therapy-related acute myeloid leukemia. Crit Rev Oncol
Hematol. 171(103607)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kaira K, Naruse I, Shinomiya S and Kagamu
H: Occurrence of hematological malignancy in long-term survivors
with advanced thymic cancer. In Vivo. 34:1511–1513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Engels EA: Epidemiology of thymoma and
associated malignancies. J Thorac Oncol. 5 (10 Suppl 4):S260–S265.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fianchi L, Pagano L, Piciocchi A, Candoni
A, Gaidano G, Breccia M, Criscuolo M, Specchia G, Maria Pogliani E,
Maurillo L, et al: Characteristics and outcome of therapy-related
myeloid neoplasms: Report from the Italian network on secondary
leukemias. Am J Hematol. 90:E80–E85. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Morton LM, Dores GM, Tucker MA, Kim CJ,
Onel K, Gilbert ES, Fraumeni JF Jr and Curtis RE: Evolving risk of
therapy-related acute myeloid leukemia following cancer
chemotherapy among adults in the United States, 1975-2008. Blood.
121:2996–3004. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open.
2(e000172)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alghanmi HA: Successful treatment of
synchronous double lung primary malignancies and colon cancer.
Cureus. 14(e22552)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hirano T and Eto K: Harlequin cells.
Blood. 132(766)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jain G, Kumar C and Chopra A: Harlequin
cell: Ubiquitous or pathognomic? Int J Lab Hematol. 42:e42–e44.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Carretta A, Ciriaco P, Muriana P, Bandiera
A and Negri G: Surgical treatment of single and multiple thymoma
recurrences. Gen Thorac Cardiovasc Surg. 68:350–356.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hamaji M, Allen MS, Cassivi SD, Nichols FC
III, Wigle DA, Deschamps C and Shen KR: The role of surgical
management in recurrent thymic tumors. Ann Thorac Surg. 94:247–254.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Miyata R, Hamaji M, Omasa M, Miyahara S,
Aoyama A, Takahashi Y, Sumitomo R, Huang CL, Hijiya K, Nakagawa T,
et al: The treatment and survival of patients with postoperative
recurrent thymic carcinoma and neuroendocrine carcinoma: A
multicenter retrospective study. Surg Today. 54:502–510.
2021.PubMed/NCBI View Article : Google Scholar
|